L
Journal of Experimental Marine Biology and Ecology, 240 (1999) 285–302
Effects of photoperiod and temperature on the regulation of
the onset of maturation in the estuarine fish Menidia
beryllina (Cope) (Atherinidae)
1 *
Marina Huber , David A. Bengtson
Department of Biological Sciences, University of Rhode Island, Kingston, RI 02881, USA Received 20 August 1998; received in revised form 2 May 1999; accepted 4 May 1999
Abstract
Menidia beryllina (Cope) is an annual species that inhabits coastal estuaries along the east coast of the United States from Cape Cod to the Gulf of Mexico. In Rhode Island, USA, its peak spawning time and the duration of the spawning vary among years and estuaries. However, the onset of gonadal maturation is more consistent (early May), suggesting that it may be regulated by more consistent cues than those regulating spawning. To determine the effects of photoperiod and temperature on the regulation of the onset of maturation two laboratory experiments were conducted and the results compared to field observations. In the first experiment, fish collected from the field in February were exposed to each of four treatments: increasing photoperiod / increasing temperature; increasing photoperiod / low temperature; low photoperiod / increasing temperature; and low photoperiod / low temperature. Only fish exposed to both increasing photoperiod and increasing temperature were able to complete maturation. Fish exposed to low photoperiod and increasing temperature responded by enlarging their livers, a response that was also observed in field fish collected in the fall. Fish exposed to the remaining two treatments neither matured their gonads nor enlarged their livers. In the second experiment fish collected from the field in early March were exposed to three treatments with different photoperiod regimes (daylight constant at 9.5 h, increasing up to 12 h, or increasing up to 15 h) and one increasing temperature regime. Fish in the 9.5-h treatment initiated maturation but were not able to complete the process, those exposed to the 12-h photoperiod matured and spawned for a short period of time before the gonads began to regress, and those exposed to the 15-h photoperiod matured and spawned large numbers of eggs throughout the remainder of the experiment. The 9.5- and 12-h photoperiod exposures also resulted in accumulation of reserves in the liver in both females and males. The 15-h photoperiod treatment resulted in liver enlargement in females, which were undergoing vitellogenesis, but not in males. Males exposed to the 9.5- and 12-h photoperiod
*Corresponding author. Department of Fisheries, Animal and Veterinary Science, University of Rhode Island, Kingston, RI 02881, USA. Tel.: 11-401-874-2668; fax: 11-401-874-4017.
E-mail address: [email protected] (D.A. Bengtson) 1
accumulated significantly more visceral fat than those exposed to the 15-h photoperiod. In females, the amount of visceral fat accumulated was inversely proportional to the hours of light. These findings suggest that this species has evolved mechanisms that enable it to anticipate the coming of winter as well as the coming of suitable breeding conditions and ensure that it exhibits the appropriate response at the appropriate time (reserve accumulation for the winter or gonad maturation in the spring). 1999 Elsevier Science B.V. All rights reserved.
Keywords: Photoperiod; Temperature; Reproduction; Gonadosomatic index; Hepatosomatic index; Visceral fat; Spawning cues; Silversides
1. Introduction
In temperate regions conditions suitable for the development of larval stages of organisms occur during restricted times of the year. Although these conditions vary among species they usually include an abundance of food organisms, a predator-minimized environment, and / or temperatures that can optimize the rate of growth and development before the cold and food-limited conditions typical of the winter occur (Balon, 1975; Hubbs, 1976). The occurrence of these conditions in a given habitat is affected by a multitude of factors and thus their exact timing may vary from year to year. Nevertheless, fish must be able to anticipate these conditions and begin the maturation process beforehand to ensure that reproduction occurs at a time when offspring can survive (DeVlaming, 1974).
Munro (1990) proposed a tentative classification for proximate controlling factors responsible for the regulation of teleost reproductive cycles. The first category includes predictive cues responsible for the initiation of maturation by triggering the reproductive axis of the fish and initiating the sequestration of resources to reproductive tissues. The second category includes synchronizing cues which signal the arrival of suitable breeding conditions and induce final maturation and spawning. The final category contains terminating cues, which end the breeding season by inducing gonadal regression. Modifying factors can alter the response of individuals to any of the cues altering the various stages of the reproductive cycle.
The present study focuses on the effect of predictive cues on the regulation of the initiation of the reproductive cycle of the inland silverside Menidia beryllina (Cope). This species is found in coastal estuaries extending from Cape Cod, Massachusetts, USA, to Tampico, Veracruz, Mexico (Bigelow and Schroeder, 1953). It is also found in freshwater systems in the United States, such as the Mississippi River drainage in Tennessee (Gosline, 1948), Lake Texoma in Oklahoma (Mense, 1967; Hubbs et al., 1971; Hubbs, 1982), and has been introduced into lakes in Northern California (Cook and Moore, 1970; Elston and Bachen, 1976). The duration of the spawning interval of
remarkably synchronized. These populations have, at any given time, only one year class and usually only one age cohort alive and provide a very useful model for the study of environmental induction of life history events. This study was carried out in the ecological and evolutionary context that M. beryllina appears to have evolved different maturation times from those of its sympatric congener Menidia menidia (Linnaeus), so that competition between the two species is minimized (Bengtson, 1984; Huber and Bengtson, 1999).
In two Rhode Island estuaries the timing and duration of M. beryllina’s spawning interval varies among years and also between estuaries within the same year (Huber, 1990). However, timing of the beginning of gonadal weight increase (early May) appears to be more consistent than the timing of peak gonad weights and / or peak gonadosomatic indices (Huber and Bengtson, 1999) suggesting that initiation of maturation is regulated by more consistent environmental cues than those regulating spawning. Temperature and photoperiod are some of the more consistent and influential environmental cues for regulating reproduction of temperate estuarine and intertidal teleost species (Scott, 1979; Wootton, 1982; Lam, 1983; Bye, 1984; Munro, 1990; Taylor, 1990). Consequently we decided to study the effects of photoperiod and temperature on the regulation of the onset of maturation of M. beryllina in two laboratory studies. In addition field studies were conducted to determine the pattern of resource allocation of this species during most of its life cycle in a Rhode Island estuary, so that natural changes experienced by the fish in the wild could be compared with the results of the experiments.
2. Methods
2.1. Field methods
Menidia beryllina were collected with a 15.2-m beach seine (mesh 2.5 mm) from the
indices were calculated by dividing the dry weights of the gonad, liver, and visceral fat respectively, by the weight of the eviscerated carcass3100. Because of the role of the liver in storing reserves intended for both reproductive and somatic purposes, throughout this paper the liver will be treated separately and the term ‘somatic’ will be used to refer to the eviscerated carcass alone.
2.2. Experiment 1
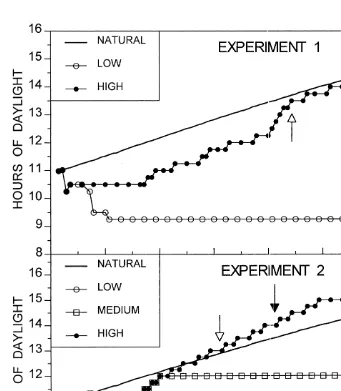
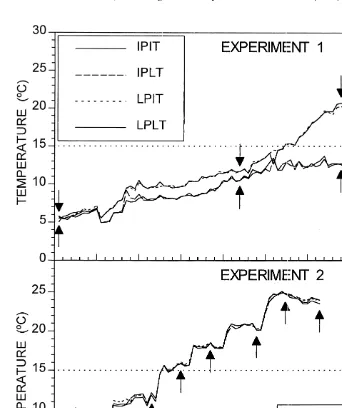
Experiment 1 consisted of four experimental treatments of differing photoperiod and temperature regimes with four replicates each. The first treatment consisted of increasing photoperiod and increasing temperature (IPIT) intended to simulate spring conditions; the second treatment of increasing photoperiod and low temperature (IPLT); the third treatment of low photoperiod and increasing temperature (LPIT); the fourth treatment of low photoperiod and low temperature (LPLT) intended to simulate winter conditions. The increasing photoperiod and increasing temperature regimes were slowly increased on a daily basis to simulate natural changes (Figs. 1 and 2).
The fish were collected from February 24–28, 1992, at the Upper Pettaquamscutt River and brought into the laboratory. During the collection period the natural photoperiod ranged from 11:01 to 11:12 hours:minutes of daylight (Mansfield, 1979). In the laboratory the fish were immediately subjected to a photoperiod of 10.5 h (Fig. 1). The temperature in the laboratory was set similar to that found in the field during the collection period (5–68C). Fish were fed live, newly hatched Artemia nauplii daily. On March 2, the fish were randomly distributed into the sixteen 75-l aquaria (29 fish per aquarium).
At the beginning of the experiment a random sample of eight females and six males was used to obtain initial average dry weights of liver, gonads, and eviscerated carcasses. The experiment started on March 3rd when the lights for the low photoperiod treatments were adjusted to 9.25 h of daylight.
Field studies in Rhode Island (Huber, 1990), New York, USA (Meade, 1988), and Florida, USA (Middaugh and Hemmer, 1992), have indicated that M. beryllina begin to mature when the water temperature increases to about 158C. The experiment was therefore designed to have the first (initial) and second samples obtained before the increasing temperature treatments reached 158C (Fig. 2). No significant differences among treatments were expected at those sampling times. The third and fourth samples were collected after the 158C threshold was crossed, and significant differences among the treatments at those times were expected. Because M. beryllina are not sexually dimorphic the sex of the fish could not be determined prior to dissection. Therefore the fish were sacrificed one at a time until three females per aquarium were obtained. The number of males sampled depended on how many had been collected by the time three females were found. The fish were dissected to obtain livers, gonads, and eviscerated carcasses which were then dried at 608C to constant weight. GSIs and HSIs were calculated as in the field data.
Fig. 1. Photoperiod regimes (hours of daylight) experienced by the various treatments used in Experiments 1 and 2. Natural photoperiod (Mansfield, 1979) is presented as well. The light arrows denote when the water temperature reached 158C in the high photoperiod treatments. The beginning of spawning in the high photoperiod treatments is denoted by the black arrows.
heated water reaching the aquarium. Initially all four treatments were allowed to warm up to 128C to minimize the mortality experienced at low temperatures (Fig. 2). However, once the water temperature reached 128C only the temperature in the two increasing temperature treatments was allowed to increase above 158C, reaching 258C by the end of the experiment.
Fig. 2. Temperature (8C) profiles for the treatments in Experiments 1 and 2. Arrows indicate the times when the fish were sampled. The treatments are indicated in the insets. The dotted lines demarcate the 158C line.
designated to represent the sunrise-to-sunset period. In addition a very dim light was placed on each side of the table to simulate dawn and dusk for a period of half hour each and also to reduce the startling of the fish caused by turning on the strong lights without a transition.
22 21
water in each aquarium when the strong lights were on was 6.89mE m s . When the
22 21
twilight-simulating lights were on the instrument recorded 0.27 mE m s .
2.3. Experiment 2
Experiment 2 consisted of three photoperiod treatments (each with three replicates): (1) constant 9.5 h of daylight, and increasing (2) up to 12, and (3) up to 15 h of daylight (Fig. 1). All three photoperiod regimes were exposed to the same temperature regime, consisting of an increase of 38C per week (Fig. 2). Four females and four males were sampled weekly from each aquarium to obtain dry weights of livers, gonads, visceral fat, and eviscerated carcasses. GSIs, HSIs and FIs were calculated as in the field data.
Fish were collected from March 1–7, 1993, when the natural photoperiod ranged from 11:15–11:31 hours:minutes of daylight (Mansfield, 1979). The fish were randomly distributed into nine 75-l aquaria with an average density of 66 fish per aquarium and exposed to a photoperiod of 11 h until March 11. At this time the photoperiod for the low photoperiod treatment was gradually decreased to 9.5 h of daylight on March 21. The two other treatments received increases of 15 min daily until 12 h of daylight were reached on March 21. After March 21 only the increasing photoperiod (up to 15 h) was increased 15 min twice a week until 15 h were reached. The numbers of fish collected for this experiment allowed for eight sampling events: three before a temperature of 158C was reached, one at 158C, and four after 158C was reached (Fig. 2).
The silicone hoses and airstones used to aerate the aquaria were checked every day for eggs. If any eggs were found they were counted and the numbers recorded in order to determine the number of eggs spawned per female. The bottoms of the aquaria were swept every few days to remove feces and any eggs appearing in the detritus were also counted.
Upon dissection of a female the diameter of one egg from the largest batch of eggs was measured. The male gonads were categorized as follows: stage 0 corresponded to the appearance of the gonad in the early fall (translucent and very thin); stage 1, enlarged, translucent testes; stage 2, testes with opaque white spots; stage 3, testes that are completely opaque white but do not release milt upon the application of gentle pressure on the abdomen; stage 4, milt is released when gentle pressure is applied.
2.4. Data analysis
The field data were analyzed for changes with time between sexes by comparing the first value to subsequent values until a significant difference was encountered. The first significantly different data point became the new base for comparison with subsequent data points. Significant differences were determined from probability values (derived from t-test comparisons) for differences between means (a 50.05) occurring with time for each sex (SAS:GLM / PDIFF) (SAS, 1985). This method was chosen because it provided comparisons among every possible combination of means for each sex, allowing comparison of all subsequent means with the previously determined significant mean while properly weighing unequal sample sizes.
for each sampling date were determined by one way analysis of variance (SAS:GLM). To determine which treatments were significantly different the probability values ( p,0.05) derived from t-tests conducted between every possible combination of treatments (SAS:GLM / PDIFF) were used. This method was chosen over the traditional multiple range tests because of the occurrence of unequal sample sizes occurring towards the end of the experiments when fewer fish were available.
3. Results
3.1. Field study
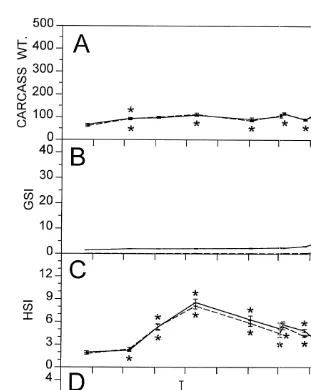
During the fall and winter the weight of the eviscerated carcass changed relatively little although some significant differences were observed over time (Fig. 3A). Rapid carcass growth occurred during the spring until the adults disappeared from the estuary, at which time they are presumed to have completed their life cycle and died. In early May both sexes began rapid maturation, although females allocated much more tissue into gonads than did males, reaching GSI values about 475% larger than those achieved by males (Fig. 3B). In October, HSI and FI of both males and females began increasing (Fig. 3C and D) and reached peaks in late November / early December. In the spring pronounced increase in HSI was detected in females only.
A comparison of the HSI of fish in the fall from three different years indicated that the timing of weight deposition into the liver was rather consistent from year to year (Fig. 4). The fish collected in fall 1992 showed an HSI pattern very similar to that of fish collected in fall 1991. However, the fish collected in fall 1994 exhibited much greater HSI values than those observed in 1991 and 1992. The higher resolution (weekly) of the 1994 collections indicated that, although significant increases in HSI were evident in early October, dramatic HSI increase began in mid October.
3.2. Experiment 1
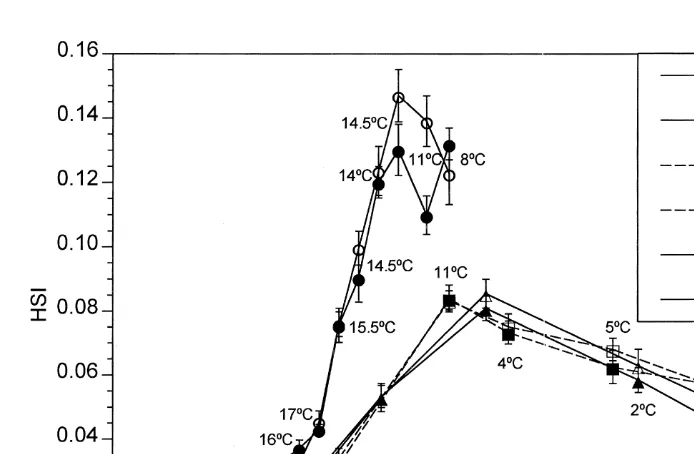
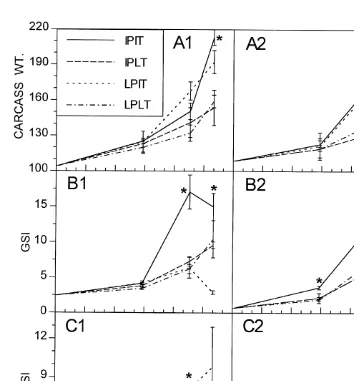
Carcass weight was affected primarily by temperature, with higher growth achieved in the warm water treatments after the 158C threshold was crossed (Fig. 5A). Increases in GSI were also observed at this time for all treatments; however, the increase was significantly higher for the IPIT for both sexes than it was for the other treatments (Fig. 5B). Eggs were first observed on the silicone tubing one day prior to the third collection time on all replicate aquaria of the IPIT while two eggs were observed in one aquarium of the LPIT treatment. Eggs were also observed in all the above mentioned aquaria the day when the third sample was taken. The particular aquarium from the LPIT treatment where eggs were found contained only two females and upon dissecting them one was found with a mature gonad while the other was immature. During the dissections the males were also checked for ripeness and 92% (N512) of those in the IPIT, 50% (N58) in the LPIT treatment, and 0% in the last two treatments had ripe gonads. Only the fish in the IPIT treatment spawned again after the third sample was taken.
Fig. 3. (A) Carcass dry weights (mg), (B) gonadosomatic indices (GSI), (C) hepatosomatic indices (HSI), and (D) visceral fat indices (FI) of M. beryllina collected in the upper Pettaquamscutt River from September 1991 to June 1992, except for fat index values, which were obtained from fish collected from September 1992 to June 1993. Females are represented by solid lines and males by dashed lines. Asterisks indicate significant changes in time from the previously marked value for each parameter measured for each sex separately. Asterisks for significant female values are placed over the line whereas those for significant male values are placed under the line.
Fig. 4. Hepatosomatic indices of field collected female (open symbols) and male (closed symbols) M. beryllina in the fall / winter of 1991 / 92 (triangles), 1992 / 93 (squares), and fall of 1994 (circles). Water temperature (8C) at time of collection is included.
were ripe; however, one did have a very large mature gonad with clear rather than pale-yellow eggs. None of the other females in this treatment had enlarged gonads.
Between the second and fourth sampling times only fish in the LPIT treatment exhibited a significantly increasing HSI (Fig. 5A). Fish exposed to the IPIT treatment responded as fish in the wild did in the spring (Fig. 3), with males showing a small decline in HSI and females showing an increase.
3.3. Experiment 2
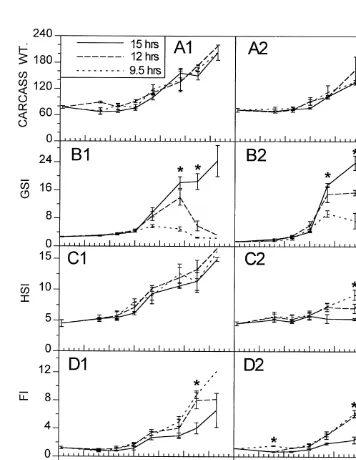
No significant changes in carcass weight, GSI, HSI, or FI (except for males on day 16) were seen among the three photoperiod treatments prior to the water temperature reaching 158C (Fig. 6). Once this temperature was attained fish in all three treatments simultaneously began to undergo maturation, reflected by increases in the gonadosomatic indices (Fig. 6B), in the egg diameter (Fig. 6E1) and in the male gonad stage (Fig. 6E2). However, the maximum GSI achieved in each treatment was directly proportional to photoperiod. The 9.5-h treatment showed the smallest increase while 15-h treatment showed the largest. The 12-h treatment showed an initial high allocation which declined towards the end of the experiment. Only fish in the 12- and 15-h treatments produced oocytes of maximum diameter (|0.92 mm; non-hydrated) (Fig. 6E), whereas the largest
Fig. 5. (A) Carcass dry weights (mg), (B) gonadosomatic indices (GSI), and (C) hepatosomatic indices (HSI) for female (1) and male (2) M. beryllina exposed to the four treatments in experiment 1. The four combinations of photoperiod and temperature are indicated in the inset. Note that GSI scale is different for females and males. Asterisks represent significant differences among treatments on a given sampling day.
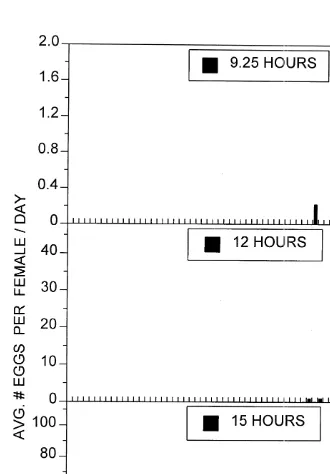
and continued until the end of the experiment at day 63. The numbers of eggs spawned per female increased with time, reaching 108 eggs / female / day near the end of the experiment. Females from the 12-h photoperiod treatment began spawning on day 44, spawned a maximum of 37 eggs per female on day 49, and ceased spawning by day 57. In the 9.5-h photoperiod treatment eggs were collected on only two days, resulting in 0.22 eggs per female on day 45 and 0.31 eggs per female on day 49. The eggs from this treatment were opaque and did not appear viable. In both experiments spawning occurred exactly two weeks after the water temperature reached 158C.
4. Discussion
The field data provided information on the overwintering strategy used by M.
beryllina. In southern Rhode Island (latitude 418 39 N) M. beryllina began allocating reserves into the liver and as visceral fat in October reaching maximum levels in late November / early December. Utilization of the reserves began thereafter and continued until late February when spring baseline levels were reached. The guts of the fish collected in early February of 1992 were empty (Huber, 1995), suggesting that stored resources continued to be of great importance at that time. A similar pattern of fall reserve accumulation has been observed in Menidia menidia (Schultz and Conover, 1997), Alosa pseudoharengus (Wilson) (Flath and Diana, 1985) and in male
Gasteros-teus aculeatus Linnaeus (females were excluded from the study) where lipid reserves in
the liver increased from August to October with subsequent decreases due to overwinter-ing energy utilization (Chellappa et al., 1989).
In the laboratory the LPIT treatments (but not the LPLT of the first experiment) induced resource allocation to the liver as occurred in juveniles in the field during the fall. These results suggest that reserve deposition in this species is induced by a low photoperiod alone as long as the temperature is above a minimum, regardless of the direction of temperature change. The inability of the fish held in the LPLT treatment to carry out this process at 128C when supplied with abundant food suggests that this process may not occur at or below this temperature. In the field accelerated deposition began in mid-October (daylength511 h) and continued until the water temperature dropped below 148C. Consequently we estimate that the temperature threshold is somewhere between 12 and 148C while the photoperiod threshold is about 11 h of daylight.
the lowest visceral fat accumulation, whereas those exposed to the lower photoperiod had significantly higher accumulation. The influence of photoperiod in the accumulation of reserves both in the liver and as visceral fat can be more clearly appreciated in the males, where the liver does not participate in reproductive functions. In the males the 15-h photoperiod induced the lowest liver and visceral fat accumulation, while the 9.5-and 12-h photoperiods induced similar accumulation patterns which were significantly higher than those of the 15-h photoperiod. The levels of reserve accumulation in the liver and as visceral fat were at times considerably higher in the laboratory experiments from those observed in the field.
Although populations of M. beryllina along their geographical distribution may be genetically distinct (Johnson, 1975), the photoperiod and temperature thresholds that regulate life history events of the species throughout its range may be the same, and responses may vary according to the schedule of occurrence of these cues at different latitudes. This level of plasticity would result in a compensatory mechanism that would ensure that appropriate responses (i.e. fattening, maturation) occurred at the appropriate time at each latitude. Fall fattening (of somatic tissues) has also been observed in M.
menidia, where populations along a latitudinal gradient (Nova Scotia, Canada; New
York, USA; and South Carolina, USA) also exhibited lipid storage levels proportional to the severity of the winter at each locality (Schultz and Conover, 1997). A common garden experiment conducted with individuals from these geographical locations suggested that differences in the rate of lipid storage have a genetic component in this species (Schultz and Conover, 1997).
In this study, when the fish brought to the laboratory were subjected to temperatures exceeding ¯158C they began maturing, regardless of the photoperiod exposure. Similar
temperature thresholds for maturation have been reported for populations throughout the range of this species (Meade, 1988; Middaugh and Hemmer, 1992). This adaptation to a temperature of ¯158C for initiation of maturation is a particularly useful tactic by this
species in this environment. Stearns (1976), in his classification of life history tactics in variable environments, indicated that in predictable cyclic environments organisms will synchronize their reproduction to occur at an optimal breeding time. In the estuaries studied in Rhode Island over several years, maturation began within the last week in April and the first week in May, when food resources were abundant (Huber, 1995) and a congener, M. menidia, had already spawned and begun its return toward the mouths of the estuaries (Bengtson, 1984), decreasing the level of competition for food and space.
Menidia menidia begins spawning earlier in the season than does M. beryllina throughout their range of overlap and the initiation of maturation presumably also begins earlier in the former. The temperature and photoperiod cues for induction of maturation by M. menidia have not been specifically studied, but M. menidia is known to spawn on salt marshes according to lunar cues at semi monthly high tides (Conover and Kynard, 1984; Middaugh, 1981; Middaugh et al., 1981, 1984). Lunar periodicity of spawning by
reported breeding at water temperatures as low as 98C in Salem Harbor, Massachusetts, USA. A striking difference between the species is that, during the maturation process,
M. beryllina allocates resources to gonadal, somatic and liver growth whereas M.
menidia allocates resources almost exclusively to gonadal growth (Huber and Bengtson,
1999). Thus, the life history differences between these congeners are expressed through both behavioral and physiological phenomena.
Although M. beryllina gonad stages were not determined in the first experiment, the initial increases in GSI observed when the water temperature reached 158C parallel those observed in the low photoperiod treatment of the second experiment, suggesting that these increases are also paralleled by advances in gonad maturity. Even though the experimental photoperiod regimes were not able to alter the occurrence of the beginning phases of secondary oocyte growth, they were able to alter events associated with final maturation and spawning. Most females in the low photoperiod treatments of both experiments were not able to achieve final maturation or spawn. Although females in the intermediate and high photoperiod exposures underwent final maturation and spawned, the former spawned for only 2 weeks and then began undergoing gonadal regression (as indicated by a decrease in oocyte diameter and a declining GSI) whereas the latter maintained high GSI and continued to spawn increasingly larger numbers of eggs. It appears that secondary oocyte growth and final maturation are regulated by different cues, with secondary oocyte growth requiring water temperature of 158C or greater to be initiated whereas later stages of gonad growth and final maturation require a high photoperiod cue in addition to warm water temperature. Photoperiod and temperature effects on maturation have been demonstrated in a number of teleosts (e.g. Scott, 1979; Baggerman, 1980; Day and Taylor, 1984).
Males in both experiments also began maturation when the water temperature reached 158C, but in contrast to females they were able to reach full maturation and produce milt in all treatments regardless of photoperiod. This suggests that the endocrine response of the males may require only the temperature cue to trigger the reproductive process. Exposure of the mummichog (Fundulus heteroclitus) to 14–208C (but not 6–108C) and 1.5 h of light also resulted in the production of spermatozoa (Burger, 1939, cited by Taylor, 1986), suggesting that a single initial cue may be sufficient to bring males of some species to full maturity whereas females may require different cues to trigger different phases of the reproductive cycle.
The laboratory experiments indicated that for both sexes GSI was a function of hours of daylight, with highest amounts allocated with the highest photoperiod exposure. Natural populations also show this pattern of allocation within the photoperiods experienced along their latitudinal distribution. In two southern locations (¯308N),
maximum female allocation to gonads was 12.7% (Lake Texoma, Oklahoma, USA; Hubbs, 1976) and 12.5% (Blackwater Bay, Florida, USA; Middaugh and Hemmer, 1992). In contrast, in two northern estuaries (¯408N) maximum female allocation to
gonads was 21.0% (Carmans R., New York / New Jersey, USA; Meade, 1988) and 22.1% (Pettaquamscutt R., Rhode Island; Huber, unpublished data). The photoperiods at the time of maximum female allocation were about 13 h for the Florida population and 15 h for the Rhode Island population. Maximum values for male allocation to gonads for these populations were 6.1% (Florida), ¯7.5% (New York / New Jersey) and 9.4%
The findings of this study cause us to suggest photoperiod and temperature thresholds required for Rhode Island M. beryllina to regulate life history events. Accelerated allocation of reserves into the liver and as visceral fat occurs with photoperiod of 11 h or less, as long as the temperature is above 12–148C. The initiation of maturation requires a water temperature of 158C or greater. Although an interplay of these environmental variables is suggested as the regulatory thresholds along the range of this species, the allocation patterns into liver, visceral fat, gonads, and carcass at different geographical locations (thus different populations) have not been studied. The function of the carcass and visceral cavity as storage organs for lipids and glycogen for overwintering purposes remains unknown. Further studies of this nature would be most useful with de-termination of proximate composition and hormonal profiles for this species.
Acknowledgements
This work was possible thanks to the following people: the late W.E.R.E. LaFarge, for allowing access to the upper Pettaquamscutt River through his property; Neal Lackie and George Morrison, for their support with facilities for conducting laboratory experiments; Lisa Shutt, Jim Kinney, Tim Gleason, Peggy Kavanaugh, Mark Tagliabue, and Graham Brawley for assistance with fish collections. The research was supported by Cooperative Agreement CR-819601 between the US Environmental Protection Agency, Environmen-tal Research Laboratory (now Atlantic EnvironmenEnvironmen-tal Division), Narragansett, RI, USA and the University of Rhode Island. This paper is Contribution Number NHEERL-NAR-X254 from AED-Narragansett.
References
Baggerman, B., 1980. Photoperiod and endogenous control of the annual reproductive cycle in teleost fishes. In: Ali, M.A. (Ed.), Environmental Physiology of Fishes, Plenum Press, New York, pp. 533–567. Balon, E.K., 1975. Reproductive guilds of fishes: a proposal and a definition. J. Fish. Res. Board Can. 32,
821–864.
Bengtson, D.A., 1984. Resource partitioning by Menidia menidia and Menidia beryllina (Osteichthyes: Atherinidae). Mar. Ecol. Prog. Ser. 18, 21–30.
Bigelow, H.B., Schroeder, W.C., 1953. Fishes of the Gulf of Maine. Fish. Bull. Fish Wildlife Ser. 53, 577. Burger, J.W., 1939. Some experiments on the relationship of the external environment to the spermatogenic
cycle of Fundulus heteroclitus. Biol. Bull. 77, 96–103.
Bye, V.J., 1984. The role of environmental factors in the timing of reproductive cycles. In: Potts, G.W., Wootton, R.J. (Eds.), Fish Reproduction: Strategies and Tactics, Academic Press, London, pp. 187–205. Chellappa, S., Huntingford, F.A., Strang, R.H.C., Thomson, R.Y., 1989. Annual variation in energy reserves in
male three-spined stickleback, Gasterosteus aculeatus L. (Pisces, Gasterosteidae). J. Fish. Biol. 35, 275–286.
Conover, D.O., Kynard, B.E., 1984. Field and laboratory observations of spawning periodicity and behavior of a northern population of the Atlantic silverside Menidia menidia (Pisces: Atherinidae). Environ. Biol. Fish. 11, 161–171.
Cook, S.F., Moore, R.L., 1970. Mississippi silversides, Menidia audens (Atherinidae) established in California. Trans. Am. Fish. Soc. 99, 70–73.
DeVlaming, V.L., 1974. Environmental and endocrine control of teleost reproduction. In: Schreck, C.B. (Ed.), Control of Sex in Fishes, Virginia Polytechnic Institute and State University, Blacksburg, VA, pp. 13–83. Elston, R., Bachen, B., 1976. Diel feeding cycle and some effects of light on feeding intensity of the
Mississippi silverside Menidia audens in Clear Lake, California. Trans. Am. Fish. Soc. 105, 84–88. Flath, L.E., Diana, J.S., 1985. Seasonal energy dynamics of the alewife in southeastern Lake Michigan. Trans.
Am. Fish. Soc. 114, 328–337.
Gleason, T.R., Bengtson, D.A., 1996. Size-selective mortality of inland silversides: evidence from otolith microstructural analysis. Trans. Am. Fish. Soc. 125, 860–873.
Gosline, W.A., 1948. Speciation of fishes in the genus Menidia. Evolution 2, 306–313.
Hubbs, C., 1976. The diel reproductive patterns and fecundity of Menidia audens. Copeia 1976, 386–388. Hubbs, C., 1982. Life history dynamics of Menidia beryllina from Lake Texoma. Am. Midl. Nat. 107, 1–12. Hubbs, C., Sharp, H.B., Schneider, J.F., 1971. Developmental rates of Menidia audens with notes on salt
tolerance. Trans. Am. Fish. Soc. 100, 603–610.
Huber, M., 1990. Aspects of reproductive ecology of Menidia menidia (L.) and Menidia beryllina (Cope) in southern Rhode Island. MS Thesis. University of Rhode Island, Kingston, Rhode Island, USA.
Huber, M. 1995. The relationship between food abundance and spawning by Menidia beryllina in Rhode Island, USA, estuaries. PhD dissertation. University of Rhode Island, Kingston, Rhode Island, USA. Huber, M., Bengtson, D.A., 1999. Interspecific differences in growth of somatic and reproductive tissues
during the breeding season in Menidia menidia and Menidia beryllina. J. Fish. Biol. (in press). Johnson, M.S., 1975. Biochemical systematics of the atherinid genus Menidia. Copeia 1975, 662–691. Lam, T.J., 1983. Environmental influences on gonadal activity in fish. In: Hoar, W.S., Randall, D.J.,
Donaldson, E.M. (Eds.), Fish Physiology, Vol. IX B, Academic Press, London, pp. 65–116.
Mansfield, R.L., 1979. Sunlight summary with a computer-generated table of the local standard times of sunrise, sunset, and the beginning and ending of twilight, for each day of the year, and for the purchaser’s own latitude, longitude, and time zone on Earth’s surface. (Generated for Pell Marine Science Library, Narragansett Bay, Rhode Island, at latitude 418 309north, longitude 718 269west), Astronomical Data Service, 3922 Leisure Lane, Colorado Springs, Colorado 80917, USA.
Meade, M.A., 1988. Life history of Menidia beryllina (Pisces: Atherinidae) in a Long Island, New York estuary. MS thesis. State University of New York, Stony Brook, NY, USA.
Mense, J.B., 1967. Ecology of the Mississippi silversides, Menidia audens Hay, in Lake Texoma. Okla. Fish. Res. Lab. Bull. 6, 32.
Middaugh, D.P., 1981. Reproductive ecology and spawning periodicity of the Atlantic silverside, Menidia menidia (Pisces: Atherinidae). Copeia 1981, 766–776.
Middaugh, D.P., Hemmer, M.J., 1992. Reproductive ecology of the inland silverside, Menidia beryllina, from Blackwater Bay, Florida. Copeia 1992, 53–61.
Middaugh, D.P., Domey, R.G., Scott, G.I., 1984. Reproductive rhythmicity of the Atlantic silverside. Trans. Am. Fish. Soc. 113, 472–478.
Middaugh, D.P., Scott, G.I., Dean, J.M., 1981. Reproductive behavior of the Atlantic silverside, Menidia menidia (Pisces: Atherinidae). Environ. Biol. Fish. 6, 269–276.
Munro, A.D., 1990. General introduction. In: Munro, A.D., Scott, A.P., Lam, T.J. (Eds.), Reproductive Seasonality in Teleosts: Environmental Influences, CRC Press, Boca Raton, FL, pp. 1–12.
SAS, 1985. SAS User’s Guide: Statistics, Version 6, SAS Institute Inc, Cary, North Carolina 27511-8000. Schultz, E.T., Conover, D.O., 1997. Latitudinal differences in somatic energy storage: adaptive responses to
seasonality in an estuarine fish (Atherinidae: Menidia menidia). Oecologia 109, 516–529.
Scott, D.B.C., 1979. Environmental timing and the control of reproduction in teleost fish. In: Miller, P.J. (Ed.), Fish Phenology: Anabolic Adaptiveness in Teleosts, Academic Press, London, pp. 105–132.
Stearns, S.C., 1976. Life-history tactics: a review of the ideas. Q. Rev. Biol. 51, 3–47.
Taylor, M.H., 1986. Environmental and endocrine influences on reproduction of Fundulus heteroclitus. Am. Zool. 26, 159–171.
Taylor, M.H., 1990. Estuarine and intertidal teleosts. In: Munro, A.D., Scott, A.P., Lam, T.J. (Eds.), Reproductive Seasonality in Teleosts: Environmental Influences, CRC Press, Boca Raton, FL, pp. 109–124. Wootton, R.J., 1982. Environmental factors in fish reproduction. In: Richter, C.J.J., Goos, H.J.T. (Eds.),