Salt tolerance of transgenic rice overexpressing yeast mitochondrial
Mn-SOD in chloroplasts
Y. Tanaka
a, T. Hibino
a, Y. Hayashi
b, A. Tanaka
b, S. Kishitani
c, T. Takabe
d,
S. Yokota
e, T. Takabe
a,f,*
aDepartment of Chemistry,Faculty of Science and Technology,Meijo Uni6ersity,Tenpaku-ku,Aichi,Nagoya468-8502,Japan bPlant Biotech Research Institute,Mitsubishi Chem.Co.,Midori-ku,Yokohama,Kanagawa227-0033,Japan cLaboratory of Plant Breeding and Genetics,Graduate School of Agricultural Science,Tohoku Uni6ersity,Aoba-ku,Yokohama,
Kanagawa227-0033,Japan
dBioScience Center,School of Agricultural Science,Nagoya Uni6ersity,Chikusa-ku,Aichi,Nagoya464-8601,Japan eDepartment of Anatomy,Yamanashi Medical School,Yamanashi409-3821,Japan
fResearch Institute of Meijo Uni6ersity,Tenpaku-ku,Aichi,Nagoya468-8502,Japan
Received 13 April 1999; received in revised form 15 June 1999; accepted 1 July 1999
Abstract
The potential role of superoxide dismutase (SOD) in the protection against salt stress was examined using transgenic rice plants. The coding region of the yeast mitochondrial Mn-SOD gene was fused with the chloroplast targetting signal of glutamine synthetase gene and introduced into rice protoplasts by electroporation. Immunogold labeling experiments revealed that the yeast Mn-SOD was accumulated in the chloroplasts of transgenic rice. The total SOD activity in the control plant was mostly attributed to the activity of cytosolic and chloroplastic Cu/Zn-SOD. Total SOD activity in the transformant was about 1.7-fold that of the control under non-stressed conditions. The photosynthetic electron transport rates of control and transgenic rice were similar under non-stressed conditions. Upon salt stress (100 mM NaCl), the SOD activities decreased in both plants, but the decrease was faster in the control plant. The activities of overexpressed Mn-SOD and cytosolic Cu/Zn-SOD did not change upon salt stress in either the transgenic or control plants, whereas the chloroplastic Cu/Zn-SOD activity in control rice decreased significantly. At high salinity, the ascorbate peroxidase activity of the transformant was about 1.5-fold higher than that in the control. These results suggest that increased levels of ascorbate peroxidase and high levels of chloroplastic SOD in the transformant are important factors for salt resistance in rice. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Rice; Mn-SOD; Oxidative stress; Rice; Salt tolerance; Transgenic rice
www.elsevier.com/locate/plantsci
1. Introduction
Accumulation of salts in irrigated soil are pri-mary factors depressing yield in crop production [1 – 3]. Since rice (Oryza sati6aL.) is a salt sensitive plant, there is keen interest to construct the salt resistant rice [2]. Under high salinity conditions, plant cells must possess specific mechanisms to
adjust their internal osmotic status [1 – 3]. One such mechanism is the ability to accumulate low molecular weight organic compatible solutes such as sugars, some amino acids and quaternary am-monium compounds [2]. Another adaptation mechanism to high salinity is the exclusion of Na+
ion from the sodium sensitive sites which has been proposed as a function of Na+/H+antiporter and
Na+ATPase [1]. Expression of compatible solutes
and heterologous sodium efflux transporters may be a useful approach to improve the salt tolerance of photosynthetic organisms. Indeed, the increase of salt tolerance or water stress tolerance of
pho-Abbre6iations: APX, ascorbate peroxidase; Chl, chlorophyll; ET, electron transport; GR, glutathione reductase; MV, methyl viologen; PSII, photosystem II; SOD, superoxide dismutase.
* Corresponding author: Tel.:+81-52-832-1151; fax:+ 81-52-832-1545.
E-mail address:[email protected] (T. Takabe)
tosynthetic organisms transformed with these genes has been demonstrated [2,4,5].
In addition to these toxic effects, salt stress may cause the induction of oxidative stress [6 – 8]. Upon salt stress, stomatal closure triggered by abscisic acid [9] limits CO2 supply to the leaf leading to overreduction of photosynthetic electron transport (ET) chain [10]. The overreduction of ET chain causes the generation of active oxygen species such as singlet oxygen, superoxide anion (O2−), hydro-gen peroxide (H2O2), and hydroxyl radical (OH) [11,12]. The first line of defense in protecting cells against oxidative stress is SOD, because its activity determines the concentrations of the Haber-Weiss reaction substrates, O2−and H2O2, which can react in the presence of Fe3+ to form OH [11,12].
Recent studies with transgenic plants overexpress-ing SOD showed the increased tolerance to MV, chilling, ozone, and water-deficit [13 – 17]. At the same time, no protective effect on the stresses to H2O2, singlet oxygen, chilling, and salt has been reported [17,18]. Therefore, it is important to clar-ify why different results were obtained. In rice, the accumulation of H2O2, decrease of SOD, and
tran-sient increases of glutathione reductase (GR) un-der salinity stress have been reported [19,20]. Hitherto, overexpression of active oxygen-scaveng-ing enzymes in rice has not been reported. There-fore, we constructed transgenic rice lines overexpressing Mn-SOD in chloroplasts and ex-amined their salt tolerance. The chloroplast is a target organism which produces the active oxygen species. Of three isoforms, Fe-SOD, Mn-SOD, and Cu/Zn-SOD, Cu/Zn-SOD is the major SOD in the chloroplast and a minor enzyme is Fe-SOD [11,12]. Since Cu/Zn-SOD enzyme activity is in-hibited by H2O2[11], we transformed rice with the yeast mitochondrial Mn-SOD of which enzyme activity is resistant to H2O2[11]. We show that the transgenic rice is more resistant to salt stress than the control plant.
2. Materials and methods
2.1. Construction of 6ector
The yeast mitochondrial Mn-SOD coding re-gion [21] was fused with the chloroplast targetting signal of glutamine synthetase gene using the
BamHI site which was flanked by the catalase first intron. The resulting gene was ligated into the
XbaI/SacI site of the binary vector pBI221 and was placed between the cauliflower mosaic virus 35S promoter and nopaline synthase terminator (Nos-ter) regions. The resulting plasmid pSc was introduced into rice protoplasts by electroporation [22]. Plants homozygous for the introduced gene were selected from R2 seeds and used for subse-quent experiments.
2.2. Growth conditions
Rice seeds (Oryza sati6aL. cv. Sasanishiki) were sterilized with 1% NaClO (1% active chlorine), soaked in water for 1 day and put onto agar plates containing 30 mg/ml hygromycin for 6 days in the
dark at 30°C. Then seedlings were grown with vermiculite in pots filled with 1/50 strength MS medium under cycles of 14 h light (200 mE m−2
s−1) at 28°C and 10-h darkness at 25°C in a plant growth chamber in which relative humidity was maintained within 70 – 80%. The concentration of MS medium was then changed to 1/20 and 1/10 for every 5 days. To induce salt stress, we
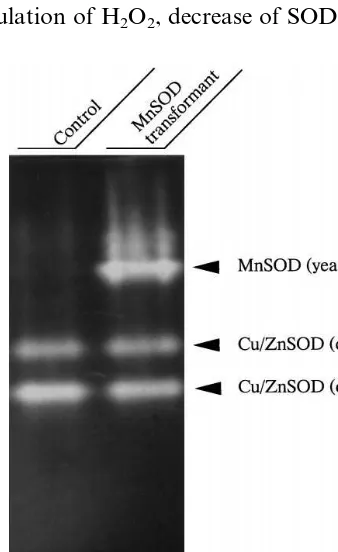
trans-Fig. 1. Expression of yeast Mn-SOD in the transformed rice plants. Proteins were extracted from the control and trans-genic rice plants. Each total soluble proteins (50 mg) were
ferred the plants to a growth medium that con-tained 100 mM NaCl.
2.3. SOD acti6ity staining and other enzyme
assays
Leaf tissue (0.03 – 0.04 g in FW) was homoge-nized with a glass homogenizer in 400ml of
extrac-tion buffer (50 mM potassium phosphate, pH 7.8, 1 mM EDTA, 5 mM ascorbic acid, and 0.2% Triton X-100). The homogenate was centrifuged at 20 000×g for 10 min at 4°C, and the supernatant was used for antioxidant enzyme assays. Total protein was determined by the method of Lowry et al. as previously described [23].
Proteins (50 mg per sample) were separated on
native PAGE with 10% acrylamide and stained for SOD activity [24]. The band intensity was quantified by using an Image Master (Pharmacia Biotec, Upsala, Sweden). The ascorbate peroxi-dase (APX) activity in the extract was determined spectrophotometrically by measuring the oxida-tion of ascorbate at 290 nm [7]. The assay mixture contained 50 mM potassium phosphate, pH 7.0, 0.2 mM H2O2and 0.2 mM ascorbate. The activity of GR was measured by monitoring the oxidation of NADPH at 340 nm. Reaction mixtures con-tained 50 mM potassium phosphate (pH 7.5), 1 mM EDTA, 0.5 mM oxidized glutathione and 50 mM NADPH. Catalase activity was assayed by monitoring the consumption of H2O2at 240 nm in a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0) and 2 mM H2O2. All spectrophotometric assays were carried out at 25°C.
2.4. Measurement of electron transport acti6ities
Electron transport (ET) rates were measured with a PAM fluorometer (Walz, Effeltrich, Ger-many) as previously described [25]. The ET rates were calculated by the equation, (Fm%−F)/Fm%× 200×0.84×0.5 [25,26]. Here, Fand Fm% represent the fluorescence levels under illumination before and after a saturating flash, respectively. The quantum efficiency of photosystem II (PSII) was calculated by the equation, (Fm−Fo)/Fm [25,26]. Where Fo and Fm represent the fluorescence levels without continuous irradiation before and after a saturating flash, respectively. Before each measure-ment, plants were adapted for 30 min in the dark.
The intensity and duration of a flash were 2000mE
m−2 s−1 and 1 s, respectively.
2.5. Methyl 6iologen treatment and other methods
Leaves cut from the control and transgenic plants were placed in the tubes containing 100mM
methyl viologen (MV). After overnight incubation in the dark, leaves were irradiated with incandes-cent fluoresincandes-cent light at the intensity of 150 mE
m−2 s−1 at 28°C. The quantum efficiency of PSII was measured as described above after 15 min of dark adaptation. The immunogold labeling experi-ments were carried out as previously described [27]. Leaves of transgenic Sc26-5 and wild-type rice were used as sample tissues. Anti yeast SOS antibody was used for the detection of Mn-SOD protein.
3. Results
3.1. Expression of Mn-SOD in rice and its localization
The mitochondrial Mn-SOD from yeast was overexpressed as described in Section 2. Twenty transformants were used for the experiments. Ten transformants showed the protective effects against the MV-induced photooxidative damages of leaf discs (data not shown). Three of them exhibited relatively high level expression of Mn-SOD and one transformant (Sc26-5) was used for salt tolerance experiments. Fig. 1 shows SOD ac-tivity staining of the transformant and control plants. Two major bands were observed in the control. The faster migrating band represents chloroplastic Cu/Zn-SOD and the slower one cy-tosolic Cu/Zn-SOD [28]. The transformant exhib-ited an aditional band corresponding to the Mn-SOD at a lower mobility region in the gel. This band was observed even after staining in the presence of H2O2 but the lower two bands were not (data not shown), supporting the above as-signments. The activity of Cu/Zn-SOD in the transformant was almost the same as that in the control plants.
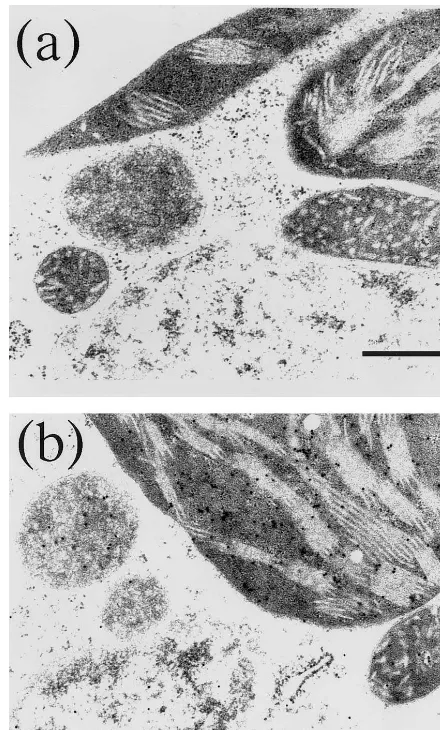
per-Fig. 2. Sections of leaves in the control and transformant rice plants incubated with anti-Mn-SOD, followed by protein A-gold complex conjugation. (a) Control rice plant; and (b) tranformant rice plant. Bar shows 0.5mm.
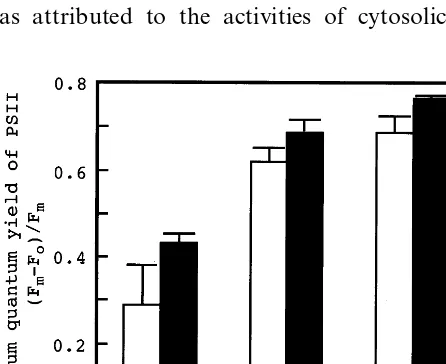
Fig. 3 shows changes in quantum efficiency of PSII during irradiation of MV-treated control and transgenic rice. Although the quantum efficiency of PSII decreased upon irradiation in both the control and in the transformant, the extent of this decline was reduced in the transgenic plants, indi-cating that the overproduction of Mn-SOD had protective effect against the photooxidative dam-age of PSII.
Next, the effects of salt stress on the ET rate was examined. The ET rates of the 5th leaf in the control and transgenic plants were nearly the same in the absence of salt stress (Fig. 4). After 4 days of salt stress, the ET rate of control leaf decreased and could not be detected after 5 days of salt stress. In contrast, the decrease of ET rate in the transformant was slower and a high activity was detected even after 4 days. The ET rate after 4 days in the transformant showed significant differ-ence (P=0.05) from the control. We also exam-ined the effects of salt stress on the ET rates at the distance of 10, 12.5 and 15 cm from the tip of the 6th leaf. The quantum efficiencies of PSII in both the control and transformant decreased from the tip to the base as shown in Fig. 5. After 10 days of salt stress, the quantum efficiencies of PSII at the positions of 10, 12.5, and 15 cm from the tip in the control plants were always lower than those in the transformant (significant at P=0.1, 0.05, and 0.05, respectively). The decreases of chlorophyll
Fig. 3. MV-induced decrease in quantum yield in rice leaves. Quantum yield was measured from the chlorophyll fluores-cence in leaves irradiated at 150 mmol m−2 s−1. The MV
concentration was 100mM., non-stressed control rice; ,
salt-stressed control rice; and , salt-stressed transformant.
Vertical bars represent SE for three samples.
oxisome and mitochondria. The gold particles in mitochondria were probably as a result of the cross-reaction of antibody with the endogenous rice Mn-SOD, whereas those in peroxisome are unknown. Moreover, the gold particles in chloro-plasts were mainly detected in the stromal space. These results suggest that the yeast Mn-SOD was successfully overexpressed in rice and accumulated in the stromal space of chloroplasts.
3.2. Effects of salt stress on the ET rates in the control and transgenic rice
Fig. 4. Time courses of the changes in ET rate in rice leaves during salt stress. The NaCl concentration was 100 mM. ET rate was calculated from the chlorophyll fluorescence in leaves irradiated with 400mmol m−2s−1 at 28°C. , control rice;
and, transformant rice. Vertical bars represent SE for three
samples.
chloroplastic Cu/Zn-SOD. Under non-stressed conditions, the Cu/Zn-SOD activity in the trans-formant was similar to that in the control, al-though the total SOD activity in the transformant was 1.7-fold higher than that in the control rice. Upon salt stress (100 mM NaCl), the cytosolic Cu/Zn-SOD activity in both the control and trans-formant was not changed, whereas the chloroplas-tic Cu/Zn-SOD activity decreased. The decrease of chloroplastic Cu/Zn-SOD was greater in the con-trol than in the transformant. The Mn-SOD activ-ity in the transformant did not change upon salt stress. Therefore, the total SOD activity in the transformant was retained at a high value under salt stress conditions, whereas the activity in the control rice significantly decreased.
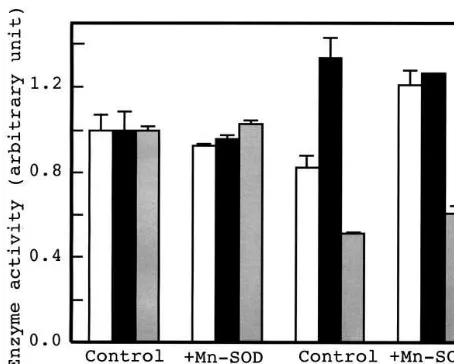
Changes in the activities of the other three active oxygen quenching enzymes upon salt stress were examined. Before salt stress, the activities of APX, GR, and catalase in the control plants were similar to that in the transformant (Fig. 7). Upon salt stress, the APX activities of the control plants decreased 17% whereas its activity in the transfor-mant increased 30%. In both the control and transformant, the catalase activity was lowered by the salt stress whereas the GR activity was increased.
levels in the control were also faster than that in the transgenic plants (data not shown). These re-sults indicate that the overexpression of Mn-SOD protects the rice chloroplast from the damage by salt stress.
Next, the effects of salt stress on the activities of SOD isozymes were examined. As shown in Figs. 1 and 6, the total SOD activity in the control plant was attributed to the activities of cytosolic and
Fig. 6. SOD activities in the 6th leaves of rice seedlings after 7 days exposure to 100 mM NaCl. White, grey, and black boxes represent the activities of cytosolic Cu/Zn-SOD, chloro-plastic Cu/Zn-SOD, and Mn-SOD, respectively. Each value shows the average of three independent measurements (SE was within 7%).
Fig. 7. APX, GR and catalase activities in leaves of the control and transformant rice grown for 8 days in the solu-tion at 100 mM NaCl. White, black, and grey boxes represent the activities of APX, GR, and catalase, respectively. Vertical bars represent SE for three samples.
than that in the control plants. They concluded that Fe-SOD-overexpressing tobacco plants were no more tolerant to salt stress than were control plants [17].
Our results indicate that the yeast mitochondrial Mn-SOD was overexpressed in rice chloroplasts, and the transformant had an increased total SOD activity and exhibited enhanced tolerance to salt stress. In the absence of salt stress, both the trans-formant and control plants exhibited nearly the same activities of chloroplastic Cu/Zn-SOD, cyto-solic Cu/Zn-SOD, APX, GR and catalase. Upon salt stress, GR activity increased in both the con-trol and transformant, whereas the activities of chloroplastic Cu/Zn-SOD and catalase decreased. The results were similar to those observed in wild-type rice [19]. In the transformant, total SOD activity was maintained at a high level, APX activ-ity increased, and the decrease of catalase activactiv-ity reduced after the exposure to salt stress. These phenomena were different from those observed in the control plants. The retention at high activity of SOD and the increased activity of APX may be correlated with the protection of the photosyn-thetic activity from the inactivation by salt stress in rice. To our knowledge, this is the first report demonstrating enhanced tolerance to salt stress in plants overexpressing SOD. As a result of the different experimental conditions between the pre-vious study [17] and ours (plant species, metal cofactors of SOD, and stress conditions), it is difficult to compare the results. One aspect to be mentioned is that under salt stress conditions, the activities of SOD and APX in the transgenic rice had 1.52.0-fold higher than that in the control rice whereas those in the transgenic Fe-SOD to-bacco were similar to that in the control toto-bacco. Since SOD produces H2O2which can react with superoxide anions to form hydroxyl radical, a highly toxic reactive oxygen species, high level of SOD might not produce salt-resistannt plants. In-creased level of APX and/or catalase may be a more important factor to acquire salt resistance. Since H2O2 has been implicated as an elicitor of several genes related to stress tolerance [30], the increased activity of SOD could lead to generation of H2O2 which then could induce APX gene expression.
Although the present results indicate that the overexpression of a yeast Mn-SOD in rice chloro-plasts conferred resistance to salt stress, its effect
4. Discussion
Several groups have generated transgenic plants that overexpress SODs in order to enhance toler-ance to oxidative stress, such as MV, chilling, ozone, and water-deficit [13 – 18]. These attempts have been successful to various degrees. From the correlation studies between the activities of antiox-idant enzymes and the oxidative stress tolerance [13,29], it has been postulated that SOD overex-pression leads to enhancement of the tolerance to oxidative stress only if one or more of antioxidant enzymes such as APX, dehydroascorbate reduc-tase, and monodehydroascorbate reducreduc-tase, is also present at high levels.
was very limited. One of the reason for the limited effect of Mn-SOD overexpression may be the lo-calization of Mn-SOD. The immunogold labeling experiments indicated that the overexpressed Mn-SOD was observed in stromal space in chloro-plasts (Fig. 2). If Mn-SOD were associated with thylakoid membranes where O2− is produced [11], more larger effects might be expected as discussed by Van Camp et al. [17]. Indeed, the association of Cu/Zn-SOD to thylakoid membranes has been detected [31]. Therefore, targetting Mn-SOD to thylakoid membranes is interesting subject to be tested.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, the High-Tech Research Center of Meijo University, the Program for Promotion of Basic Research Activities for Innovative Biosciences (PRO-BRAIN), the JSPS Project ‘Research for Future’, and the Biodesign Project of the Japanese Ministry of Agriculture, Forestry and Fisheries.
References
[1] R. Serrano, Salt tolerance in plants and microorganisms: toxicity targets and defense responses, Int. Rev. Cytol. 165 (1996) 1 – 52.
[2] T. Takabe, T. Nakamura, M. Nomura, Y. Hayashi, M. Ishitani, Y. Muramoto, et al., Glycinebetaine and the genetic engineering of salinity tolerance in plants, in: K. Satoh, N. Murata (Eds.), Stress Responses of Photosyn-thetic Organisms, Elsevier Science, Amsterdam, 1998, pp. 115 – 131.
[3] K.M. Volkmar, Y. Hu, H. Steppuhn, Physiological re-sponses of plant to salinity: a review, Can. J. Plant Sci. 78 (1998) 19 – 27.
[4] M. Nomura, M. Ishitani, T. Takabe, A.K. Rai, T. Takabe, Synechococcus sp. PCC7942 transformed with
Escherichia colibet genes produces glycine betaine from choline and aquires resistance to salt stress, Plant Phys-iol. 107 (1995) 703 – 708.
[5] M.C. Tarczynski, R.G. Jensen, H.J. Bohnert, Stress pro-tection of transgenic tobacco by production of the os-molyte mannitol, Science 259 (1993) 508 – 510.
[6] R.H. Burdon, D. O’Kane, N. Fadzillah, V. Gill, P.A. Boyd, R.P. Finch, Oxidative stress and responses in
Arabidopsis thalianaandOryza sati6asubjected to
chill-ing and salinity stress, Biochem. Soc. Trans. 24 (1996) 468 – 472.
[7] D.R. Gossett, E.P. Millhollon, M.C. Lucas, Antioxidant response to salt stress in salt-tolerant and salt-sensitive cultivars of cotton, Crop Sci. 34 (1994) 706 – 714. [8] J.A. Hernandez, E. Olmos, F.J. Corpas, F. Sevilla, L.A.
del Rio, Salt-induced oxidative stress in chloroplasts of pea plants, Plant Sci. 105 (1995) 151 – 167.
[9] I.S. Fendina, T.D. Tsonev, E.I. Guleva, ABA as a mod-ulator of the response ofPisum sati6umto salt stress, J. Plant Physiol. 143 (1994) 245 – 249.
[10] C.B. Osmond, S.C. Grace, Perspectives on photoinhibi-tion: and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosyn-thesis?, J. Exp. Bot. 46 (1995) 1351 – 1362.
[11] K. Asada, Production and action of active oxygen spe-cies in photosynthetic tissues, in: C.H. Foyer, P.M. Mullineaux (Eds.), Causes of Photooxidative Stress in Plants and Amelioration of Defense Systems, CRC Press, Boca Raton, FL, 1994, pp. 77 – 104.
[12] C. Bowler, M. Van Montagu, D. Inze, Superoxide dis-mutase and stress tolerance, Annu. Rev. Plant Physiol. 43 (1992) 83 – 116.
[13] C.H. Foyer, P. Descourvieres, K.J. Kunert, Protection against oxygen radicals: an important defence mecha-nism studied in transgenic plants, Plant Cell Environ. 17 (1994) 507 – 523.
[14] A.S. Gupta, R.P. Webb, A.S. Holaday, R.D. Allen, Overexpression of superoxide dismutase protects plants from oxidative stress, Plant Physiol. 103 (1993) 1067 – 1073.
[15] W. Van Camp, H. Willekens, C. Bowler, M. Van Mon-tagu, D. Inze, P. Reupold-Popp, et al., Elevated levels of superoxide dismutase protect transgenic plants against ozone damage, Biotechnology 12 (1994) 165 – 168. [16] B.D. McKersie, S.R. Bowley, E. Harjanto, O. Leprince,
Water-deficient tolerance and field performance of trans-genic alfalfa overexpressing superoxide dismutase, Plant Physiol. 111 (1996) 1171 – 1177.
[17] W. Van Camp, K. Capiau, M. Van Montagu, D. Inze, L. Slooten, Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts, Plant Physiol. 112 (1996) 1703 – 1714.
[18] P. Payton, R.D. Allen, N. Trolinder, A.S. Holaday, Over-expression of chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum L., cv. Coker 312) does not alter the reduction of photosynthesis after short exposures to low temperature and high light inten-sity, Photosynth. Res. 52 (1997) 233 – 244.
[19] S. Singha, M.A. Choudhuri, Effect of salinity (NaCl) stress on H2O2metabolism inVignaandOryzaseedlings,
Biochem. Physiol. Pflanzen 186 (1990) 69 – 74.
[20] N.M. Fadzilla, R.P. Finch, R.H. Burdon, Salinity, oxi-dative stress and antioxidant responses in shoot cultures of rice, J. Exp. Bot. 48 (1997) 325 – 331.
[22] K. Shimamoto, R. Terada, T. Izawa, H. Fujimoto, Fer-tile transgenic rice plants regenerated from transformed protoplasts, Nature 338 (1989) 274 – 276.
[23] T. Hibino, A. Douwe de Boer, P.J. Weisbeek, T. Takabe, Reconstitution of mature plastocyanin from precursor apo-plastocyanin expressed in Escherichia coli, Biochim. Biophys. Acta 1058 (1991) 107 – 112.
[24] C.O. Beauchamp, I. Fridovich, Isozymes of superoxide dismutase from wheat germ, Biochim. Biophys. Acta 317 (1973) 50 – 64.
[25] Y. Tanaka, S. Katada, H. Ishikawa, T. Ogawa, T. Takabe, Electron flow from NAD(P)H dehydrogenase to Photosystem I is required for adaptation to salt shock in cyanobacterium Synechocystissp. PCC 6803, Plant Cell Physiol. 38 (1997) 1311 – 1318.
[26] G.H. Krause, E. Weis, Chlorophyll fluorescence and phytosynthesis: the basics, Annu. Rev. Plant Physiol. 42 (1991) 313 – 349.
[27] S. Yokota, H. Tsuji, K. Kato, Localization of lysosomal and peroxisomal enzymes in the specific granules of rat intestinal eosinophil leukocytes revealed by
immunoelec-tron microscopic techniques, J. Histochem. Cytochem. 32 (1984) 267 – 274.
[28] S. Kanematsu, K. Asada, Characteristic amino acid se-quences of chloroplast and cytosol isozymes of CuZn-su-peroxide dismutase in spinach, rice and horsetail, Plant Cell Physiol. 31 (1990) 99 – 112.
[29] L. Slooten, K. Capiau, W. Van Camp, M. Van Mon-tagu, C. Sybesma, D. Inze, Factors affecting the en-hancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts, Plant Physiol. 107 (1995) 737 – 750. [30] A. Levine, R. Tenhaken, R. Dixon, C. Lamb, H2O2from
the oxidative burst orchestrates the plant hypersensitive disease resistance response, Cell 79 (1994) 583 – 593. [31] K. Ogawa, S. Kanematsu, K. Takabe, K. Asada,
Attach-ment of CuZn-superoxide dismutase to thylakoid mem-branes at the site of superoxide generation (PSI) in spinach chloroplasts: detection by immuno-gold labeling after rapid freezing and substitution method, Plant Cell Physiol. 36 (1995) 565 – 573.