Genetic diversity in viruses is shaped by high rates of recombination and is constrained by host defenses and the requirements of transmission. Recent studies of insect-transmitted plant viruses demonstrate highly conserved molecular motifs in viral genomes that regulate the specificity of insect transmission. In contrast, advances in our understanding of host plant response to virus infection reveal some
generalized patterns of host defense to a diversity of viruses.
Addresses
Department of Ecology and Evolutionary Biology, Corson Hall, Cornell University, Ithaca, New York 14853, USA;
e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:336–340 1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations
HC helper component
PTGS post-transcriptional gene silencing
TYLCV tomato yellow leaf curl geminivirus
Introduction
Plant viruses are known for their remarkable genetic diver-sity, both within and between species. Among RNA plant viruses, genetic diversity arises from error-prone replica-tion mechanisms that result in high mutareplica-tion rates, as well as from recombination and reassortment. The evidence for virus recombination among RNA viruses comes from both experimental systems and virus molecular phyloge-nies [1–3,4•]. In DNA viruses, recombination also appears to be common, and some diversity may reflect a lack of postreplication repair [1]. Recent phylogenetic analyses indicate that recombination has been particularly impor-tant in the evolution of some DNA viruses, particularly the pareretroviruses [5] and the geminiviruses [6,7,8•,9•]. Yet patterns of specificity in insect transmission and evidence from molecular studies indicate that the high genetic diversity generated by mutation and recombination are sig-nificantly constrained by the requirements of insect transmission. This review summarizes recent studies of transmission specificity, with special emphasis on advances in geminivirus and potyvirus research. A brief, selective overview of recent research on host defenses against viral invasion is also provided.
Virus diversity and host defense
The genetic diversity generated by high mutation rates and frequent recombination allows the rapid evolution of viruses in response to host defenses. The small size of the virus population that invades a new host may, however, result in bottlenecks that cause lower viral fitness over successive transfers (i.e. Muller’s ratchet [10]). Although a few studies with phages or animal viruses have shown that RNA viruses
can lose fitness through Muller’s ratchet, there are no exper-imental examples of declining RNA-virus fitness in the plant literature. However, Fraile et al. [11] suggest that Muller’s ratchet may be partially responsible for the displacement of tobacco mosaic tobamovirus by tobacco mild green mosaic tobamovirus in Australian Nicotiana glaucaover the past cen-tury. In an interesting comparison of the genetic diversity of geminiviruses infecting sexual and asexual populations of the host plant Eupatorium, Ooi and Yahara [12] found lower overall infection rates but greater virus genetic diversity in the sexual host populations, suggesting that the viruses had evolved in response to host genetic diversity despite the potential for evolutionary bottlenecks.
How do plants defend themselves against the evolutionary potential of invading viruses? A variety of defense responses have been reviewed recently (e.g. [13]), but one of the most exciting areas of current research is post-transcriptional gene silencing (PTGS). Recent work on PTGS in plants has pro-vided evidence that this mechanism functions as a general defense against virus invasion. Viral invasion can induce gene silencing and provide cross-protection against sec-ondary virus infection [14••,,15,16]. At the same time, suppression of gene silencing is a general strategy used by a broad range of DNA and RNA plant viruses. Successful virus infection results from a virus’ ability to prevent PTGS-medi-ated degradation of its genome, either by directly incapacitating the plant’s PTGS response or by moving through the plant more quickly than the PTGS response or both [17••••]. The virus-encoded proteins responsible for sup-pressing PTGS are known in at least two cases: the helper component (HC) proteinase of potyviruses [18–21] and the 2b protein of cucumoviruses ([19,21,22••,23]).
Experiments with a diversity of unrelated viruses that infect a single host plant, Nicotiana benthamiana,found that many viruses suppressed the gene-silencing defenses of this plant [24••••]. Moreover, in situanalysis of the response of pea embryonic tissue to virus infection has demonstrat-ed a common pattern of host gene regulation in response to infection by viruses from four different virus fami-lies [25••••]. These results support the notion of a generalized host response to viral invasion that might facil-itate the adaptation of viruses to multiple hosts.
Transmission by vectors
The evolution of viruses that rely on a vector to move between hosts is constrained not only by adaptation to hosts and host defenses, but also by the requirements of vector compatibility. Unlike animal viruses, many of which can depend upon host movement for direct transmission to new hosts, most plant viruses are transmitted by vectors, the majority by insects. All transmission requires some specificity between virus and vector, though the degree of
Insect transmission of plant viruses: a constraint on virus variability
specificity varies dramatically among viruses. We are beginning to recognize that even those viruses that are simply carried on the mouthparts of vectors, and appear to have the least specific relations with their vectors, still depend on a complex interaction between viral proteins and vector-associated compounds [26••]. Thus, the diversi-ty of virus populations is constrained by the need to retain specific interactions with their vectors.
In a number of well known examples, expansion of the host range of insect vectors has been shown to increase the host range of the viruses that these vectors transmit (e.g. [8••,27]), which implies that virus distribution is constrained more by the specificity of virus–vector relations than by the speci-ficity of virus–host-plant relations. A recent quantitative comparison of specificity in virus–host and virus–vector rela-tions among over 400 vector-borne plant viruses demonstrated that these viruses have much more specific relations with their vectors than with their hosts [28]. Whereas many viruses have a very narrow range of vectors but a large host range, no viruses have a narrow range of host plants if they have many vector species. The host range of the vector largely determines the host range of the virus, suggesting that viruses can adapt to new hosts fairly readily.
These patterns indicate that most viruses are able to take advantage of a relatively narrow set of vectors. Indeed, Nault [29] suggests that transmission mode is a stable evolutionary trait for virus genera. The transmission modes of insect-vectored plant viruses have been catego-rized according to the intimacy of their association with the vector. Stylet-borne viruses are carried on the mouth-parts of the vectors and are known as ‘nonpersistent’ because they are lost once a vector has fed on a host. Foregut-borne viruses appear to enter the foregut of the vector and are ‘semi-persistent’ in their vectors. Circulative ‘persistent’ viruses pass through the insect gut into the hemolymph and then into the salivary glands
via highly specific transport mechanisms and can be transmitted over a long period. Propagative viruses are circulative viruses that replicate in the insect vector as well as in the plant host, and their relations with vectors are highly specific.
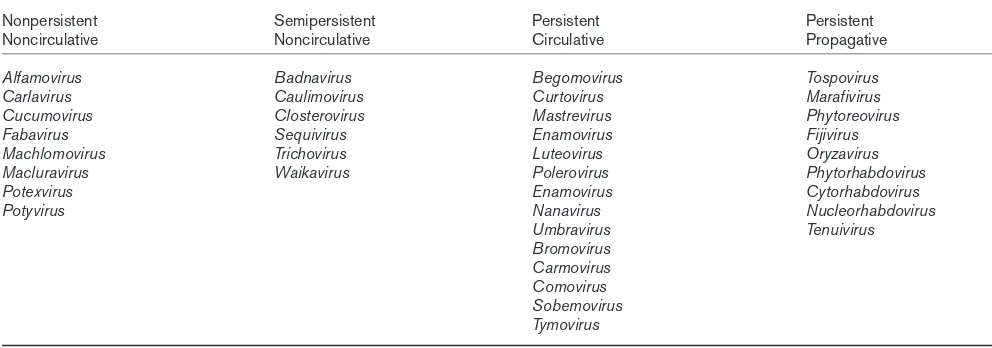
Although some genera of viruses can be transmitted by insects from more than one family, no virus species is capa-ble of being transmitted by insects from more than one family. Furthermore, there are no documented examples of different members of a virus genus having different insect transmission modes, for example, one species of virus being transmitted in a stylet-borne manner and another being transmitted in a circulative mode (though some stylet-borne viruses may also be seed-transmitted). This specificity of transmission mode appears to be a consistent evolutionary constraint, such that virus genera can be assigned to a par-ticular insect transmission mode (Table 1). The consistency of transmission mode within a virus genus and the general-ly greater specificity of vector relations compared to host relations suggest that selection imposed by a requirement for efficient vectors may be more severe than that imposed by host plant defenses. Recent work with geminiviruses and potyviruses, which differ significantly in their genetics and ecology, illustrates how transmission constrains virus evolu-tion despite the high genetic diversity of viruses.
Transmission constraints on geminivirus variation
The geminiviruses are unipartite or bipartite single-strand-ed DNA viruses that are dividsingle-strand-ed into three genera (i.e. Begomovirus, Curtovirus and Mastrevirus). They are transmitted by whiteflies, leafhoppers and treehoppers in a persistent, circulative manner. There is one report that a Begomovirus(tomato yellow leaf curl geminivirus [TYLCV]) may propagate within its whitefly vectors [30], but this possibility is yet to be definitively established [26••,29]. Additional lines of evidence for the propagation of TYLCV within whiteflies are provided by several studies carried Table 1Plant virus genera organized according to transmission mode by insects.
Nonpersistent Semipersistent Persistent Persistent
Noncirculative Noncirculative Circulative Propagative
Alfamovirus Badnavirus Begomovirus Tospovirus
Carlavirus Caulimovirus Curtovirus Marafivirus
Cucumovirus Closterovirus Mastrevirus Phytoreovirus
Fabavirus Sequivirus Enamovirus Fijivirus
Machlomovirus Trichovirus Luteovirus Oryzavirus
Macluravirus Waikavirus Polerovirus Phytorhabdovirus
Potexvirus Enamovirus Cytorhabdovirus
Potyvirus Nanavirus Nucleorhabdovirus
Umbravirus Tenuivirus
Bromovirus Carmovirus Comovirus Sobemovirus Tymovirus
out by Czosnek and colleagues, which suggest that transo-varial transmission of the virus from female whiteflies to their offspring [31] and sexual transmission of the virus among whiteflies [32] take place. Several authors have sug-gested that propagative viruses originated with insect hosts and adapted secondarily to plants [33,34]. This hypothesis is based on the fact that propagative viruses rarely cause disease in their vectors and can be transmitted in the germline from parent to offspring in insects but not in plants. Thus, it could be argued that the observation that vector whitefly longevity and fecundity are reduced by car-rying TYLCV is evidence against the propagation of this virus in whiteflies [35]. In any case, transovarial transmis-sion and sexual transmistransmis-sion among vectors, with or without propagation within vectors, would seem to impose significant constraints on virus variability.
Although geminiviruses are typically transmitted by a sin-gle species of vector, many have large host ranges. The range of diseases incited by begomoviruses has increased dramatically over the past decade, largely because of the introduction of the Old World B-biotype Bemesia tabaci whitefly into the Americas. Recent phylogenetic analyses using mitochondrial DNA [36••] support earlier genetic, biochemical and behavioral evidence that has been used to seperate whiteflies according to geographic origin, and pro-vide epro-vidence that B. tabaciis a highly cryptic group of sister species [37]. B-biotype B. tabaci have an unusually broad host range and can transmit begomoviruses among host plants that did not previously share insect vec-tors [37]. The introduction of this new vector to the New World has apparently provided the opportunity for pre-existing viruses to be transmitted to a variety of crops from their original hosts, which include wild plants. In addition, the high frequency of mixed infections of begomoviruses owing to broad vector–host range undoubtedly provides opportunities for the emergence of new viruses arising from recombination among strains or species [6,7].
Recent molecular analyses of the whitefly-transmitted begomoviruses indicate striking levels of genetic diversity within virus species [38,39••]. Populations of cotton leaf curl geminivirus are extremely genetically diverse. Nevertheless, no genetic differentiation has been detected between isolates from different host plant species or dif-ferent geographic locations within Pakistan [39••]. The regions of the genome encoding the coat protein are, how-ever, much less variable than the regions encoding the replication protein. Nucleotide diversity values for the coat protein, which determines vector transmission specificity [40,41], are similar to those of other highly conserved virus proteins [39••]. These data suggest that the genetic vari-ability of the virus is constrained by the requirements of maintaining effective whitefly transmission.
A number of recent studies support the hypothesis that whitefly-transmitted begomoviruses have evolved along three separate branches of differing geographic origin, a
pattern that mirrors whitefly genetic variation [42–45]. Phylogenetic analyses of begomovirus coat protein sequences divide these viruses into three groups, from the Americas, Asia/Australia, and Africa/Mediterranean/Middle East. It is striking that no relationships based on common host plants have been detected. For example, a bego-movirus infecting tobacco in the New World is much more similar to other New World begomoviruses than to bego-moviruses infecting tobacco in Zimbabwe [45]. This geographically associated genetic variation appears to reflect the spatial isolation of the three virus groups com-bined with their ready adaptation to a diversity of host plants. It also appears to reflect geographically related selection pressure exerted by whitefly vectors as a given begomovirus is transmitted more efficiently by a whitefly biotype from the same region than by a whitefly biotype from a different region [46]. In surveys of the tomato yellow leaf curl virus in Spain, the displacement of one strain by another appeared to be driven largely by the higher effi-ciency of transmission of the displacing strain by local whitefly biotypes [47], although the availability of reservoir hosts may also have influenced virus success. There was no competitive advantage for either strain within the host plant, which suggests that virus–host interactions did not drive displacement.
Transmission constraints on potyvirus variation
Potyviruses are single-stranded RNA viruses that are charac-terized by their nonpersistent stylet-borne transmission by aphids. Potyviruses appear to have evolved a novel strategy for overcoming the bottleneck of insect transmission by encoding a ‘helper component’, a non-structural protein that is produced by the infected plant. HCs aid the virus in bind-ing to the aphid mouthparts, thereby facilitatbind-ing transmission. Several recent studies [48,49] have shown that the HC regu-lates the specificity and efficiency of potyvirus transmission. In some systems, HC encoded by one virus may enable the transmission of a different virus, although homologous virus–HC combinations appear to be required for high trans-mission efficiency [49]. Pirone and Blanc [50] have argued that the ability of a given HC to facilitate transmission of more than one virus (i.e. heterologous as well as homologous combinations) makes viruses that use helper-assisted trans-mission less prone to severe evolutionary bottlenecks than stylet-borne viruses that rely only on direct virus binding.does not appear to hold for other potyviruses [54]. In gener-al, the pattern of highly conserved motifs that are responsible for insect transmissibility suggests that the requirements of vector transmission exert significant selection pressure that limits the diversity of coat protein sequences.
Conclusions
Recent molecular studies of geminiviruses and potyviruses, and of patterns of virus transmission by insects, suggest that insect transmission imposes significant constraints on the evolution of plant viruses. The accelerating pace of molecular studies of viruses will undoubtedly add to our understanding of the genetic regulation of transmission specificity. In addition, evolutionary explanations for this specificity should be explored. Obviously, transmission is an essential part of virus life history. Yet, it is not obvious why it should be significantly more difficult for a virus to increase its range of efficient vectors than to increase its host range. Future research should address this question.
Acknowledgements
Financial support from the United States Department of Agriculture is gratefully acknowledged.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest •• of outstanding interest
1. Roosinck MJ: Mechanisms of virus evolution.Annu Rev Phytopathol 1997, 35:191-209.
2. Domingo E, Holland JJ: RNA virus mutations and fitness for survival.Annu Rev Microbiol 1997, 51:151-178.
3. Aaziz R, Tepfer M: Recombination in RNA viruses and in virus-resistant transgenic plants.J Gen Virol 1999, 80:1339-1346. 4. Aaziz R, Tepfer M: Recombination between genomic RNAs of two • ucumoviruses under conditions of minimal selection pressure.
Virology 1999, 263:282-289.
This study demonstrated the occurrence of RNA recombination between two closely related, nondefective viruses under natural conditions of little or no selection pressure. Tobacco plants were inoculated with cucumber mosaic virus and tomato aspermy virus, and multiple recombinants were detected in three of 82 plants.
5. Chenault KD, Melcher U: Phylogenetic relationships reveal recom-bination among isolates of cauliflower mosaic virus.J Mol Evol 1994, 39:496-505.
6. Zhou X, Liu Y, Calvert L, Munoz C, Otim-Nape GW: Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination.
J Gen Virol 1997, 78:2101-2111.
7. Brown JK, Ostrow KM, Idris AM, Stenger DC: Biotic, molecular, and phylogenetic characterization of bean calico mosaic virus, a dis-tinct Begomovirus species with affiliation in the squash leaf curl virus cluster.Phytopathol 1999, 89:273-280.
8. Harrison BD, Robinson DJ: Natural genomic and antigenic variation • in whitefly-transmitted geminiviruses (Begomoviruses).Annu Rev
Phytopathol 1999, 37:369-398.
This is an excellent review of genetic variability in the begomoviruses that provides useful insights into the evolution of this emerging group of viruses. 9. Paddidam M, Sawyer S, Fauquet CM: Possible emergence of new • geminiviruses by frequent recombination.Virology 1999,
265:218-225.
The authors use a phylogenetic approach to study recombination. The approach is based on detecting chimeric sequences by searching for anom-alies in the distribution of polymorphic sites in a sequence alignment. The
results indicate that frequent recombination is distributed throughout the genome and has contributed significantly to virus evolution.
10. Muller HJ: The relation of recombination to mutational advance.
Mutat Res 1964, 1:2-9.
11. Fraile A, Escriu F, Aranda MA, Malpica JM, Gibbs AJ, Garcia-Arenal F:
A century of tobamovirus evolution in an Australian population of Nicotiana glauca.J Virol 1997, 71:8316-8320.
12. Ooi K, Yahara T: Genetic variation of geminiviruses: comparison between sexual and asexual host plant populations.Mol Ecol 1999, 8:89-97.
13. Carrington JC, Whitham SA: Viral invasion and host defense: strategies and counter-strategies.Curr Opin Plant Biol 1998,
1:336-341.
14. Ratcliff FG, MacFarlane SA, Baulcombe DC: Gene silencing without • DNA: RNA-mediated cross-protection between viruses.Plant Cell
1999, 11:1207-1215.
Studies of a tobravirus and a potexvirus showed that RNA-mediated cross-protection was functionally equivalent to PTGS in plants. This research pro-vides additional evidence that PTGS is a general defense mechanism that is induced by a wide range of viruses.
15. Baulcombe DC: Viruses and gene silencing in plants.Arch Virol 1999, 15(suppl):189-201.
16. Ding SW: RNA silencing.Curr Opin Biotechnol 2000, 11:152-156. 17. Waterhouse PM, Smith NA, Wang MB: Virus resistance and gene •• silencing: killing the messenger.Trends Plant Sci 1999, 4:452-457.
The authors review recent research on RNA-mediated virus resistance, co-suppression of resistance by viral transgenes, and interactions between viruses and their hosts. They provide a superb discussion of the various models that have been proposed to explain these phenomena.
18. Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB: A viral suppressor of gene silencing in plants.Proc Natl Acad Sci USA 1998, 95:13079-13084.
19. Brigneti G, Voinnet O, Li WX, Ji LH, Ding SW, Baulcombe DC:
Viral pathogenicity determinants are suppressers of transgene silencing in Nicotiana benthamiana.EMBO J 1998,
17:6739-6746.
20. Kasschau KD, Carrington JC: A counter-defensive strategy of plant viruses: suppression of post-transcriptional gene silencing.Cell 1998, 95:461-470.
21. Lucy AP, Guo HS, Li WX, Ding SW: Suppression of post-transcrip-tional gene silencing by a plant viral protein localized in the nucleus. EMBO J 2000, 19:1672-1680.
22. Li HW, Lucy AP, Guo HS, Li WX, Ji LH, Wong SM, Ding SW: Strong • host resistance targeted against a viral suppressor of the plant
gene silencing defense mechanism.EMBO J 1999,
18:2683-2691.
This study provides a nice demonstration of the complexity of virus–host interactions. Research on the 2b protein, which is encoded by cucu-moviruses, indicates that this viral suppressor of gene silencing may activate an independent host resistance mechanism other than gene silencing. 23. Mayers CN, Palukaitis P, Carr JP: Subcellular distribution analysis
of the cucumber mosaic virus 2b protein.J Gen Virol 2000,
81:219-226.
24. Voinnet O, Pinto YM, Baulcombe DC: Suppression of gene •• silencing: a general strategy used by diverse DNA and RNA
viruses of plants.Proc Natl Acad Sci USA 1999, 96:14147-14152. Experiments with a diverse set of DNA and RNA viruses demonstrate the generality of suppression of post-transcriptional gene silencing by viruses and lead to the identification of three suppressors. The spatial pattern and degree of suppression, however, varies among viruses.
25. Escaler M, Aranda MA, Thomas CL, Maule AJ: Pea embryonic •• tissues show common responses to the replication of a wide
range of viruses.Virology 2000, 267:318-325.
This study extends earlier work on the response of pea embryonic tissue to an advancing front of virus infection. In this work, plant responses to infec-tion by viruses from four different families show similar patterns of inducinfec-tion of some host genes and downregulation of others.
26. Gray SM, Banerjee N: Mechanisms of arthropod transmission of • plant and animal viruses.Microbiol Mol Biol Rev 1999, 63:128-148.
27. Goldbach R, Peters D: Possible causes of the emergence of tospovirus diseases.Semin Virol 1994, 5:113-120.
28. Power AG, Flecker AS: Species expendability in disease systems.
In When are Species Expendable?Edited by Levin SA, Kareiva P. Princeton: Princeton University Press; 2000:in press.
29. Nault LR: Arthropod transmission of plant viruses: a new synthesis.Ann Entomol Soc Am 1997, 90:521-541.
30. Mehta P, Wymna JA, Nakle MK, Maxwell DP: Transmission of tomato yellow leaf curl geminivirus by Bemesia tabaci(Homoptera: Aleyrodidae).J Econ Entomol 1994, 87:1291-1297.
31. Ghanim M, Morin S, Zeidan M, Czosnek H: Evidence for transovarial transmission of tomato yellow leaf curl virus for its vector, the whitefly Bemesia tabaci.Virology 1998, 240:295-303. 32. Ghanim M, Czosnek H: Tomato yellow leaf curl geminivirus
(TYLCV-Is) is transmitted among whiteflies (Bemesia tabaci) in a sex-related manner.J Virol 2000, 74:4738-4745.
33. Matthews REF: Plant Virology, edn 3. New York: Academic Press; 1991. 34. Nault LR: Transmission biology, vector specificity and evolution of
planthopper-transmitted viruses.In Planthoppers: their Ecology and Management. Edited by Denno RF, Perfect TG. New York: Chapman & Hall; 1994.
35. Rubinstein G, Czosnek H: Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemesia tabaci: effect on the insect transmission capacity, longevity and fecundity.J Gen Virol 1997, 78:2683-2689.
36. Frolich DR, Torres-Jerez I, Bedford ID, Markham PG, Brown JK: A • phylogenetic analysis of the Bemesia tabacispecies complex
based on mitochondrial DNA markers.Mol Ecol 1999, 8:1683-1691. This phylogenetic study adds to the considerable body of evidence support-ing the hypothesis that Bemesia tabaciis a cryptic group of sibling species. 37. Brown JK, Frolich DR, Rosell RC: The sweetpotato or silverleaf
whiteflies: biotypes of Bemisia tabacior a species complex?
Annu Rev Entomol 1995, 40:511-534.
38. Ooi K, Ohshita S, Ishii I, Yahara T: Molecular phylogeny of geminivirus infecting wild plants in Japan.J Plant Res 1997, 110:247-257. 39. Sanz AI, Fraile A, Gallego JM, Malpica JM, Garcia-Arenal F: Genetic • variability of natural populations of cotton leaf curl geminivirus, a
single-stranded DNA virus.J Mol Evol 1999, 49:672-681. Extremely high levels of genetic variability were found in natural populations of cotton leaf curl geminivirus, but no differentiation between isolates from different host plants was detected. The results suggest that recombination is a significant factor in genetic variation, but that coat protein variability is constrained by whitefly transmission.
40. Briddon RW, Pinner MS, Stanley J, Markham P: Geminivirus coat protein gene replacement alters insect specificity.Virology 1990, 177:85-94. 41. Hofer P, Bedford ID, Markham PG, Jeske H, Frischmuth T: Coat protein
gene replacement results in whitefly transmission of an insect nontransmissible geminivirus isolate.Virology 1997, 236:288-295.
42. Hong YG, Harrison BD: Nucleotide sequences from tomato leaf curl viruses from different countries: evidence for three geographically separate branches in evolution of the coat protein of whitefly-transmitted geminiviruses.J Gen Virol 1995, 76:2043-2049. 43. Czosnek H, Laterrot H: A worldwide survey of tomato yellow leaf
curl viruses.Arch Virol 1997, 142:1391-1406.
44. Zhou XP, Liu YL, Robinson DJ, Harrison BD: Four DNA-A variants among Pakistani isolates of cotton leaf curl virus and their affinities to DNA-A of geminivirus isolates from okra.J Gen Virol 1998, 79:915-923.
45. Paximadis M, Idris AM, Torres-Jerez I, Villarreal A, Rey MEC, Brown JK:
Characterization of tobacco geminiviruses in the Old and New World.Arch Virol 1999, 144:703-717.
46. McGrath PF, Harrison BD: Transmission of tomato leaf curl geminiviruses by Bemesia tabaci. Effects of virus isolate and vector biotype.Ann Appl Biol 1995, 126:307-316.
47. Sanchez-Campos S, Navas-Castillo J, Camero R, Soria C, Diaz JA, Moriones E: Displacement of tomato leaf curl virus (TYLCV)-Sr by TYLCV-Is in tomato epidemics in Spain.Phytopathology 1999,
89:1038-1043.
48. Wang RY, Powell G, Hardie J, Pirone TP: Role of the helper component in vector-specific transmission of potyviruses.J Gen Virol 1998, 79:1519-1524.
49. Flasinski S, Cassidy BG: Potyvirus aphid transmission requires helper component and homologous coat protein for maximal efficiency.Arch Virol 1998, 143:2159-2172.
50. Pirone TP, Blanc S: Helper-dependent vector transmission of plant viruses. Annu Rev Phytopathol1996, 34:227-247.
51. Blanc S, Lopez-Moya JJ, Wang R, Garcia-Lampasona S, Thornbury DW, Pirone TP: A specific interaction between coat protein and helper component correlates with aphid transmission of a potyvirus.Virology 1997, 231:141-147.
52. Peng YH, Kadoury D, Gal-On A, Huet H, Wang Y, Raccah B:
Mutations in the HC-Pro gene of zucchini yellow mosaic potyvirus: effects on aphid transmission and binding to purified virions.J Gen Virol 1998, 79:897-904.
53. Lopez-Moya JJ, Wang RY, Pirone TP: Context of the coat protein DAG motif affects potyvirus transmissibility by aphids.J Gen Virol 1999, 80:3281-3288.
54. Andrejeva J, Puurand U, Merits A, Rabenstein F, Jarvekulg L, Valkonen JPT: Potyvirus helper component-proteinase and cost protein (CP) have coordinated functions in virus–host interactions and the same CP motif affects virus transmission and
accumulation.J Gen Virol 1999, 80:1133-1139.