Summary We identified a novel 66 kDa boiling-stable pro-tein (BspA) in cultured shoots of aspen (Populus tremula L.) which was highly expressed in response to gradual water stress. The BspA protein, which was highly expressed as early as 1 h after initiation of a drought treatment, accumulated during progressive water stress, decreased on rehydration, and was expressed in response to abscisic acid (ABA) application, as detected by SDS-PAGE protein analysis and Western blotting. Anti-BspA antibodies also cross-reacted with a 119 kDa pro-tein. The 119 kDa protein was also induced by water stress, but it was detected only in the total protein fraction and not in the heat-stable fraction. The BspA protein cross-reacted with an-tibodies raised against a water-stress-responsive protein iso-lated from the African resurrection plant Craterostigma plantagineum Hochst. The N-terminal amino acid sequence of BspA was determined and exhibited high homology with the wheat germins GF-2.8 and GF-3.8. The BspA protein was the only major, water-stress-responsive boiling-stable protein de-tected in aspen.
Keywords: ABA, boiling-stable protein, drought, water stress.
Introduction
Water loss from plant tissues under drought conditions results in inhibition of growth as well as several other metabolic and physiological changes. These include accumulation of abscisic acid (ABA) (Zeevaart and Creelman 1988), stomatal closure resulting in reduced transpiration rates, a decrease in the water potential of plant tissues, and decreased photosynthesis (Boyer and Bowen 1970, Hsiao 1973, Turner 1986). Changes in gene expression in response to drought, as indicated by the synthe-ses of new proteins and mRNAs, have also been noted (Gomez et al. 1988, Mundy and Chua 1988, Close et al. 1989, Piatkowsky et al. 1990, Vierling and Kimpel 1992). These proteins and mRNAs seem to respond similarly to the applica-tion of ABA (Mundy and Chua 1988, Skriver and Mundy 1990, Vilardell et al. 1990, Bradford and Chandler 1992) and cold stress (Lin et al. 1990, Thomashow 1990). Some drought-in-duced proteins (e.g., dehydrins) are highly hydrophilic and
remain soluble after boiling (Close et al. 1989), a characteristic that has been termed ‘‘boiling stability’’ (Jacobsen and Shaw 1989). Nucleotide sequencing of dehydrin genes from several species, such as rab 17 in maize (Vilardell et al. 1990) and rab 16 in rice (Mundy and Chua 1988), indicates conservation of lysine-rich regions. The conservation of these sequences in several plant species and the induction of dehydrins by desic-cation have led to the suggestion that these proteins serve to protect membranes and other cellular proteins in the event of water loss (Baker et al. 1988, Close et al. 1989, Close and Chandler 1990, Skriver and Mundy 1990). The expression of these proteins has been studied in herbaceous plants; however, many of the underlying control mechanisms, as well as their role in drought tolerance, remain obscure.
Although the physiological drought-avoidance charac-teristics of many commercially important woody plants have been documented, there is little information on the molecular basis of drought tolerance in these species. Efforts to improve growth and productivity of forest trees under drought condi-tions could benefit from an understanding of the expression of specific desiccation-induced proteins, which may contribute to drought tolerance. This is especially relevant in deciduous trees, such as many poplars and aspens, that can survive pro-longed droughts. In this study, we report on the temporal expression, response to ABA, and partial characterization of a boiling-stable protein found in gradually water-stressed shoot cultures and detached leaves of aspen (Populus tremula L.).
Materials and methods
Culture conditions
Fifty to 100 shoot explants of an aspen clone were routinely subcultured every 5--6 weeks on half-strength Murashige and Skoog basal medium supplemented with 500 mg l−1 casein enzymatic hydrolysate (Nadel et al. 1992). The shoot cultures, which formed roots 1--2 weeks after being subcultured, were maintained in a growth room at 24 °C, in a 16-h photoperiod provided by cool-white fluorescent lights supplying 50--60
µmol m−2 s−1.
Characterization of BspA, a major boiling-stable,
water-stress-responsive protein in aspen (Populus tremula)
DAN PELAH,
1ODED SHOSEYOV
1,2and ARIE ALTMAN
1,2,31
The Kennedy Leigh Center for Horticultural Research, The Hebrew University of Jerusalem, P.O. Box 12, Rehovot 76100, Israel 2
The Otto Warburg Center for Agricultural Biotechnology, Faculty of Agriculture, The Hebrew University of Jerusalem, P.O. Box 12, Rehovot 76100, Israel
3
Author to whom correspondence should be addressed
Received August 12, 1994
Water-stress treatments
Shoots were excised from cultured rooted plantlets (3--4 weeks after subculturing), wilted on a balance by continuous moni-toring of weight loss at room temperature to 80 or 60% of their original fresh weight, then kept in plastic bags for an additional 4 h. In some experiments, excised shoots were wilted to 80% of their fresh weight and kept in bags for an additional 1 or 4 h. For the rehydration experiments, shoots were wilted to 80% of their fresh weight, then kept enclosed in humidified plastic bags for an additional 4 h. Freshly excised shoots maintained in plastic bags for 4 h without prior wilting served as controls. Shoots were removed from the plastic bags at the end of the experiment, immediately frozen in liquid nitrogen, and stored at −80 °C.
Abscisic acid treatment
Shoots were excised from cultured rooted plantlets (3--4 weeks after subculturing) and sprayed with 1 mM ABA (Sigma Chemical Co., St. Louis, MO). Each shoot was then placed in an Eppendorf tube containing 1 ml of 1 mM ABA, and all tubes were enclosed in a plastic bag for 4 h. Control shoots were sprayed with water, and each shoot was placed in an Eppendorf tube containing 1 ml of water, and all tubes were kept in a plastic bag for 4 h.
Analysis of total and boiling-stable proteins
Shoot cultures were frozen in liquid nitrogen and homoge-nized with a chilled mortar and pestle in extraction buffer (50 mM Tris-HCl, pH 7) containing water-insoluble poly-vinylpolypyrrolidone (1% w/v). Crude extracts were centri-fuged at 10,000 g for 10 min, and total protein in the supernatant was determined with a BioRad protein assay kit at 595 nm, with bovine serum albumin as a standard (Bradford 1976). Protein samples (800 µg) for SDS-PAGE were boiled for 10 min, kept on ice for 5 min, and centrifuged at 10,000 g for 10 min. Boiling-stable proteins in the supernatant were precipitated by adding four volumes of cold acetone, then centrifuged at 10,000 g for 10 min, and redissolved in SDS-PAGE sample application buffer (125 mM Tris-HCl, 4% w/v SDS, 20% v/v glycerol, 0.002% w/v bromophenol blue, and 5% v/v 2-mercaptoethanol). Before loading, the samples were heated at 100 °C for 5 min.
Electrophoresis and Western blot analysis
Proteins were separated by 10% SDS-PAGE (Laemmli 1970). Each lane was loaded with the equivalent of 800 µg total protein and run at 200 V for 45 min (on a mini-gel), or overnight at 12 mA (on a standard gel). Gels were stained with 0.125% w/v Coomassie Blue in 50% v/v methanol and 10% v/v acetic acid, and destained in 50% methanol containing 10% acetic acid. For Western blotting, proteins were electro-transferred to a nitrocellulose membrane, reacted with poly-clonal antibodies raised against Craterostigma plantagineum Hochst. protein Dsp14 (a gift from Dr. Bartels, Max-Planck Institute, Köln, Germany) or rabbit polyclonal anti-66-kDa protein, then visualized by means of goat-antirabbit IgG con-jugated to alkaline phosphatase (Harlow and Lane 1988).
Pre-stained molecular weight markers were used (Kit No. P-1677, Sigma).
Production of polyclonal antibodies
Approximately 2 mg of the 66 kDa protein was electroeluted from 10% SDS-PAGE gels to a Biotrap (Schleicher and Schuell BT-100, Dassel, Germany), and aliquots of 500 µg protein + complete Freund’s adjuvant were injected into a rabbit. A final injection of antigen (250 µg) in incomplete adjuvant was performed after 21 days, followed by antisera collection (Anilab, Rehovot, Israel). Antibody specificity was determined using preimmune serum as a control.
Protein sequencing
Eluted protein (10 µg) was run on a 10% SDS-polyacrylamide electrophoresis gel, which had been prerun with 0.1 mM thio-glycolate, and then transferred to a PVDF membrane. The protein sequence was determined with a gas-phase protein sequencer (Model 470A, Applied Biosystems, Foster City, CA).
Results and discussion
Determination of boiling-stable proteins in wilting shoots
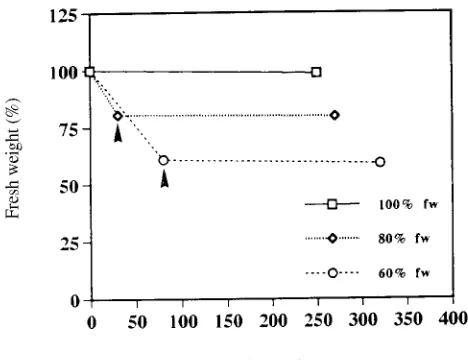
Under ambient laboratory conditions, aspen shoots detached from in-vitro-cultured plantlets wilted rapidly (Figure 1), los-ing 20 and 40% of their initial fresh weight after 30 and 80 min, respectively. They maintained those fresh weights when kept in plastic bags for 4 h. Similar findings were obtained with leaves detached from potted aspen plants (data not shown).
Wilting of aspen shoots was accompanied by a rapid reduc-tion in total protein content, i.e., 10.1 mg protein gFW−1 in control shoots compared with 7.3 mg protein gFW−1 in shoots
that had lost 40% of their initial fresh weight (data not shown). This reduction in total protein content may have been caused by a decrease in protein synthesis, as has been found in pine callus cultures (Valluri et al. 1988) and in pea roots and maize mesocotyls (Bewely and Larsen 1980). Alternatively, it could have been caused by protein breakdown resulting from in-creased proteolytic activity, as found in drought-stressed Phaseolus and Vigna leaves (Roy-Macauley et al. 1992). An analysis of the effect of boiling on the stability of proteins produced in response to moderate water stress of aspen shoots (20% loss of fresh weight) revealed that about 7% of the total proteins were boiling stable and remained in solution follow-ing 2 to 10 min of boilfollow-ing. In cotton seeds (Hughs and Galau 1987) and in seeds of other species, late-embryogenesis-abun-dant (LEA) polypeptides with similar properties may comprise more than 30% of the total soluble seed proteins just before seed desiccation. Because no changes in total protein profiles were detected, only boiling-stable protein profiles are shown.
Temporal expression, response to ABA, and immunodetection of BspA
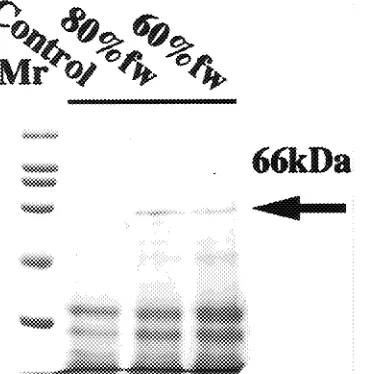
Fresh, nonstressed shoots contained several low molecular mass (less than 36 kDa) boiling-stable proteins that were also present in water-stressed shoots (Figure 2). These boiling-stable proteins were not responsive to water stress, and their content varied among different plant samples, irrespective of the treatments. However, a water-stress-induced boiling-stable protein, having a molecular mass of 66 kDa, accumulated in shoots that had lost 20 or 40% of their fresh weight (Figure 2). Western blot analysis, using polyclonal antibodies raised against the purified 66 kDa boiling-stable protein revealed this protein to be in the total protein fraction as well as in the boiled
protein fraction (Figure 3). Antibodies raised against the 66 kDa boiling-stable protein also cross-reacted with a protein having a molecular mass of 119 kDa. The 119 kDa protein was also induced by water stress; however, unlike the 66 kDa protein, it was detected only in the total protein fraction, not in the boiling-stable fraction (Figure 3). It is possible that the boiling-unstable 119 kDa protein is a precursor of the mature 66 kDa portion, suggesting that boiling stability is only con-ferred on mature proteins.
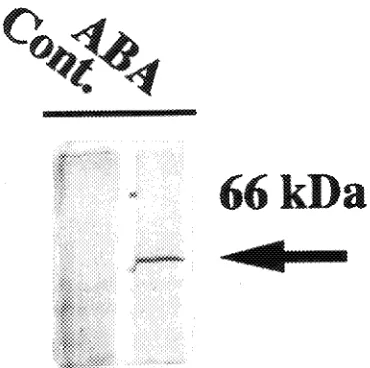
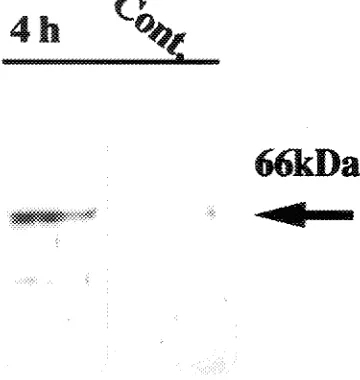
Accumulation of the 66 kDa protein was rapid and occurred within 1--2 h after the initiation of water stress (Figure 4). Similar rapid responses have been observed in other water-stress-induced proteins and mRNAs expressed in, for example, Brassica napus L. leaves (Reviron et al. 1992) and pea shoots (Guerrero et al. 1990). The 66 kDa boiling-stable protein was also expressed when leaves detached from potted aspen plants were exposed to water stress (data not shown). Accumulation of the 66 kDa boiling-stable protein increased with duration of the water stress (Figure 4). This protein also accumulated in response to application of ABA (Figure 5), and in a more recent study using Western blots, we observed that the 66 kDa protein accumulated in osmotic- and cold-stressed callus cultures (data not shown). The ABA content increases in tissues sub-jected to osmotic stress by desiccation, salt or cold, and under these conditions, specific genes are expressed that can also be induced by the application of exogenous ABA (Skriver and Mundy 1990).
On rehydration of the water-stress-treated shoots, the 66 kDa boiling-stable protein almost completely disappeared (Figure 6). Similar results have been found in other studies of proteins responsive to drought, salt or PEG treatment, or re-lated to the onset of senescence (Reviron et al. 1992).
Immunological cross-reactivity and sequence relationships
A water-stress protein (Dsp14) has been isolated from the African resurrection plant C. plantagineum (Bartels et al.
Figure 2. The SDS-PAGE profiles of boiling-stable proteins from water-stressed aspen shoots. Each lane was loaded with boiling-stable proteins (equivalent to 800 µg total protein). Control = nonstressed shoots held in plastic bags for 4 h; 80% fw = shoots wilted to 80% of their initial fresh weight and then held in plastic bags for 4 h; 60% fw = shoots wilted to 60% of their initial fresh weight and then held in plastic bags for 4 h.
1990, Schneider et al. 1993). Western blot analysis, with anti-Dsp14 antibodies, revealed immuno-cross-reactivity between this protein and the 66 kDa aspen protein (Figure 7), indicating possible structural homology between the two water-stress-in-duced proteins. Craterostigma plantagineum leaves can be desiccated to a relative water content of 1% and remain viable after rehydration (Gaff 1971, Bartels et al. 1990).
Slash pine (Pinus elliotti Engelm.) callus cultures exposed to mannitol-induced water stress also express a 66 kDa protein (Valluri et al. 1988). More recently, a boiling-stable protein with a molecular mass of 85 kDa was found in spinach (Neven et al. 1993) exposed to water stress. However, in many species, water-stress proteins have a low molecular mass, from 14.2 kDa for a barley dehydrin (Close et al. 1989) to 28 kDa for a Dsp21 protein in C. plantagineum (Bartels et al. 1990). Water-stressed aspen shoots expressed a few boiling-stable proteins in that range of molecular masses, but they were also expressed in the nonstressed shoots. Unlike the other systems that have been investigated to date, the 66 kDa boiling-stable protein in aspen was the only major protein that was induced by water stress. It could be that the 66 kDa water-stress-induced protein, along with several other (low molecular mass) boiling-stable proteins, participates in the initial rapid response to water stress. We named the 66 kDa boiling-stable protein BspA (boiling-stable protein A).
The N-terminal amino acid sequence of BspA showed a high degree of similarity with the N-terminal sequence of the ma-Figure 4. An SDS-PAGE analysis of boiling-stable proteins from
water-stressed aspen shoots. Each lane was loaded with boiling-stable proteins (equivalent to 800 µg total protein). 4 h Cont. = nonstressed shoots held in plastic bags for 4 h; 1 h 80% fw = shoots wilted to 80% of their initial fresh weight and held in plastic bags for 1 h; 4h 80% fw = shoots wilted to 80% of their initial fresh weight and held in plastic bags for 4 h.
Figure 5. An SDS-PAGE analysis of boiling-stable proteins from ABA-treated aspen shoots. Each lane was loaded with boiling-stable proteins (equivalent to 800 µg total protein). Cont. = aspen shoots treated with water and then held in humid plastic bags for 4 h; ABA = aspen shoots treated with 1 mM ABA for 4 h and then held in humid plastic bags for 4 h.
ture-protein coding sequences of the wheat germins GF-2.8 and GF-3.8 (Table 1) (Lane et al. 1991). Wheat germins also share homology with spherulin, a putative cell-wall protein in the slime mold Physarum polycephalum (strains M3C and LU 648X LU688) that increases during spherulation in response to osmotic stress (Lane et al. 1991). Additional similarity was detected between the N-terminal amino acid sequence of BspA and a cold responsive protein (pA086) isolated from barley (Cattivelli and Bartels 1990) (Table 1). Recently, a germin-like protein homologous to GF-2.8 and GF-3.8 has been found in salt-stressed barley (Hordeum vulgare L.) roots (Hurkman et al. 1994). Sequence similarities were also found between GF-2.8 and GF-3.8 cDNAs and the amino acid sequence of barley oxalate oxidase (Lane et al. 1993).
We conclude that water stress induces specific metabolic changes in shoots and in detached leaves of aspen, leading to the induction of BspA. Although we were unable to elucidate the function of this protein, we identified several novel
charac-teristics including an ability to remain water-soluble on boil-ing, and the finding that, in contrast to other systems, BspA was the only major boiling-stable protein in aspen shoots that accumulated in response to water stress and disappeared fol-lowing shoot rehydration.
Acknowledgments
This research was funded in part by the German--Israeli Agricultural Research Agreement for the benefit of the Third World (GIARA), and by the Land Development Authority (Keren Kayemet LeIsrael). The gift of antibodies from Dr. D. Bartels, MPI, Köln, is gratefully ac-knowledged.
References
Baker, J.C., C. Steele and L. Dure. 1988. Sequence and charac-terization of 6 LEA proteins and their genes from cotton. Plant Mol. Biol. 11:277--291.
Bartels, D., K. Schneider, G. Terstappen, D. Piatkowski and F. Salamini. 1990. Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant
Craterostigma plantagineum. Planta 181:27--34.
Bewely, J.D. and K.M. Larsen. 1980. Cessation of protein synthesis in water-stressed pea roots and maize mesocotyls without loss of polyribosomes. Effects of lethal and non-lethal water stress. J. Exp. Bot. 31:1245--1256.
Boyer, J.S. and B.L. Bowen. 1970. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 45:612--615.
Bradford, K.J. and P.M. Chandler. 1992. Expression of ‘‘dehydrin-like’’ proteins in embryos and seedlings of Zizinia palustris and
Oryza sativa during dehydration. Plant Physiol. 99:488--494.
Bradford, M.M. 1976. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248--254.
Cattivelli, L. and D. Bartels. 1990. Molecular cloning and charac-terization of cold-regulated genes in barley. Plant Physiol. 93:1504--1510.
Close, T.J and P.M. Chandler. 1990. Cereal dehydrins: serology, gene mapping and potential functional roles. Aust. J. Plant Physiol. 17:333--344.
Close, T.J., A.A. Kortt and P.M. Chandler. 1989. A cDNA based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol. Biol. 13:95--108.
Gaff, D.F. 1971. Desiccation-tolerant flowering plants in southern Africa. Science 174:1033--1034.
Gomez, J., D. Sanchez-Martinez, V. Stiefel, J. Rigau, P. Puigdomenech and M. Pages. 1988. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature 334:262--264.
Guerrero, F.D., J.T. Jones and J.E. Mullet. 1990. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol. Biol. 15:11--26.
Harlow, H. and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, p 505. Hsiao, T.C. 1973. Plant responses to water stress. Annu. Rev. Plant
Physiol. 24:519--570.
Hughs, D.W. and G.A. Galau. 1987. Translation efficiency of lea mRNAs in cotton embryos: minor changes during embryogenesis and germination. Plant Mol. Biol. 9:301--303.
Figure 7. Western blot analysis of boiling-stable proteins from water-stressed aspen shoots, using polyclonal antibodies against C.
plantagi-neum protein Dsp14. Cont. = boiling-stable proteins (equivalent to 800
µg total protein) extracted from nonstressed shoots held in plastic bags for 4 h; 4 h = boiling-stable proteins (equivalent to 800 µg total protein) extracted from shoots that wilted to 60% of their initial fresh weight and were held in plastic bags for 4 h.
Hurkman, W.J., B.G. Lane and C.K. Tanaka. 1994. Nucleotide se-quence of a transcript encoding a germin-like protein that is present in salt-stressed barley (Hordeum vulgare L.) roots. Plant Physiol. 104:803--804.
Jacobsen, J.V. and D.C. Shaw. 1989. Heat-stable proteins and abscisic acid action in barley aleurone cells. Plant Physiol. 91:1520--1526. Laemmli, U.K. 1970. Cleavage of structural proteins during the
as-sembly of the head of the bacteriophage T4. Nature 227:680--685. Lane, B.G., F. Bernier, E. Dratewka-Kos, R. Shafai, T.D. Kennedy,
C. Pyne, J.R. Munro, T. Vaughan, T. Walters and F. Altomare. 1991. Homologies between members of the germin gene family in hexaploid wheat and similarities between these wheat germins and certain Physarum spherulins. J. Biol. Chem. 266:10461--10469. Lane, B.G., J.M. Dunwell, J.A. Ray, M.R. Schmitt and A.C. Cuming.
1993. Germin, a protein marker of early plant development, is an oxalate oxidase. J. Biol. Chem. 268:12239--12242.
Lin, C., W.W. Gau, E. Everson and M.F. Tomashow. 1990. Cold acclimation in Arabidopsis and wheat. A response associated with expression of related genes encoding ‘‘boiling-stable’’ polypeptides. Plant Physiol. 94:1078--1093.
Mundy, J. and N.-H. Chua. 1988. Abscisic acid and water stress induce the expression of a novel rice gene. EMBO J. 7:2279--2286. Nadel, B.L., G. Hazan, R. David, A. Hutterman and A. Altman. 1992.
In vitro propagation of Populus species: response to growth regula-tors and media composition. Acta Hortic. 314:61--68.
Neven, L.G., D.W. Haskell, A. Hofig, Q.-B. Li and C.L. Guy. 1993. Characterization of a spinach gene responsive to low temperature and water stress. Plant Mol. Biol. 21:291--305.
Piatkowsky, D., K. Schneider, F. Salamini and D. Bartels. 1990. Characterization of five abscisic acid-responsive cDNA clones iso-lated from the desiccation-tolerant plant Craterostigma
plantagi-neum and their relationship to other water-stress genes. Plant
Physiol. 94:1682--1688.
Reviron, M.P., N. Vartanian, M. Sallantin, J.C. Huet, J.C. Pernollet and D. de Vienne. 1992. Characterization of a novel protein induced by progressive or rapid drought and salinity in Brassica napus leaves. Plant Physiol. 100:1486--1493.
Roy-Macauley, H., Y. Zuily-Fodil, M. Kidric, A.T. Pham Thi and J. Vieira da Silva. 1992. Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiol. Plant. 85:90--96.
Schneider, K., B. Wells, E. Scmelzer, F. Salamini and D. Bartels. 1993. Desiccation leads to the rapid accumulation of both cytosolic and chloroplastic proteins in the resurrection plant Craterostigma
plan-tagineum Hochst. Planta 189:120--131.
Skriver, K. and J. Mundy. 1990. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2:503--512.
Thomashow, M.F. 1990. Molecular genetics of cold acclimation in higher plants. Adv. Genet. 28:99--131.
Turner, N.C. 1986. Adaptation to water deficits: a changing perspec-tive. Aust. J. Plant Physiol. 13:175--190.
Valluri, J.V., W.J. Treat, R.J. Newton, B.G. Cobb and E.J. Soltes. 1988. Protein synthesis in slash pine callus cultures exposed to water stress. Tree Physiol. 4:181--186.
Vierling, E. and J.A. Kimpel. 1992. Plant response to environmental stress. Curr. Opin. Biotechnol. 3:164--170.
Vilardell, J., A. Goday, M.A. Freire, M. Torrent, C. Martinez, J.M. Torne and M. Pages. 1990. Gene sequence, developmental expres-sion, and protein phosphorylation of RAB-17 in maize. Plant Mol. Biol. 14:423--432.