APPLIED ANDENVIRONMENTALMICROBIOLOGY, Apr. 2011, p. 2527–2530 Vol. 77, No. 7 0099-2240/11/$12.00 doi:10.1128/AEM.02577-10
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
Accumulation of Sulfonamide Resistance Genes in Arable Soils Due
to Repeated Application of Manure Containing Sulfadiazine
䌤
Holger Heuer,
1Qodiah Solehati,
1Ute Zimmerling,
1Kristina Kleineidam,
2Michael Schloter,
2Tanja Mu
¨ller,
3Andreas Focks,
3So
¨ren Thiele-Bruhn,
4and Kornelia Smalla
1*
Julius Ku¨hn-Institut, Federal Research Centre for Cultivated Plants (JKI), Department of Epidemiology and Pathogen Diagnostics, Messeweg 11-12, 38104 Braunschweig, Germany1; Helmholtz Zentrum Mu¨nchen, Department of Terrestrial Ecogenetics,
Ingolsta¨dter Landstr. 1, 85764 Neuherberg, Germany2; Universita¨t Osnabru¨ck, Institute of Environmental Systems Research,
Barbarastr. 12, 49076 Osnabru¨ck, Germany3; and University of Trier, Department of Soil Science,
Behringstr. 21, 54286 Trier, Germany4
Received 2 November 2010/Accepted 29 January 2011
Two soils were amended three times with pig manure. The abundance of sulfonamide resistance genes was determined by quantitative PCR 2 months after each application. In both soils treated with sulfadiazine-containing manure, the numbers of copies ofsul1andsul2significantly increased compared to numbers after treatments with antibiotic-free manure or a control and accumulated with repeated applications.
The transmission of antibiotic resistance through agriculture may have a greater impact on human health than hospital transmission of resistance (25), as gene transfer and the envi-ronmental spread of antibiotic resistance genes might substan-tially contribute to antibiotic resistance in the human micro-biome, which exacerbates the threat of infections with antibiotic-resistant bacteria (10). Antibiotic resistance genes on transferable plasmids are introduced via manure into agro-ecosystems and can persist regardless of the viability of the introduced host cell due to horizontal gene transfer (9). Pig manure often contains considerable amounts of administered antibiotics that pose a selective pressure on bacterial commu-nities during storage of manure and after application to soil (5, 27). Sulfonamides are among the most widely used veterinary antibiotics in the European Union (19), of which especially sulfadiazine (SDZ) has a high usage and potential to enter the environment (6). Resistance to sulfonamides is mediated mainly by the genessul1andsul2, coding for dihydropteroate synthases which are insensitive to sulfonamides (24). The genes occur in a wide range of bacterial species, because they are often located on transposable elements of self-transferable or mobilizable broad-host-range plasmids (8, 16, 23). Feeding experiments performed with 14C-labeled SDZ showed that
more than 96% of the SDZ administered to pigs was excreted within 10 days and did not decrease during storage of the manure over 6 months (13). A survey of 15 field-scale piggery manures showed that piggery manure represents a hot spot for antibiotic resistance genes as well as broad-host-range plas-mids (2–4). In a soil microcosm experiment, significant effects of manure containing SDZ on the abundance of the sulfon-amide resistance genes sul1 and sul2 were detected even 2 months after application, compared to the abundance in un-treated soil or soil un-treated with manure devoid of antibiotics (14, 15). Also, the frequency of capturing mobile genetic
ele-ments conferring SDZ resistance was increased by manure containing SDZ. The effects of a single manure application on bacterial resistance levels decreased over time due to dimin-ished selective pressure, and eventually the resistance level of untreated soil was restored. Farmers typically apply manure to their fields several times a year (22), which might lead to an accumulation of resistance genes and antibiotic compounds in soil. However, this has not been investigated so far (9). Only Knapp et al. showed increases in the numbers of resistance genes over recent decades in archived soil samples from agri-cultural sites but could not correlate them with manure appli-cation due to confounding factors (17). Therefore, it was the objective of this study to test whether repeated applications of manure containing SDZ results in an accumulation ofsulgenes in soil bacterial communities.
Manure from healthy mature pigs was applied to topsoil samples (Ap horizon) of two German agricultural soils that substantially differed physically and chemically (20). Soil M was a silt loam (Orthic Luvisol) which had no history of pre-vious manure applications. Soil K was a loamy sand (Gleyic Cambisol) fertilized in previous years with manure. Four treat-ments were prepared in pots with 100 g of 2-mm-sieved soil. To each pot, 4 g of manure with or without SDZ was added, so that an initial concentration of 0, 10, or 100 mg kg⫺1
soil was achieved for treatment S0, S10, or S100, respectively. The amount of manure roughly corresponded to 30 m3
ha⫺1
, and treatment S10 corresponded to a realistic maximal input of sulfonamides according to agricultural practice (13, 21). The soil moisture content was set to 55% of the soil’s water-holding capacity. All treatments were set up individually in five inde-pendent replicates per soil and per sampling time. The loosely covered pots were incubated at 15°C in the dark. The weight loss of the microcosms was compensated for by adding water to the soil surface twice a week. Manure was applied at day 0, day 63, and day 133. The soil samples analyzed were taken 60 days after each manure application. Total community DNA was extracted using the FastPrep FP120 bead-beating system for cell lysis and the FastDNA spin kit for soil (Q-Biogene, Carls-bad, CA). The copy numbers of sul1, sul2, and 16S rRNA
* Corresponding author. Mailing address: JKI, Messeweg 11-12, 38104 Braunschweig, Germany. Phone: 49531299-3814. Fax: 49531299-3006. E-mail: kornelia.smalla@jki.bund.de.
䌤Published ahead of print on 4 February 2011.
genes were quantified by 5⬘-nuclease assays using quantitative real-time PCR as previously described (14, 15). Copy numbers ofsulgenes were related to 16S rRNA gene copy numbers and log transformed (Fig. 1). Treatment effects were analyzed by analysis of variance (ANOVA) using the MIXED procedure for repeated measures of SAS package 9.2 (SAS Institute, Cary, NC).
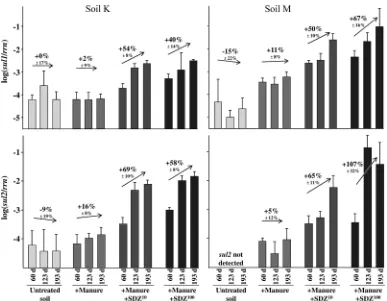
Two months after each application of antibiotic-free ma-nure, the levels ofsul1genes in soil M were significantly in-creased compared to levels in untreated soil by about 1 order of magnitude (P⬍0.0001). The abundance ofsul2genes was below the detection limit in untreated soil M at all time points analyzed but became detectable after the addition of manure (Fig. 1). Probably due to the long history of manure fertiliza-tion, soil K responded less to manure, which had no effect on
sul1 (P ⫽ 0.2) and only a slight effect on sul2 (P ⫽ 0.03) abundances. The treatments with SDZ-containing manure (S10, S100) resulted in significantly higher abundance levels of both sulgenes than were obtained with the treatments with antibiotic-free manure (P⬍0.0001), and this was observed in both soils.
Linear-regression analysis revealed no change over time or a trend toward a slight decrease insulgene copy numbers for both untreated soils (Fig. 1). Only moderate increases insul
gene abundance were detected during the course of repeated applications of antibiotic-free manure, which were within the
error range forsul1in soil K andsul2in soil M. In contrast, significant accumulations of bothsulgenes were observed for both soils in the course of repeated applications of SDZ-con-taining manure. The increases ranged between 40% and 107% of log units per manure application. Two months after the third application of SDZ-containing manure to soil M, the abundance ofsul1increased by more than 3 orders of magni-tude in comparison to the abundance in untreated soil M. A maximum of⫺1 log ofsul1andsul2per ribosomal gene copy was reached, meaning that roughly 10% of the soil bacteria became resistant to sulfonamides. The accumulation was less strong in soil K but still exceeded 1 order of magnitude. For soil M, but not for soil K, the accumulation was more pro-nounced after treatment S100 than after S10. This indicated that the bacterial community of soil M that had previously received only mineral fertilizer was less adapted to manure-SDZ treatment and thus responded more strongly.
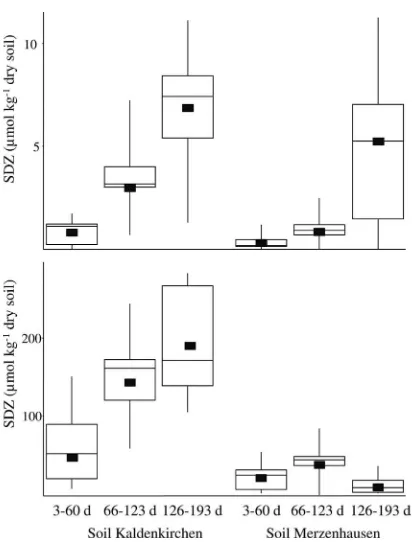
The desorbable and hence potentially bioavailable fraction of SDZ (26) was sequentially extracted with 0.01 M CaCl2and
methanol and quantified by high-performance liquid chroma-tography (12). As previously shown (13), the easily extractable fraction of SDZ rapidly declined after the manure was spread on soil but was still detectable 2 months after application (data not shown). The average concentration of SDZ in soil K in-creased after each manure application of treatments S10 and S100 and in soil M after treatment S10 (Fig. 2). The dissipation
FIG. 1. Effects of antibiotic-free manure and manure containing sulfadiazine (SDZ10or SDZ100, 10 or 100 mg kg⫺1soil) on the abundances
of the sulfonamide resistance genessul1andsul2relative to ribosomalrrngene abundances in two soils, as quantified by 5⬘-nuclease assays using quantitative real-time PCR. Manure was applied at days 0, 63, and 133. Samples were analyzed 2 months after each application (days 60, 123, and 193). Error bars indicate the standard deviations (n⫽5). Values over arrows show the percentages of alterations due to the repeated applications of the respective manures within a treatment (⫾estimated errors of slopes), as revealed by linear-regression analysis.
of SDZ in the soil matrix did not occur quickly enough to prevent the accumulation of effective concentrations in soil. Notably, adsorption and/or absorption of SDZ by microorgan-isms cannot be excluded as a putative mechanism of dissipa-tion. In contrast to what occurred with the other samples, after the S100 treatment of soil M, the concentration of SDZ de-clined in the period after the third manure application. This might indicate the development of microbial SDZ degraders in this treatment. The contribution of reversibly sequestered res-idues to the bioavailable fraction is not yet clear, but they might well serve as a long-term reservoir for SDZ in soils treated with SDZ-containing manure (11).
The results obtained from the soil microcosm experiment supported the hypothesis that repeated applications of manure increase the abundance of sulfonamide resistance genes in soil. Obviously, this was not so much caused by the addition ofsul
genes originating from manure (S0 treatment) but rather by the selective pressure exerted by bioavailable SDZ in soil. Manure typically contains substantial numbers of resistant bac-teria, with 10⫺3 to 10⫺2 sul1copies and 10⫺2 to 10⫺1 sul2
copies per 16S rRNA gene copy (1, 14, 15). These numbers were high enough to result in a temporary increase insulgenes in soil after manure application in earlier studies (14, 15). The substrate from manure allows for the growth of populations carryingsulgenes under selective pressure of SDZ. Increased selection rates could be observed even at low antibiotic con-centrations (13, 18), but without the addition of the substrate,
the bacteriostatic SDZ could not affect soil bacterial commu-nities (7, 28). Compared to the rate of vertical transmission of
sulgenes by the growth of resistant populations, the rate of horizontal transmission in soil is presumably very low. How-ever, the horizontal transfer of resistance is an important fac-tor in the dissemination of resistance, as bacteria from manure may not be well adapted to the soil environment (9, 12, 13). The horizontal transfer ofsulgenes may have initially resulted in intraspecific, resistant subpopulations in soil, if they were not already present, and subsequentlysulgenes may have be-come relatively more abundant by the selective effect of SDZ-containing manure. This hypothesis is supported by the signif-icant increase in the number of resistance genes found in the soil treated with SDZ after the repeated applications, whereas the levels ofsulgenes in the soil treated with only manure were rather constant and independent of the number of applications of manure.
Overall, our data indicated that the accumulation of resis-tance genes in environmental bacterial communities and the consequences of the potential long-term persistence of active pharmaceutical residues in soil should be considered more in risk assessments, which previously not only have been impeded by a shortage of suitable data but also have rather ignored important mechanisms, like the cross-species spread of resis-tance by plasmid transfer. As humans are continuously ex-posed to bacteria in the environment, the accumulation of resistance genes in soil due to the spreading of manure is likely to contribute to the threat of antimicrobial resistance in the therapy of infectious diseases.
This study was funded by the Deutsche Forschungsgemeinschaft (FOR566: “Veterinary Medicines in Soils: Basic Research for Risk Analysis”).
REFERENCES
1.Binh, C. T. T., H. Heuer, N. C. M. Gomes, M. Kaupenjohann, and K. Smalla. 2010. Similar bacterial community structure and high abundance of sulfon-amide resistance genes in field-scale manures, p. 141–166.InC. S. Della-guardia (ed.), Manure: management, uses and environmental impacts. Nova Science Publishers, Hauppauge, NY.
2.Binh, C. T. T., et al.2007. Short-term effects of amoxicillin on bacterial communities in manured soil. FEMS Microbiol. Ecol.62:290–302. 3.Binh, C. T. T., H. Heuer, M. Kaupenjohann, and K. Smalla.2008. Piggery
manure used for soil fertilization is a reservoir for transferable antibiotic resistance plasmids. FEMS Microbiol. Ecol.66:25–37.
4.Binh, C. T. T., H. Heuer, M. Kaupenjohann, and K. Smalla.2009. Diverse
aadA gene cassettes on class 1 integrons introduced into soil via spread manure. Res. Microbiol.160:427–433.
5.Boxall, A. B., et al.2004. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol.180:1–91.
6.Boxall, A. B. A., et al.2003. Prioritisation of veterinary medicines in the UK environment. Toxicol. Lett.142:207–218.
7.Brandt, K. K., O. R. Sjøholm, K. A. Krogh, B. Halling-Sørensen, and O. Nybroe.2009. Increased pollution-induced bacterial community tolerance to sulfadiazine in soil hotspots amended with artificial root exudates. Environ. Sci. Technol.43:2963–2968.
8.Byrne-Bailey, K. G., et al.2009. Prevalence of sulfonamide resistance genes in bacterial isolates from manured agricultural soils and pig slurry in the United Kingdom. Antimicrob. Agents Chemother.53:696–702.
9.Chee-Sanford, J. C., et al.2009. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual.38:1086–1108.
10.Croft, A. C., A. V. D’Antoni, and S. L. Terzulli.2007. Update on the anti-bacterial resistance crisis. Med. Sci. Monitor13:RA103–RA118.
11.Fo¨rster, M., et al.2009. Sequestration of manure-applied sulfadiazine resi-dues in soils. Environ. Sci. Technol.43:1824–1830.
12.Hammesfahr, U., H. Heuer, B. Manzke, K. Smalla, and S. Thiele-Bruhn. 2008. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biol. Biochem.40:1583–1591. 13.Heuer, H., et al.2008. Fate of sulfadiazine administered to pigs and its FIG. 2. Sulfadiazine concentrations in soil microcosms after
treat-ment S10 (upper graph) and S100 (lower graph) during the 60-day periods after each of three manure applications. Black boxes represent the temporal averages for each period, which were calculated from interpolated concentrations with a time step of 1 day. Box whisker plots show the medians, quartiles, and ranges of measured concentra-tions.
quantitative effect on the dynamics of bacterial resistance genes in manure and manured soil. Soil Biol. Biochem.40:1892–1900.
14.Heuer, H., C. Kopmann, C. T. T. Binh, E. M. Top, and K. Smalla.2009. Spreading antibiotic resistance through spread manure: characteristics of a novel plasmid type with low %G⫹C content. Environ. Microbiol.11:937–949. 15.Heuer, H., and K. Smalla.2007. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol.9:657–666.
16.Heuer, H., et al.2004. The complete sequences of plasmids pB2 and pB3 provide evidence for a recent ancestor of the IncP-1group without any accessory genes. Microbiology150:3591–3599.
17.Knapp, C. W., J. Dolfing, P. A. Ehlert, and D. W. Graham.2010. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol.44:580–587.
18.Knapp, C. W., et al.2008. Indirect evidence of transposon-mediated selec-tion of antibiotic resistance genes in aquatic systems at low-level oxytetra-cycline exposures. Environ. Sci. Technol.42:5348–5353.
19.Kools, S. A. E., J. F. Moltmann, and T. Knacker.2008. Estimating the use of veterinary medicines in the European Union. Regul. Toxicol. Pharmacol. 50:59–65.
20.Kotzerke, A., et al.2008. Alterations in soil microbial activity and N-trans-formation processes due to sulfadiazine loads in pig-manure. Environ. Pol-lut.153:315–322.
21.Lamsho¨ft, M., P. Sukul, S. Zu¨hlke, and M. Spiteller.2010. Behaviour of C-14-sulfadiazine and C-14-difloxacin during manure storage. Sci. Total En-viron.408:1563–1568.
22.Montforts, M. H. M. M., D. F. Kalf, P. L. A. van Vlaardingen, and J. B. H. J. Linders.1999. The exposure assessment for veterinary medicinal products. Sci. Total Environ.225:119–133.
23.Schlu¨ter, A., et al.2003. The 64 508 bp IncP-1antibiotic multiresistance plasmid pB10 isolated from a waste-water treatment plant provides evidence for recombination between members of different branches of the IncP-1
group. Microbiology149:3139–3153.
24.Sko¨ld, O.2000. Sulfonamide resistance: mechanisms and trends. Drug Re-sist. Updat.3:155–160.
25.Smith, D. L., J. Dushoff, and J. G. Morris.2005. Agricultural antibiotics and human health—does antibiotic use in agriculture have a greater impact than hospital use? PLoS Med.2:731–735.
26.Thiele-Bruhn, S., and M. O. Aust.2004. Effects of pig slurry on the sorption of sulfonamide antibiotics in soil. Arch. Environ. Contam. Toxicol.47:31–39. 27.Witte, W.2000. Ecological impact of antibiotic use in animals on different complex microflora: environment. Int. J. Antimicrob. Agents14:321–325. 28.Zielezny, Y., J. Groeneweg, H. Vereecken, and W. Tappe.2006. Impact of sulfadiazine and chlorotetracycline on soil bacterial community structure and respiratory activity. Soil Biol. Biochem.38:2372–2380.