www.elsevier.com / locate / bres
Research report
Evaluation of combined fibroblast growth factor-2 and moderate
hypothermia therapy in traumatically brain injured rats
a a a b a
Hong Qu Yan , Jianyun Yu , Anthony E. Kline , Peter Letart , Larry W. Jenkins ,
a a ,
*
Donald W. Marion , C. Edward Dixon
a
Brain Trauma Research Center, Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, PA 15260, USA
b
Department of Neurosurgery, Loyola University Chicago, Maywood, IL 60153, USA Accepted 19 September 2000
Abstract
Both the exogenous administration of fibroblast growth factor-2 (FGF-2) or the induction of moderate hypothermia have been shown to attenuate histopathology and improve functional outcome after traumatic brain injury (TBI). Since combined therapeutic strategies may be more beneficial than single therapies, we examined the potential synergistic effect of FGF-2 combined with moderate hypothermia treatment induced 10 min after TBI on functional and histological outcome following controlled cortical impact (CCI) injury. Fifty male Sprague–Dawley rats were randomized to one sham and four CCI treatment groups: Sham1vehicle (VEH); FGF-2 (45mg / kg / h for 3 h i.v.)1Normothermia (3760.58C); FGF-21Hypothermia (3260.58C for 3 h); VEH1Norm; VEH1Hypo. Vestibulomotor performance on the beam balance and beam-walk (BW) tasks on post-operative days 1–5 and spatial memory acquisition in the Morris water maze (MWM) on days 14–18 were assessed. After 4 weeks survival, histological evaluations (CA and CA cell counts and lesion volume)1 3
were performed. MWM performance improved in all treatment groups, but combined treatment was not more efficacious than either alone. The FGF-21Hypo group performed significantly better than the other injured treatment groups in the BW task. Lastly, no significant group differences in beam balance or histological outcome were observed. These data suggest a suboptimal and incomplete synergy of combined FGF-2 and hypothermia treatment. These data may indicate that either our dose of FGF-2 or combination therapy was not optimized in our model. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Trauma
Keywords: Controlled cortical impact; Neurotrophin; MWM
1. Introduction hypothermia after TBI reduces both neuronal death and axonal injury [6,13] while providing functional benefit [5], The experimental evidence that moderate hypothermia is others have shown that moderate hypothermia initiated neuroprotective when given before or even after traumatic following CCI improves motor and spatial memory re-brain injury (TBI) or cerebral ischemia is overwhelming covery despite no significant effect on structural pathology (for general review see [14,35,36]) and occurs across a as reflected by cortical necrosis or hippocampal cell variety of different models. Specifically, hypothermia has survival [18]. Thus, hypothermia may independently re-improved outcome following experimental TBI via central duce both lethal and sublethal TBI damage. Despite or lateral fluid percussion [5,9,34], weight drop-induced positive preliminary reports using moderate hypothermia in diffuse injury [29], and controlled cortical impact (CCI) clinical TBI [8,36], recent results from the multicenter [7,18,36]. While some TBI studies have shown that National Brain Injury Study: Hypothermia (NABIS:H) clinical trial appear disappointing (personal communica-tion). The results suggest that more refinement of the
*Corresponding author. Tel.: 11-412-383-2188; fax: 1
1-412-624-clinical application of hypothermia to severe TBI is needed
0943.
E-mail address: [email protected] (C.E. Dixon). and perhaps the beneficial actions of hypothermia need to
be better clarified and enhanced while minimizing potential 4% isoflurane (Anaquest, Memphis, TN) in oxygen and the nonspecific effects, which can reduce therapeutic efficacy. rats intubated with a 14-gauge angiocatheter and me-Consequently, the best protocol and patient populations for chanically ventilated with 2.0% isoflurane / 66% N O / bal-2 the use of hypothermia in clinical TBI remain to be defined ance O2 for surgery. Venous and arterial catheters were and optimized. One approach that may prove useful is to placed in the tail for FGF-2 infusion and the continuous combine other treatments with hypothermia that are in- monitoring of blood pressure and arterial glucose and dependently effective and have other beneficial specific blood gas sampling. A rectal probe was inserted for mechanistic actions. The use of exogenous trophic factors continuous monitoring of body temperature, and a micro-is one class of candidates, especially since there micro-is probe thermistor (Type MT-29 / 1, Physiotemp Instruments evidence that TBI alters neurotrophin receptors after injury Inc., Clifton, NJ) was inserted into a temporalis muscle for [39] and hypothermia treatment can reduce the endogenous indirect monitoring of brain temperature. A midline inci-neurotrophic response of the brain after experimental CCI sion was made, the soft tissues reflected, and a 7 mm
[11,23,24]. craniotomy was made between lambda and bregma and
A number of studies in experimental TBI have shown centered 5 mm laterally on the right side of the central that neurotrophins attenuate functional deficits or his- suture. The rats received a CCI through the craniotomy at a topathology [16,44–46]. One of several neurotrophic fac- velocity of 4 m / s with a tissue deformation depth of 2.6 tors, fibroblast growth factor-2 (FGF-2) has been shown mm. Shams underwent identical surgical procedures, but protective in a number of in vitro injury models [33,37,52], did not receive the impact. After injury, the scalp incision and in vivo models such as fluid percussion TBI was closed with interrupted nylon sutures and the rat was [12,25,38], stroke [27,28], and spinal cord injury [31,40]. removed from the injury device and returned to the heating Importantly, FGF-2 is effective when administered after pad. The rat was stabilized on anesthesia for 30 min after injury, and retards functional recovery when its endogen- which arterial blood gas (ABG), glucose, and hematocrit
ous expression is inhibited [42]. were checked just before injury. Mean arterial pressure
Given the previous beneficial effects of FGF-2, we was kept above 90 mm Hg, brain and body temperature combined FGF-2 treatment with moderate hypothermia were kept at 3760.58C, and pCO at 402 65 mm Hg. therapy to determine if synergistic effects occur by
evaluating the effect of individual or combined FGF-2 and 2.3. Injury device moderate hypothermia therapy on motor, cognitive, and
histological outcome following a moderate CCI injury in The CCI injury device [15] consisted of a small (1.975
rats. cm) bore, double-acting, stroke-constrained, pneumatic
cylinder with a 5.0 cm stroke. The cylinder was rigidly mounted in an angled vertical position on a crossbar. The 2. Material and methods lower rod end had an impactor tip attached (i.e., that part of the shaft that comes into contact with the exposed dura)
2.1. Subjects and the upper rod end was attached to the transducer core
of a linear velocity displacement transducer (LVDT). The Fifty adult male Sprague–Dawley rats weighing 370– velocity of the impactor shaft was controlled by gas 400 g on the day of surgery were used. Animals were pressure and measured directly by the LVDT (Shaevitz group-housed (two per cage) in standard steel / wire mesh Model 500 HR; Macro Sensors, Pennsauken, NJ), which cages with ad libitum access to food and water. All produced an analog signal that was recorded by a PC-procedures carefully conformed to the guidelines outlined based data acquisition system (Axoscope; Axon Instru-in the Guide for the Care and Use of Laboratory Animals ments, Inc., Foster City, CA) for analysis of time / displace-from the U.S. Department of Health and Human Services ment parameters of the impact.
and were approved by the University of Pittsburgh
Institu-tional Animal Care and Use Committee. The animals were 2.4. FGF-2 infusion and hypothermia induction randomized to one sham or four CCI groups: Sham1
vehicle (VEH); FGF-2 (45 mg / kg / h for 3 h i.v.)1 Ten minutes after injury, rats randomized to the hypo-Normothermia (3760.58C); FGF-2 (same as above)1 thermic groups were cooled to a brain temperature of hypothermia (3260.58C for 3 h); VEH1Norm; VEH1 3260.58C with ice packs; those randomized to
normother-Hypo. mic groups were maintained at 3760.58C with a heating
pad. Hypothermic rats were maintained at 3260.58C for 3
2.2. Surgical and injury protocols h, and then gradually (over a 1 h period) rewarmed to
135 mg / kg of FGF-2. The sham rats were infused with before being placed in a heated incubator between trials (4 vehicle at the same rate and for the same time as the CCI min intertrial interval).
groups. All lines were subsequently removed at 4 h,
anesthesia was discontinued, and the animals were allowed 2.6. Tissue preparation to regain consciousness before being returned to their
home cages. Four weeks after injury, animals were deeply
anes-thetized with pentobarbital (Nembutal, 80–100 mg / kg;
2.5. Functional assessments Abbott Laboratories, North Chicago, IL) and transcardially
perfused with 100 ml of 0.1 M phosphate buffered saline
2.5.1. Motor performance with 5 U / ml heparin pH 7.4, followed by 500 ml 4%
Gross vestibulomotor function was assessed on a beam- paraformaldehyde. After perfusion, the brain was removed balance task that consisted of placing the animal on a and immersed in 4% paraformaldehyde in 0.1 M phosphate suspended, narrow wooden beam (1.5 cm wide) and buffer pH 7.4 at 48C overnight. The brain was next recording the duration it remained on the beam for a transferred to 15% sucrose in 0.1 M phosphate buffer pH maximum of 60 s. Training prior to injury consisted of 7.4 at 48C for 24 h then to 30% sucrose in 0.1 M three trials, during which baseline measurements were phosphate buffer pH 7.4 at 48C until sunken. The cryop-taken. More complex vestibulomotor function and coordi- rotected rat brain was frozen and two series of 7mm thick nation were assessed using a modified beam-walking task. coronal sections were cut on a cryostat (Jung CM 1800; Briefly, this task consisted of training rats to escape a Brodersen Instruments, Valencia, PA) and mounted on bright light and high decibel white noise (Lafayette gelatinized glass slides. One set of sections was stained Instruments, Inc., Layfaette, IN, Model [15800C) by with Cresyl violet for morphological analysis and the other
traversing a narrow wooden beam (2.53100 cm) and set was prepared for immunohistochemistry. entering a darkened goal box at the opposite end. Noxious
stimuli were terminated when the rat entered the goal box. 2.6.1. Morphology
Four pegs (3.0 mm diameter, 4.0 cm high) were placed in Treatment efficacy on the survival of vulnerable neurons an alternating sequence along the beam to increase the in the hippocampal CA and CA regions was evaluated1 3 difficulty of the task. Performance was assessed by the from coronal sections underlying the area of contusion, latency to traverse the beam. Data for each session approximately 3.5 mm posterior to bregma, using a double consisted of the mean of three trials. All rats were pre- blind analysis for each of the following groups (Sham1
trained prior to injury. VEH56; FGF-21Norm54; VEH1Norm54; FGF-21
Hypo54, and VEH1Hypo55). To reduce counting errors
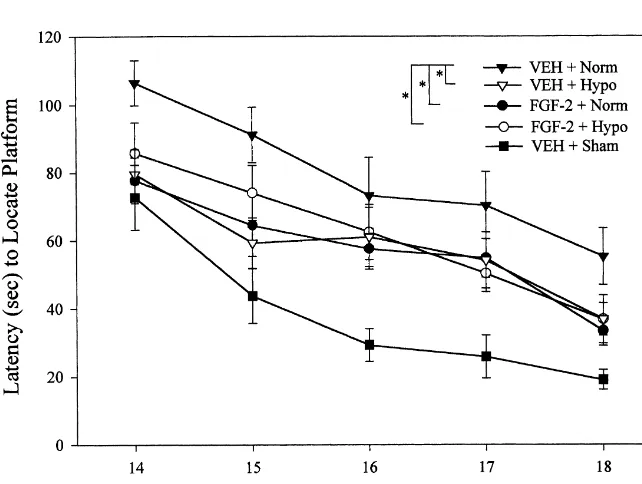
2.5.2. Cognitive performance associated with false positive identification of dying
neu-A Morris water maze task variant was used to compare rons, the total number of CA and CA surviving neurons1 3 acquisition rates between groups. The maze consisted of a were counted using a Nikon Eclipse E600 microscope plastic pool (180 cm in diameter and 60 cm in depth) filled (Nikon Corporation, Tokyo, Japan). All data are reported with water to a depth of 28 cm with a clear Plexiglas stand as the percent of total percent neurons in the ipsilateral (10 cm in diameter and 26 cm high, i.e., 2 cm below the (injured) CA and CA regions relative to the contralateral1 3 water’s surface) used as the hidden goal platform. The pool side. Cortical volume was determined by outlining both the was located in a 2.532.5 m room with numerous extra- contralateral and ipsilateral (injured) hemispheres (MCID, maze cues (e.g., posters) that remained constant throughout Imaging Research, Ontario, Canada) then subtracting the the experiment. Water maze testing began on day 14 area of the ipsilateral hemisphere from the contralateral post-injury in order to avoid possible confounds with the and summing the differences to calculate a mean volume. motor deficits observed in the first few days following
injury. The rats were given four trials per day for five 2.6.2. Immunohistochemistry
ml; Jackson ImmunoResearch Laboratories, West Grove, 3. Results PA) were used to visualize immunoreactivity with 2% NRS
and TX-PBS at 48C for 3 h. Tissue slides were rinsed 3.1. Physiology between all steps with TX-PBS three times for at least 10
min each time. The peroxidase reaction was developed Arterial blood gases were measured at 10, 30, 60, 120, with DAB Substrate Kit (Vector; Burlingame, CA) until a 180 and 240 min post injury (Table 1). Mean arterial blood dark brown reaction product was evident. Sections were pressure (MABP) was never below 90 mmHg in any rat rinsed in water, dehydrated in alcohols, defatted in although some slight group differences were seen (Fig. 1). xylenes, and coverslipped for light microscopic analysis. As seen in Table 1, slight elevations in blood glucose Some sections were counterstained with hematoxylin levels were observed during hypothermia before returning (Vector; Burlingame, CA). At least three sections through near baseline after rewarming (240 min after TBI) in both the hippocampus of each animal were processed for hypothermic groups. Hypothermia is known to increase immunostaining. Control experiments were run in parallel circulating catecholamines [26] which is likely responsible to confirm specificity. Primary antibody was omitted and for the slight increase in glucose seen in our hypothermic polyclonal antibody to GFAP (DAKO, Denmark) was used rats.
for comparison.
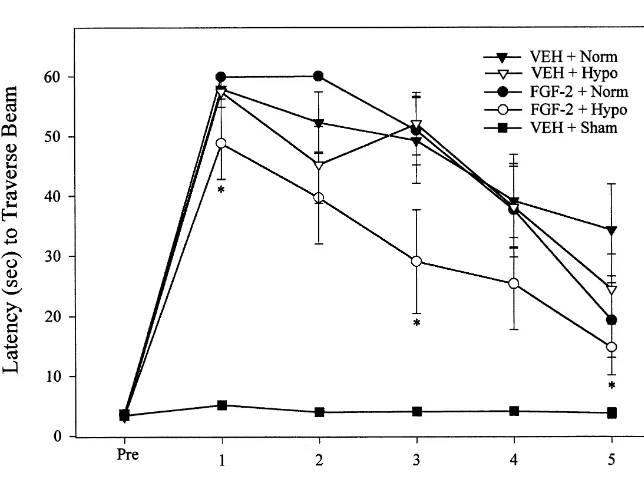
3.2. Motor performance
2.7. Statistical analysis A repeated-measures ANOVA on beam balancing ability
revealed a significant group difference (F4,4554.066, P5 Analyses were performed using Statview software on a 0.0067), a significant time (days) difference (F5,2255 Macintosh computer. Behavioral data were analyzed using 28.397, P,0.0001), and a significant group3day inter-repeated-measures analysis of variance (ANOVA). If a action (F20,22553.614, P,0.0001). Post-hoc analysis re-significant effect was found with the ANOVA, individual vealed that all CCI rats had motor deficits compared to the group comparisons were made with Fisher’s PLSD post- Sham1VEH group (P,0.001). However, there were no hoc tests. Histological and physiological data were ana- significant differences among the injured groups (Fig. 2). lyzed with unpaired t-tests. Data were expressed as In contrast, a repeated measures ANOVA of the beam mean6S.E.M. and a significance level of P,0.05 was walking task also showed a significant group (F4,455
used. 17.181, P,0.0001), time (F5,225586.633, P,0.0001), and
Table 1
Systemic physiological parameters
Baseline VEH1Sham VEH1Norm FGF-21Norm VEH1Hypo FGF-21Hypo
pH 7.4260.01 7.4360.01 7.4360.01 7.4460.01 7.4460.01
Fig. 1. Mean arterial blood pressure (MABP) data before and 4 h after the injury in all the animal groups. Values are mean (6S.E.M.).
group3day interaction (F20,22556.488, P,0.001). Post- 3.3. Cognitive performance hoc analysis showed that the combined FGF-21hypo
group displayed significantly improved beam walking A repeated measures ANOVA revealed significant dif-function compared to the other groups (P,0.0042 vs. ferences in the latency to find a hidden platform among FGF-21Norm, P,0.0021 vs. VEH1Norm, and P,0.0153 groups (F4,4559.42, P,0.0001) and time (F4,180537.869,
vs. VEH1Hypo). P,0.0001), but there was no significant group3day
interaction (F16,18050.609, P50.874). Post-hoc analyses groups. The percentage of CA1 neuronal survival was showed that all injured groups had deficits (P,0.001). 98.4965.68 (Sham1VEH), 35.4364.13 (FGF-21Norm), Additionally, all treated but injured groups showed re- 34.9467.69 (VEH1Norm), 35.4364.13 (FGF-21Hypo), duced deficits compared with untreated injured animals and 43.6269.71 (VEH1Hypo). All injured groups were (P,0.05) however, there were no differences seen be- significantly different from the Sham1VEH group (P, tween treatment groups (Fig. 3). Post-hoc analysis showed 0.05), but no significant differences were observed among significant differences at days 14, 15, and 18 with both the injured (P.0.05, unpaired t-test). The mean percentage of FGF-21Norm and VEH1Hypo groups performing better CA3 neuronal survival was 104.8866.22 (Sham1VEH), than the VEH1Norm group (P,0.05). The FGF-21Hypo 54.2967.45 (FGF-21Norm), 53.5166.75 (VEH1Norm), group exhibited a significantly reduced latency to find the 73.3268.92 (FGF-21Hypo), and 76.7569.67 (VEH1 hidden platform compared to the VEH1Norm group only Hypo). An unpaired t-test on cortical tissue loss did not at post-operative day 18 (P,0.029, Fisher). In addition, all reveal a significant difference among injured groups (P. injured groups with or without treatment demonstrated 0.05, n.s.). The mean (6S.E.M.) cortical volume was
3 3
significantly shorter swim latencies (P,0.05) during the 22.3463.07 mm (FGF-21Norm), 23.4163.30 mm 3
visible platform trials suggesting that their performance (VEH1Norm), 21.1063.23 mm (FGF-21Hypo), and 3
deficits during the hidden platform trials were not con- 20.4761.62 mm (VEH1Hypo). founded by non-specific deficits such as visual processing
and motivation.
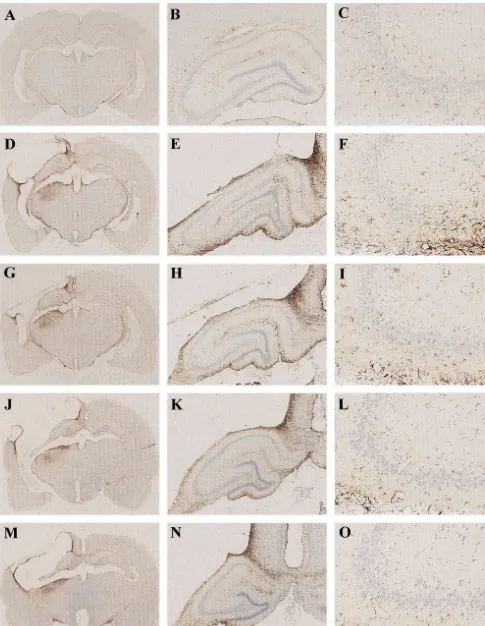
3.4.2. Immunohistochemistry
3.4. Histology GFAP staining showed marked astrogliosis in all injured
brains (Fig. 4). An increase in GFAP-immunoreactivity
3.4.1. Neuronal density was observed in the ipsilateral hippocampus cortex and
Hippocampal CA and CA neuronal density underlying1 3 thalamus in all injured groups (Fig. 4). GFAP staining in the contusion was examined from each group. TBI pro- the ipsilateral CA3 corresponded directly to the area of duced significant reductions in CA1 and CA3 surviving maximal hippocampal cell loss caused by CCI injury. neurons in the hippocampus ipsilateral to impact compared However, FGF-2 and / or hypothermia-treated animals to the contralateral hippocampus in all the CCI-injured showed an attenuation of GFAP expression compared to
the injured VEH1Norm-treated control by immunohisto- produces less severe histological damage. These data
chemistry (Fig. 4). showed that the histopathological consequences of these
different types of injury are quite different. It may be that FGF-2 histological protection is limited by injury intensity, 4. Discussion but that functional benefit can still be obtained by
modulat-ory FGF-2 actions with less overt pathology.
In the present study, we demonstrate that behavioral In addition to the traditional role of trophic factors in the morbidity after CCI in the rat can be reduced by either CNS as survival, growth, and differentiation factors, recent moderate hypothermia or FGF-2 treatment in an injury studies have shown acute modulatory effects of trophic model that results in enduring motor and MWM dysfunc- factors on axonal branching and arborization, ion channel tion and histopathological damage. However, additional function, and synaptic efficacy [20]. Furthermore, the benefit by the combination of moderate hypothermia with expression and secretion of trophic factors are regulated by FGF-2 treatment was only observed in one of several electrical activity, suggesting that many activity-dependent functional assessments, suggesting that optimal combined interactions at the synapse may be mediated by trophic treatment may not have been obtained. The exogenous signals [20]. Trophic signal cascades interact with excitat-administration of FGF-2 [12,38] or the induction of ory and inhibitory neurotransmitter systems producing both moderate hypothermia [4,5,9,12,13,29,34,47] have previ- transient and persistent changes in neuronal signaling. ously been shown to reduce injury when given alone There are many examples of relatively fast modulation of following experimental TBI. However, to our knowledge, pre- and postsynaptic inhibitory and excitatory transmis-our study is the first to investigate the combined effects of sion by neurotrophins [43]. Additionally, important roles FGF-2 and hypothermia after TBI. We found improve- for trophic factors in synaptic plasticity such as long term ments in beam walking and MWM performance in animals potentiation (LTP) and memory and learning are also well receiving FGF-2, hypothermia or combined optimal in- established [43], which suggests one possible explanation dividual therapy. Additional benefit was found in com- for why FGF-2 provides behavioral protection in the bined treatment, but only in the beam walking task, absence of structural protection after TBI in our CCI suggesting the possibility that optimal combined treatment model.
was not obtained, or ineffective on higher-order func- The trophic actions of exogenous trophic factor treat-tioning. Importantly, while there was minimal synergy ment may reduce excitotoxic damage by buffering in-seen with the one trial of combined therapy used in the creases in free radicals and intracellular calcium [37]. present study, a therapeutic benefit was still seen that was However, exogenous trophic factor administration may comparable or better to either treatment alone. These data acutely contribute to excitotoxicity by further activating suggest that the two treatments were at least compatible phospholipases D (PLD) and A2 (PLA2) via protein with each other and further study may be required to tyrosine kinase (PTK) pathways [22,49], which in turn, determine if optimal conditions for combined therapy can transiently increase neuronal free radicals and cellular
exist. calcium by the excessive production of diacylglycerol and
FGF-2 is an 18 kD, 154-amino acid protein with potent arachidonic acid cascades. Evidence in support of detri-trophic actions on neurons, and glia. Neuroprotection has mental diacylglycerol and arachidonic acid cascades after been reported with FGF-2 in vitro [30,41] and in vivo TBI is provided by studies showing CDP-choline treatment following focal [3,28] or global ischemia [10], spinal cord reduces brain edema and blood–brain barrier breakdown injury [1,31,40,48] traumatic brain injury [12,51], and [2] as well as attenuate water maze performance deficits
seizures [32]. [17]. CDP-choline treatment has been shown to prevent the
[3] A. Bethel, J.R. Kirsch, R.C. Koehler, S.P. Finklestein, R.J.
It has been shown in various ischemia and trauma
Traystman, Intravenous basic fibroblast growth factor decreases
studies that the duration of hypothermia is critical in
brain injury resulting from focal ischemia in cats, Stroke 28 (1997)
whether or not neuroprotection is demonstrated in a 609–615, discussion 615-606.
particular model. In the present study, no histopathologic [4] H.M. Bramlett, W.D. Dietrich, E.J. Green, R. Busto, Chronic histopathological consequences of fluid-percussion brain injury in
protection was seen with either FGF-2 treatment alone or
rats: effects of post-traumatic hypothermia, Acta Neuropathol.
in combination with 3 h of moderate hypothermia. This
(Berl.) 93 (1997) 190–199.
result is consistent with our previously reported study [5] H.M. Bramlett, E.J. Green, W.D. Dietrich, R. Busto, M.Y. Globus, showing that moderate hypothermia for 3 h exhibits M.D. Ginsberg, Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits, J. Neurotrauma
protective effects on behavioral deficits but not
histo-12 (1995) 289–298.
pathological improvement following CCI injury in the rat
[6] A. Buki, H. Koizumi, J.T. Povlishock, Moderate posttraumatic
[18]. It is possible that contusion volume, especially hypothermia decreases early calpain-mediated proteolysis and con-following CCI, is the ultimate morphological expression of comitant cytoskeletal compromise in traumatic axonal injury, an irreversible pathological process leading to cell death Neurol. Res. 21 (1999) 585–592.
[7] R.S. Clark, P.M. Kochanek, D.W. Marion, J.K. Schiding, M. White,
within regions of focal necrosis. These pathological
pro-A.M. Palmer, S.T. DeKosky, Mild posttraumatic hypothermia
cesses may be insensitive to a variety of therapeutic
reduces mortality after severe controlled cortical impact in rats, J.
interventions including moderate hypothermia [18]. It is Cereb. Blood Flow Metab. 16 (1996) 253–261.
also possible that a longer duration of hypothermia may be [8] G.L. Clifton, S. Allen, P. Barrodale, P. Plenger, J. Berry, S. Koch, J. Fletcher, R.L. Hayes, S.C. Choi, A phase II study of moderate
more protective and thereby attenuate histopathology.
hypothermia in severe brain injury, J. Neurotrauma 10 (1993)
Finally, studies of hypothermia or FGF-2 have employed a
263–271, discussion 273.
variety of hypothermic protocols, injury models, and [9] G.L. Clifton, J.Y. Jiang, B.G. Lyeth, L.W. Jenkins, R.J. Hamm, R.L. magnitudes as well as histopathological endpoints that Hayes, Marked protection by moderate hypothermia after ex-perimental traumatic brain injury, J. Cereb. Blood Flow Metab. 11
could contribute to different results. These observations
(1991) 114–121.
highlight the need for rigorously controlled studies that
[10] P. Cuevas, F. Carceller, I. Munoz-Willery, G. Gimenez-Gallego,
systematically examine the effects of moderate hypother- Intravenous fibroblast growth factor penetrates the blood–brain mia or FGF-2 on different magnitudes of TBI and associ- barrier and protects hippocampal neurons against ischemia-reperfu-ated histopathological damage. sion injury, Surg. Neurol. 49 (1998) 77–83, discussion 83-74.
[11] S.T. DeKosky, J.R. Goss, P.D. Miller, S.D. Styren, P.M. Kochanek,
In summary, our data indicate that FGF-2 and
hypo-D. Marion, Upregulation of nerve growth factor following cortical
thermia treatments, while producing some benefit on their
trauma, Exp. Neurol. 130 (1994) 173–177.
own, were only slightly more effective when combined. [12] W.D. Dietrich, O. Alonso, R. Busto, S.P. Finklestein, Posttreatment The lack of a synergistic effect in all behaviors may be with intravenous basic fibroblast growth factor reduces
histopatho-related to the dose and timing of combined treatments, but logical damage following fluid-percussion brain injury in rats, J. Neurotrauma 13 (1996) 309–316.
at least the combined approach is compatible in that
[13] W.D. Dietrich, O. Alonso, R. Busto, M.Y. Globus, M.D. Ginsberg,
therapeutic benefit was still obtained. Further work is
Post-traumatic brain hypothermia reduces histopathological damage
necessary to determine if different timing and dose reg- following concussive brain injury in the rat, Acta Neuropathol. imens exhibit greater synergistic effects. (Berl.) 87 (1994) 250–258.
[14] W.D. Dietrich, R. Busto, M.Y. Globus, M.D. Ginsberg, Brain damage and temperature: cellular and molecular mechanisms, Adv. Neurol. 71 (1996) 177–194, discussion 194-197.
Acknowledgements
[15] C.E. Dixon, G.L. Clifton, J.W. Lighthall, A.A. Yaghmai, R.L. Hayes, A controlled cortical impact model of traumatic brain injury in the
The authors thank Scios (Mountain View, CA) for the rat, J. Neurosci. Methods 39 (1991) 253–262.
[16] C.E. Dixon, P. Flinn, J. Bao, R. Venya, R.L. Hayes, Nerve growth
generous donation of FGF-2 and vehicle supplied as two
factor attenuates cholinergic deficits following traumatic brain injury
blinded samples. The authors thank Dr. Elizabeth
Hooghe-in rats, Exp. Neurol. 146 (1997) 479–490.
Peters for critical reading of this manuscript. Supported by
[17] C.E. Dixon, X. Ma, D.W. Marion, Effects of CDP-choline treatment
grants from the Copeland Fund, NIH-NS33150, NIH- on neurobehavioral deficits after TBI and on hippocampal and
NS30318, and NIH-NS40125. neocortical acetylcholine release, J. Neurotrauma 14 (1997) 161–
169.
[18] C.E. Dixon, C.G. Markgraf, F. Angileri, B.R. Pike, B. Wolfson, J.K. Newcomb, M.M. Bismar, A.J. Blanco, G.L. Clifton, R.L. Hayes, References Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat, [1] R. Baffour, K. Achanta, J. Kaufman, J. Berman, J.L. Garb, S. Rhee, J. Neurotrauma 15 (1998) 95–103.
P. Friedmann, Synergistic effect of basic fibroblast growth factor and [19] R.V. Dorman, Z. Dabrowiecki, L.A. Horrocks, Effects of methylprednisolone on neurological function after experimental CDPcholine and CDPethanolamine on the alterations in rat brain spinal cord injury, J. Neurosurg. 83 (1995) 105–110. lipid metabolism induced by global ischemia, J. Neurochem. 40 [2] M.K. Baskaya, A. Dogan, A.M. Rao, R.J. Dempsey, Neuroprotec- (1983) 276–279.
tive effects of citicoline on brain edema and blood–brain barrier [20] R.M. Fitzsimonds, M.-M. Poo, Retrograde signaling in the develop-breakdown after traumatic brain injury, J. Neurosurg. 92 (2000) ment and modification of synapses, Physiol. Rev. 78 (1998) 143–
[21] W.J. Friedman, L.A. Greene, Neurotrophin signaling via Trks and traumatic brain injury: mechanisms of action and implications for p75, Exp. Cell Res. 253 (1999) 131–142. therapy, J. Neurotrauma 11 (1994) 3–33.
[22] K.B. Glaser, D. Mobilio, J.Y. Chang, N. Senko, Phospholipase A2 [38] K.L. McDermott, R. Raghupathi, S.C. Fernandez, K.E. Saatman, enzymes: regulation and inhibition, TIPS 14 (1993) 92–98. A.A. Protter, S.P. Finklestein, G. Sinson, D.H. Smith, T.K. McIn-[23] J.R. Goss, M.E. O’Malley, L. Zou, S.D. Styren, P.M. Kochanek, S.T. tosh, Delayed administration of basic fibroblast growth factor DeKosky, Astrocytes are the major source of nerve growth factor (bFGF) attenuates cognitive dysfunction following parasagittal fluid upregulation following traumatic brain injury in the rat, Exp. Neurol. percussion brain injury in the rat, J. Neurotrauma 14 (1997) 191–
149 (1998) 301–309. 200.
[24] J.R. Goss, S.D. Styren, P.D. Miller, P.M. Kochanek, A.M. Palmer, [39] N.M. Oyesiku, C.O. Evans, S. Houston, R.S. Darrell, J.S. Smith, D.W. Marion, S.T. DeKosky, Hypothermia attenuates the normal Z.L. Fulop, C.E. Dixon, D.G. Stein, Regional changes in the increase in interleukin 1 beta RNA and nerve growth factor expression of neurotrophic factors and their receptors following following traumatic brain injury in the rat, J. Neurotrauma 12 (1995) acute traumatic brain injury in the adult rat brain, Brain Res. 833
159–167. (1999) 161–172.
[25] K.Z. Guluma, K.E. Saatman, A. Brown, R. Raghupathi, T.K. [40] A.G. Rabchevsky, I. Fugaccia, A. Fletcher-Turner, D.A. Blades, McIntosh, Sequential pharmacotherapy with magnesium chloride M.P. Mattson, S.W. Scheff, Basic fibroblast growth factor (bFGF) and basic fibroblast growth factor after fluid percussion brain injury enhances tissue sparing and functional recovery following moderate results in less neuromotor efficacy than that achieved with mag- spinal cord injury, J. Neurotrauma 16 (1999) 817–830.
nesium alone, J. Neurotrauma 16 (1999) 311–321. [41] J.J. Ramirez, S.P. Finklestein, J. Keller, W. Abrams, M.N. George, T. [26] S.B. Jones, F. Depocas, C.C. Chan, Plasma catecholamines in rats Parakh, Basic fibroblast growth factor enhances axonal sprouting
during rewarming from induced hypothermia, J. Appl. Physiol. 57 after cortical injury in rats, Neuroreport 10 (1999) 1201–1204. (1984) 808–814. [42] S. Rowntree, B. Kolb, Blockade of basic fibroblast growth factor [27] T. Kawamata, N.E. Alexis, W.D. Dietrich, S.P. Finklestein, Intracis- retards recovery from motor cortex injury in rats, Eur. J. Neurosci. 9
ternal basic fibroblast growth factor (bFGF) enhances behavioral (1997) 2432–2441.
recovery following focal cerebral infarction in the rat, J. Cereb. [43] E.M. Schuman, Neurotrophin regulation of synaptic transmission, Blood Flow Metab. 16 (1996) 542–547. Curr. Opin. Neurobiol. 9 (1999) 105–109.
[28] T. Kawamata, W.D. Dietrich, T. Schallert, J.E. Gotts, R.R. Cocke, [44] G. Sinson, B.R. Perri, J.Q. Trojanowski, E.S. Flamm, T.K. McIn-L.I. Benowitz, S.P. Finklestein, Intracisternal basic fibroblast growth tosh, Improvement of cognitive deficits and decreased cholinergic factor enhances functional recovery and up-regulates the expression neuronal cell loss and apoptotic cell death following neurotrophin of a molecular marker of neuronal sprouting following focal cerebral infusion after experimental traumatic brain injury, J. Neurosurg. 86 infarction, Proc. Natl. Acad. Sci. USA 94 (1997) 8179–8184. (1997) 511–518.
[29] H. Koizumi, J.T. Povlishock, Posttraumatic hypothermia in the [45] G. Sinson, M. Voddi, T.K. McIntosh, Nerve growth factor adminis-treatment of axonal damage in an animal model of traumatic axonal tration attenuates cognitive but not neurobehavioral motor dysfunc-injury, J. Neurosurg. 89 (1998) 303–309. tion or hippocampal cell loss following fluid-percussion brain injury [30] M. Kusumoto, E. Dux, K.A. Hossmann, Effect of trophic factors on in rats, J. Neurochem. 65 (1995) 2209–2216.
delayed neuronal death induced by in vitro ischemia in cultivated [46] G. Sinson, M. Voddi, T.K. McIntosh, Combined fetal neural hippocampal and cortical neurons, Metab. Brain Dis. 12 (1997) transplantation and nerve growth factor infusion: effects on neuro-113–120. logical outcome following fluid-percussion brain injury in the rat, J. [31] T.T. Lee, B.A. Green, W.D. Dietrich, R.P. Yezierski, Neuroprotective Neurosurg. 84 (1996) 655–662.
effects of basic fibroblast growth factor following spinal cord [47] W.C. Taft, K. Yang, C.E. Dixon, G.L. Clifton, R.L. Hayes, Hypo-contusion injury in the rat, J. Neurotrauma 16 (1999) 347–356. thermia attenuates the loss of hippocampal microtubule-associated [32] Z. Liu, P.A. D’Amore, M. Mikati, A. Gatt, G.L. Holmes, Neuro- protein 2 (MAP2) following traumatic brain injury, J. Cereb. Blood
protective effect of chronic infusion of basic fibroblast growth factor Flow Metab. 13 (1993) 796–802.
on seizure-associated hippocampal damage, Brain Res. 626 (1993) [48] Y.D. Teng, I. Mocchetti, A.M. Taveira-DaSilva, R.A. Gillis, J.R. 335–338. Wrathall, Basic fibroblast growth factor increases long-term survival [33] J.C. Louis, E. Magal, W. Gerdes, W. Seifert, Survival-promoting and of spinal motor neurons and improves respiratory function after protein kinase C-regulating roles of basic FGF for hippocampal experimental spinal cord injury, J. Neurosci. 19 (1999) 7037–7047. neurons exposed to phorbol ester, glutamate and ischaemia-like [49] N.T. Thompson, R.W. Bosner, L.G. Garland, Receptor-coupled conditions, Eur. J. Neurosci. 5 (1993) 1610–1621. phospholipase D and its inhibition, TIPS 12 (1991) 404–408. [34] B.G. Lyeth, J.Y. Jiang, S. Liu, Behavioral protection by moderate [50] G. Trovarelli, G.E. de Medio, R.V. Dorman, G.L. Piccinin, L.A.
hypothermia initiated after experimental traumatic brain injury, J. Horrocks, G. Porcellati, Effect of cytidine diphosphate choline Neurotrauma 10 (1993) 57–64. (CDP-choline) on ischemia-induced alterations of brain lipid in the [35] D.W. Marion, Y. Leonov, M. Ginsberg, L.M. Katz, P.M. Kochanek, gerbil, Neurochem. Res. 6 (1981) 821–833.
A. Lechleuthner, E.M. Nemoto, W. Obrist, P. Safar, F. Sterz, S.A. [51] V. Valouskova, A. Gschanes, Effects of NGF, b-FGF, and cere-Tisherman, R.J. White, F. Xiao, H. Zar, Resuscitative hypothermia, brolysin on water maze performance and on motor activity of rats: Crit. Care Med. 24 (1996) S81–89. short- and long-term study, Neurobiol. Learn. Mem. 71 (1999) [36] D.W. Marion, L.E. Penrod, S.F. Kelsey, W.D. Obrist, P.M. Kochanek, 132–149.