*Corresponding author. Tel.:#32-2-627-43-05; fax:#32-2-627-41-32.
E-mail address:[email protected] (J.L. BoeveH)
The secretion of the ventral glands in
Cladius
,
Priophorus
and
Trichiocampus
saw#y larvae
Jean-Luc Boeve
H
!
,
*
, Sylvie Heilporn
"
, Konrad Dettner
#
,
Wittko Francke
$
!De&partement d'Entomologie, Institut Royal des Sciences Naturelles de Belgique, Rue Vautier 29, B-1000 Bruxelles, Belgium
"Laboratoire de Chimie bio-organique, Universite& Libre de Bruxelles, Avenue F.-D. Roosevelt 50, B-1050 Bruxelles, Belgium
#Lehrstuhl fu(r Tiero(kologie II, Universita(t Bayreuth, Postfach 10 12 51, D-95440 Bayreuth, Germany
$Institut fu(r Organische Chemie, Universita(t Hamburg, Martin-Luther-King-Platz 6, D-20146 Hamburg, Germany
Received 22 July 1999; accepted 18 November 1999
Abstract
The volatile secretion from ventral glands of the larvae ofCladius pectinicornis,Priophorus morio,P. pallipesandTrichiocampus grandiswas found to be principally composed of long-chain acetogenins, in majority of the esters and hydrocarbons, with more than 15 carbon atoms. The scarcity of more volatile compounds may be considered as plesiomorphic for the tribe Cladiini to which the four species belong. Further chemotaxonomic signi"cance and chemical ecological implications of the glandular secretions are discussed. Moreover, the function of the well-developed pubescence covering the body of Cladiini larvae is discussed as a part of their defensive mechanism. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Cladius; Priophorus; Trichiocampus; Tenthredinidae; Hymenoptera; Saw#y larvae; Ventral glands; Defensive secretion; Acetogenins; Chemotaxonomy
1. Introduction
Of the tribes Cladiini and Nematini, belonging to the subfamily Nematinae
(Hy-menoptera, Tenthredinidae), the former might be more `primitivea (Ross, 1937),
namely representing an earlier lineage of the Nematinae, and is obviously less rich in species. The Cladiini comprise about 30 species, worldwide, occurring predominantly
in holarctic regions. Three genera exist,CladiusIlliger, 1807, PriophorusDahlbom,
1835, andTrichiocampusHartig, 1837.
Cladiuslarvae feed exclusively on leaves of Rosaceae, especiallyRosaandFragaria
(e.g., Taeger et al., 1998). In contrast, a species such asPriophorus pallipesis obviously
polyphagous. It feeds on 11 plant genera belonging to the Rosales, Laurales and Fagales. The larvae of both genera feed at the underside of leaves, and they are cryptic.
The visual appearance of the body is greenish for Cladius and dorsally dark for
Priophorus(from which not all species are described at larval stage). The larvae of both genera are solitary. However, they can be found more or less aggregated on an
individual plant. Trichiocampus larvae generally feed only on Salicaceae, at the
underside of leaves. They possess a true gregarious habit and are brightly coloured. The larval body is yellowish and covered with black spots.
A morphological characteristic of all Cladiini larvae is the well-developed pubes-cence distributed on the body. The setae do not seem to possess a secretory function,
but they present"ne hooks on their surface (Lorenz and Kraus, 1957, p. 151). On the
other hand, the larvae possess ventral glands (BoeveH and Pasteels, 1985). Such glands,
present in all nematines, are of relatively small size in the Cladiini; this is probably
related to the primitive phylogenetic position of the tribe (BoeveH, 1988). Some Cladiini
species emit an odour which can be perceived by the human nose (Downes, 1925;
BeHique, 1961; Lorenz and Kraus, 1957), and which can be described as `acrid and
unpleasanta(Downes, 1925) or`unpleasant and with a pronounced heavy notea(J.-L.
BoeveH, personal description). Thus, like in other nematines, the ventral glands of
Cladiini larvae can produce volatile compounds, the chemical nature of which remained, however, unknown.
In the present paper, secretions of the following species have been analysed by
GC-MS:C. pectinicornis(Geo!roy, 1785),P. brulleiDahlbom, 1835 ["P. morioauct.;
P. tener(Zaddach, 1859)],P. pallipes (Serville, 1823) andT. grandis (Serville, 1823) ["T.viminalis (FalleHn, 1808)] (for nomenclature, see Blank and Taeger, 1998). The
results will be discussed from a phylogenetic and chemical ecological point of view.
2. Material and methods
2.1. Glandular secretion
A"rst series of saw#y larvae was collected in the"eld around Bayreuth (Germany): C. pectinicornisonRosaspp.,P. morioonRubusspp.,P. pallipesonCrataegusspp., and T. grandisonPopulus tremulaL. The larvae were identi"ed with Lorenz and Kraus (1957) and adults, obtained by rearing, with Benson (1958) and Zhelochovstev (1952). The methods concerning gland preparation (i.e., solid sample injection) and
com-pound identi"cation as given in Table 1 were already described in BoeveH et al. (1992).
For each species, 2}3 samples were analysed by EI and 1}2 by CI. The data from the
equipped with a polar capillary column (CP-Wax-52-CB, 10 m, 0.32 mm id) and the
temperature program was 60}2503C with 103C min~1. All analyses were carried out
in split/splitless-mode with a delay of 30 s.
2.2. Total extract
The gland size of Cladiini larvae is highly reduced, presenting a secretory area
below 0.1 mm2per gland (BoeveH and Pasteels, 1985). Results obtained by solid sample
injections were, therefore, con"rmed for three of the four species already studied, by
analyzing extracts of whole larvae.C. pectinicorniswas collected onRosaspp. around
Berne (Switzerland),T. grandisonPopulusspp. andP. pallipesonPrunus avium(L.) L.
at Grammont (Belgium). Living larvae of the two former species and frozen larvae of the latter were extracted by successively submerging individuals in pentane, each one
for 5 min, then removing it. The extracts of C. pectinicornis and P. pallipes were
submitted to GC-MS analysis using a Carlo Erba 2150 (polar capillary column:
FFAP, 50 m, 250lm id) connected to a Finnigan 311 A mass spectrometer (70 eV).
The temperature was programmed 2 min at 603C, then up to 2303C with 33C min~1.
The GC-MS system for the extract of T. grandis was a Carlo Erba 8000 (apolar
column OV1 701 Rescom, 25m, 250lm id) and connected to a Micromass AutoSpec
3F mass spectrometer. The temperature program was 1 min at 503C, then up to 2803C
with 53C min~1.
3. Results
3.1. Glandular secretion
A total of about 40 compounds was identi"ed by analysing dissected glands. Most
compounds were long-chain acetogenins with 16}29 carbon atoms. Principally, they
comprised alkanes and esters (Table 1).
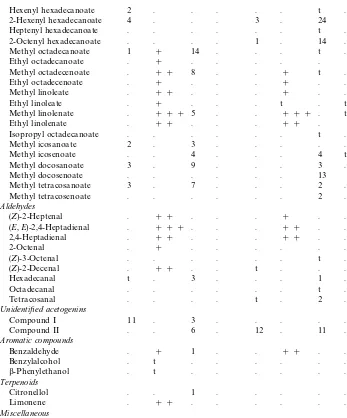
The secretion of each Cladiini species contained major compounds, i.e. representing
more than 5% of the total secretion in the respective species: inC. pectinicornisthree
alkanes, hexadecyl pentanoate and an unidenti"ed compound I; in P. morio octyl
octanoate,"ve saturated and unsaturated methyl esters with 18, 22 and 24 carbon
atoms, and compound II; inP. pallipesthe same three alkanes as inC. pectinicornis,
and compound II; and inT. grandishexadecyl pentanoate, 2-hexenyl hexadecanoate,
octenyl hexadecanoate, methyl dodecenoate, and compound II. Compounds I and II
remain unidenti"ed acetogenins.
2-Hexenyl hexadecanoate was detected in several species (Table 1) and could be
identi"ed since its mass spectrum showed characteristic fragments at m/z82
(hexa-diene) and m/z239 (hexadecanoyl), as described by Honkanen et al. (1963). Mass
spectral data by EI-MS 70 eV [m/z(%)] were as follows: 338 (1) [M`.], 309 (5) [M`.
!29], 239 (16) [M`.!99], 155 (4), 113 (5), 99 (9), 97 (10), 85 (30), 83 (32), 82 (34), 71
3.2. Total extract
Compared to the contents of ventral glands, extracts of whole larvae of C.
pec-tinicornis, P. pallipes and T. grandis showed di!erences (Table 1). Most of the additional compounds were present in small or medium amounts. However, large
amounts of (E,E)-2,4-heptadienal and methyl linolenate were detected inC.
pectinicor-nis, while the latter methylester, acetic and pentanoic acid were found inP. pallipes,
and an alkane inT. grandis.
Some branched alkanes were detected in the total extracts of larvae ofT. grandis.
They were identi"ed according to the literature. For both 2-methyltetracosane and
2-methylhexacosane (Herlan, 1964; Bartelt et al., 1984), the characteristic peaks arose from the methyl (15 Da) and isopropyl (43 Da) loss from the molecular ion. A minor compound in our samples was supposed to be 2-methyldocosane (not listed in Table
1) for the same reasons, but the relative intensity of both characteristic peaks di!ered
when compared to the literature. In the case of 3-methylpentacosane (Lenoir et al.,
1997; Nelson et al., 1980), the characteristic peaks were due to an ethyl loss (M`.
!28) and to the isobutyl ion (57 Da). 13-Methylheptacosane was identi"ed due to its
characteristic peaks at 196/197 and 224/225 (Lenoir et al., 1997; Nelson et al., 1980). Two other methylated hydrocarbons (not listed in Table 1) were also tentatively
identi"ed as 13-methylpentacosane that showed the same characteristic spectrum as
its homologue, 13-methylheptacosane, and 5-methylpentacosane that was
character-ised by diagnostic signals atm/z309 and 84.
4. Discussion
In the four Cladiini species studied, the glandular secretion was mostly composed of acetogenins with more than 15 carbon atoms (Table 1). In the higher evolved group of
the Nematini, however, more volatile components occur frequently (e.g., BoeveH et al.,
1992). Therefore, the relative rarity of such compounds in the Cladiini could be related with the primitive phylogenetic position of this group. A similar trend towards a higher volatility of defensive secretions during evolution is known in staphylinid beetles (Steidle and Dettner, 1993). Moreover, compounds which belong to a relatively low number of chemical types are considered plesiomorphically characteristic within
tenebrionid beetles (Tschinkel, 1975). This seems to be true for the nematine saw#ies
as well; the Nematini frequently emit long-chain acetogenins (as do the Cladiini, Table
1), but also short-chain acetogenins, aromatic compounds and terpenoids (e.g., BoeveH
et al., 1992). Thus, from a chemotaxonomic point of view, our results support the opinion that the Cladiini might be an early lineage within the nematines. However, it will be more critical to use the glandular secretion as a taxonomic cue within the
Cladiini. Indeed, the secretions of both Priophorus species varied qualitatively as
much between them as when compared to the secretions ofCladiusandTrichiocampus
(Table 1).
Di!erent chemical compositions were obtained between analyses by solid sample
di!erences could be due to the fact that in the latter case cuticular products covering
the body of the larva were extracted as well. Moreover, the host}plant species and
geographic origin of the larvae as well as the type of capillary column used also
di!ered between both sets of analyses. Acids, for instance, were only detected in
extracts of whole larvae chromatographed on a polar column.
The defence strategies of Cladiini larvae comprise several aspects. As all members of
its genus,T. grandislarvae are gregarious and brightly coloured. Birds reject them in
bio-assays (Downes, 1925; Carrick, 1936; BoeveH and Pasteels, 1985) and were never
observed to prey upon them in the "eld (BeHique, 1961). The defensive mechanism
underlying this unpalatability is not known. It might be due to (an aposematism
associated to) the glandular secretion as supposed by Downes (1925) and BeHique
(1961), the pubescence of the body and/or the hemolymph (and other body parts) that
would contain distasteful chemicals. Concerning the pubescence, T. grandis larvae
drop o!the plant when touched by spiders, falling being perhaps induced by a tactile
contact with the pubescence (Benson, 1950: p. 75). Falling is also induced when larvae are bitten by ants; the pubescence constitutes here rather a physical barrier, due to
which the mandibles of an ant worker reach the integument of a larva with di$culty
(BoeveH and Pasteels, 1985). This mechanical protection seems to be overcome by
pentatomids as they possess a rostrum that renders an attack possible at a certain
distance. BeHique (1961) mentiones the pentatomidApateticus cynicus(Say) as the most
frequent predator on the larvae of T. grandis, whereas other predators as well as
parasitoids are rare. In comparison to T. grandis, less is known on the defence
strategies of Cladius and Priophorus larvae. Their pubescence probably possesses
a similar function as tactile cue (e.g., McC. Callan, 1978). Thus, it is likely that all Cladiini larvae are similarly defended by their pubescence, and glandular secretion
(Table 1), towards a series of arthropod predators. The more speci"c unpalatability of
T. grandis, and probably other congenerics as well, towards birds should then rely on
still unknown allelochemicals of the hemolymph. This would also con"rm that the
glandular secretion of nematine saw#ies evolved primarily as a defence, not against
vertebrate, but against invertebrate predators (BoeveH and Pasteels, 1985; BoeveH et al.,
1992).
The Cladiini are most likely monophyletic. They constitute a homogenous group of species showing a clear similarity in morphological characteristics at both larval and adult stages (Lorenz and Kraus, 1957; Goulet, 1992), and in the chemical composition of the larval glandular secretions by a dominance of long-chain acetogenins (Table 1).
The larvae ofTrichiocampus, compared to Cladiusand Priophorus, are di!erent in
their visual appearance, gregariousness, general host plants (see Introduction) as well as by some characters of adult morphology (Goulet, 1992). Moreover, some amounts of aromatic compounds can be detected in the glandular secretion of larvae of both CladiusandPriophorus(Table 1). Thus, there is a segregation into the two taxonomic
bulks of Trichiocampus and Cladius}Priophorus. The question arises whether the
Cladiini evolved from an ancestral group either moreTrichiocampus-like from which
Cladius}Priophorusare derived, or the reverse. We presently perform a revision of the Cladiini, comprising biological as well as morphological characteristics, that will
Table 1
Distribution of components in the glandular secretion of Cladiini larvae and in extracts from whole larvae!
Table 1 (Continued)
!(C. pect.) Cladius pectinicornis, (P. br.)Priophorus brullei, (P. pall.)P. pallipes, (T. gr.)Trichiocampus grandis. (GS) Glandular secretion, with amounts expressed as a percentage of the total secretion in the respective species. (TE) Total extract, with presence mentioned as in (###) large, (##) medium, or (#) small amounts. (t) Trace amounts.P. brulleinot studied by total extracts.
Acknowledgements
References
Bartelt, R.J., Krick, T.P., Jones, R.L., 1984. Cuticular hydrocarbons of the yellow headed spruce saw#y
Pikonema alaskensis. Insect Biochem. 14, 209}213.
BeHique, R., 1961. Etude sur la mouche-a`-scie du peuplierTrichiocampusviminalis(Fall.) (HymeHnopte`re: Tenthredinidae). Can. Entomol. 93, 1085}1097.
Benson, R.B., 1950. An introduction to the natural history of British saw#ies. Trans. Soc. Br. Entomol. 10, 45}142.
Benson, R.B., 1958. In: Handbooks for the Identi"cation of British Insects: Hymenoptera 2. Symphyta 6, Part 2 (c). Royal Entomol. Soc, London, pp. 1}49.
Blank, S.M., Taeger, A., 1998. Comments on the taxonomy of Symphyta (Hymenoptera) (Preliminary studies for a catalogue of Symphyta, part 4). In: Taeger, A., Blank, S.M. (Eds.), P#anzenwespen Deutschlands (Hymenoptera, Symphyta). Kommentierte Bestandsaufnahme. Verlag Goecke & Evers, Keltern, pp. 141}174.
BoeveH, J.-L., 1988. StrateHgies deHfensives des larves de neHmatines (Hymenoptera, Tenthredinidae) vis-a`-vis de leurs preHdateurs. Ph.D. Thesis. UniversiteH Libre de Bruxelles.
BoeveH, J.-L., Pasteels, J.M., 1985. Modes of defense in nematine saw#y larvae. E$ciency against ants and birds. J. Chem. Ecol. 11, 1019}1036.
BoeveH, J.-L., Dettner, K., Francke, W., Meyer, H., Pasteels, J.M., 1992. The secretion of the ventral glands in
Nematussaw#y larvae. Biochem. Syst. Ecol. 20, 107}111.
Carrick, R., 1936. Experiments to test the e$ciency of protective adaptations in insects. Trans. Royal Entomol. Soc. London 85, 131}139.
Downes, W., 1925. The poplar saw#y (Trichiocampusviminalis(FalleHn)). Proc. Entomol. Soc. Brit. Col. 22, 26}32.
Goulet, H., 1992. The genera and subgenera of the saw#ies of Canada and Alaska. Hymenoptera: Symphyta. In: The Insects and Arachnids of Canada (Part 20). Agriculture Canada, Ottawa, 235 pp. Herlan, A., 1964. Massenspektren von Para$nen mit 10 bis 24 C-Atomen. I. 2-Methylpara$ne. 1. Teil.
Brennsto!-Chemie 45, 244}249.
Honkanen, E., Moisio, T., Ohno, M., Hatanaka, A., 1963. Mass spectra of seven isomeric hexen-1-ols. Acta Chem. Scand. 17, 2051}2054.
Lenoir, A., Malosse, C., Yamaoka, R., 1997. Chemical mimicry between parasitic ants of the genus
Formicoxenusand their hostMyrmica(Hymenoptera,Formicidae). Biochem. Syst. Ecol. 25, 379}389. Lorenz, H., Kraus, M., 1957. Die Larvalsystematik der Blattwespen (Tenthredinoidea und
Megalodon-toidea). Akademie Verlag, Berlin.
McC Callan, E., 1978. Biological notes on the introduced saw#yPriophorus morio(Lepeletier) (Hymenopt-era: Tenthredinidae) in Australia. J. Aust. Entomol. Soc. 17, 23}24.
Nelson, D.R., Fatland, C.L., Howard, R.W., McDaniel, C.A., Blomquist, G.J., 1980. Re-analysis of the cuticular methylalkanes ofSolenopsis invictaandS. richteri. Insect Biochem. 10, 409}418.
Ross, H.H., 1937. A generic classi"cation of the Nearctic saw#ies (Hymenoptera, Symphyta). Illinois Biol. Monogr. 34, 1}173.
Steidle, J.L.M., Dettner, K., 1993. Quantitative composition of the defensive secretion ofBlediusspecies (Coleoptera: Staphylinidae: Oxytelinae) is adapted to naturally occurring predators. Chemoecology 4, 63}71.
Taeger, A., Altenhofer, E., Blank, S.M., Jansen, E., Kraus, M., Pschorn-Walcher, H., Ritzau, C., 1998. Kommentare zur Biologie, Verbreitung und GefaKhrdung der P#anzenwespen Deutschlands (Hymenoptera, Symphyta), In: Taeger, A., Blank, S.M. (Eds). P#anzenwespen Deut (Hymenoptera. Symphyta). Kommentierte Bestandsaufrahme. Verlag Goeche & Evers. Keltern. pp. 49}135. Tschinkel, W.R., 1975. A comparative study of the chemical defensive system of tenebrionid beetles:
chemistry of the secretions. J. Insect Physiol. 21, 753}783.