Biomineralization of Schlumbergerella
floresiana, a significant carbonate-producing
benthic foraminifer
ARTICLE
in
GEOBIOLOGY · JULY 2014
Impact Factor: 3.83 · DOI: 10.1111/gbi.12085
CITATION
1
READS
74
10 AUTHORS
, INCLUDING:
Anna Sabbatini
Università Politecnica delle Marche
20PUBLICATIONS242CITATIONS
SEE PROFILE
Arul Marie
Muséum National d'Histoire Naturelle
51PUBLICATIONS781CITATIONS
SEE PROFILE
Annachiara Bartolini
Muséum National d'Histoire Naturelle
69PUBLICATIONS1,304CITATIONS
SEE PROFILE
Gusti Ngurah Mahardika
Udayana University
42PUBLICATIONS
110CITATIONS
Biomineralization of
Schlumbergerella floresiana
, a
significant carbonate-producing benthic foraminifer
A . S A B B A T I N I ,1 , 2 L . BED O U E T , 3 A . M A R I E ,4 A . B A R T O L I N I ,2L . L A N D E M A R R E ,5 M . X . W E B E R ,6 , 7 I . G U S T I N G U R A H K A D E M A H A R D I K A ,8 S . B E R L A N D ,3 F . Z I T O9 A N D M . - T . VEN E C - P E Y R E21Department of Life and Environmental Sciences (Di.S.V.A.), Polytechnic University of Marche, Ancona, Italy 2Centre de Recherche sur la Pal
eobiodiversite et les Paleoenvironnements, UMR 7207 CNRS MNHN UPMC, Museum National d’Histoire Naturelle, Paris Cedex 05, France
3Biologie des Organismes et Ecosyst
emes Aquatiques, UMR CNRS 7208/IRD 207, Museum National d’Histoire Naturelle, Paris Cedex 05, France
4D
epartement Regulation Developpement et Diversite Moleculaire, UMR CNRS 7245, Plateforme de Spectrometrie de masse et de Proteomique, Museum National d’Histoire Naturelle, Paris Cedex 05, France
5GLYcoDiag, UFR Sciences, Orl
eans Cedex 2, France
6Department of Biology, Pennsylvania State University, 208 Mueller Lab, University Park, PA 16802, USA 7Unidad Acad
emica de Sistemas Arrecifales, Instituto de Ciencias del Mar y Limnologia, Universidad Nacional Autonoma de Mexico, Puerto Morelos, Q. Roo, Mexico
8The Animal Biomedical and Molecular Biology Laboratory, Udayana University, Bali, Indonesia 9Institut de Biologie Physico-Chimique, CNRS/Universite Paris-7 UMR 7099, Paris, France
ABSTRACT
Most foraminifera that produce a shell are efficient biomineralizers. We analyzed the calcitic shell of the large tropical benthic foraminifer Schlumbergerella floresiana. We found a suite of macromolecules con-taining many charged and polar amino acids and glycine that are also abundant in biomineralization pro-teins of other phyla. As neither genomic nor transcriptomic data are available for foraminiferal biomineralization yet,de novo-generated sequences, obtained from organic matrices submitted toMS BLAST database search, led to the characterization of 156 peptides. Very few homologous proteins were matched in the proteomic database, implying that the peptides are derived from unknown proteins present in the foraminiferal organic matrices. The amino acid distribution of these peptides was queried against the UNI-PROTdatabase and the molluskUNIPROTdatabase for comparison. The mollusks compose a well-studied phy-lum that yield a large variety of biomineralization proteins. These results showed that proteins extracted from S. floresianashells contained sequences enriched with glycine, alanine, and proline, making a set of residues that provided a signature unique to foraminifera. Three of the de novo peptides exhibited sequence similarities to peptides found in proteins such as pre-collagen-P and a group of P-type ATPases including a calcium-transporting ATPase. Surprisingly, the peptide that was most similar to the collagen-like protein was a glycine-rich peptide reported from the test and spine proteome of sea urchin. The molecules, identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry analyses, included acid-soluble N-glycoproteins with its sugar moieties represented by high-mannose-type glycans and carbo-hydrates. Describing the nature of the proteins, and associated molecules in the skeletal structure of living foraminifera, can elucidate the biomineralization mechanisms of these major carbonate producers in marine ecosystems. As fossil foraminifera provide important paleoenvironmental and paleoclimatic information, a better understanding of biomineralization in these organisms will have far-reaching impacts.
Received 12 June 2013; accepted 25 February 2014
INTRODUCTION
Foraminifera are eukaryotic unicellular micro-organisms that live in almost all marine environments. Most species construct tests (or shells). During the course of their evo-lution, they experimented with different materials to build their tests, namely organic material, calcium carbonate (CaCO3), opaline silica, and agglutinating sediment grains.
Calcitic foraminifera are among the major calcium carbonate producers in the oceans (Langer et al., 1997; Hohenegger, 2006; Langer, 2008; Ziegler & Uthicke, 2011) with intricate and beautiful test morphologies. Their biomineralization processes are still poorly understood. However, the elemental and isotopic composition of car-bonate tests can be used to trace environmental signals, including variations in ocean temperature, productivity, oxygen level, and salinity. Foraminifera constitute an important tool for paleoenvironmental and paleoclimatic reconstructions because of their abundance and nearly continuous fossil record (Gooday, 2003).
Calcification in foraminifera is likely to be a biologically controlled process similar to hard-part (shell, skeleton) for-mation observed in many metazoan taxa (e.g., mollusks, cor-als, sea urchins). In foraminifera, two main models of calcification have been proposed. The first model concerns the imperforate high-Mg calcite foraminifera (order Miliol-ida). In this group, calcification occurs in the intracellular ves-icles, where calcite needles are formed and later transported to the site of formation of the new chamber (Towe & Cifelli,
1967). The second model applies to the bilamellar perforate hyaline calcitic foraminifera (orders Buliminida, Rotaliida, and Globigerinida). In these groups, the calcification occurs
in situ. An organic matrix (Hemleben et al., 1977; Be et al., 1979), also called the ‘primary organic lining’ (Angell, 1979, 1980), initiates calcification. Precipitation likely occurs in a con-fined extracellular space, where ion concentration can be regu-lated behind membranes (Angell, 1979; Hemlebenet al., 1986; Erez, 2003; Weiner & Dove, 2003; Bentov & Erez, 2005, 2006; Bentovet al., 2009; de Nooijeret al., 2009; Fig. 1).
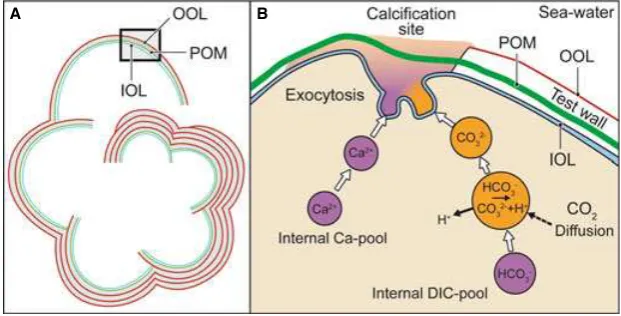
This bilamellar model likely contributed to the evolu-tionary success of these foraminifera, which are incredibly abundant and diverse in past and present oceans. The test consists of a series of chambers that are constructed sequentially. The protoplasm of the cell pervades the exist-ing chambers and extends outside the test, functionexist-ing in excretion, food capture, and chamber construction. Aided by ectoplasmic pseudopods, the first step in building a new chamber is the delineation of a space that partially isolates the organism from its environment. In this space, an organic matrix organized in a thin layer, called the primary organic membrane (POM; Erez, 2003; Weiner & Dove, 2003), may act as template or mold for the nucleation and the precipitation of CaCO3on both its sides, resulting in inner and outer layers of calcite (Fig. 1). With the forma-tion of every newly secreted chamber, the whole of the pre-existing test is covered with an outer layer of calcite, which precipitates on an organic layer called the organic outer layer (OOL). Another major organic layer associated
A B
Fig. 1Schematic model describing the calcification mechanism in bilamellar perforate hyaline calcitic foraminifera. (A) Lamination scheme modified, from Ku-niokaet al.(2006); the test consists of a series of chambers that are constructed sequentially, each newly added chamber being composed of two layers of calcite. With the formation of each newly secreted chamber, the entire pre-existing test is covered with an outer layer of calcite, which precipitates on an organic layer called the organic outer layer (OOL). Another major organic layer associated with the test, the inner organic layer (IOL), covers the internal sur-face of the carbonate chamber. (B) Detail of A) describing the building of a new chamber. The cytoplasm of the cell pervades the existing chambers and extends outside the test, delimiting space that partially isolates the organism from its environment. In this space, an organic matrix organized in a thin layer, called the primary organic membrane (POM), may act as template or mold for the nucleation and the precipitation of CaCO3on both its sides, resulting in
inner and outer layers of calcite. This process involves vacuolization of seawater by endocytosis; various pumps and possibly channels operate to increase the pH, Ca2+, and inorganic carbon pool in these vacuoles. These modified seawater vacuoles are exocytosed into the delimited biomineralization space where
with the test, the inner organic layer (IOL), covers the internal surface of the carbonate chamber. However, this inner layer appears to be separate from the protoplasm of the foraminifer. It is not known whether these organic lay-ers (POM, IOL, OOL) are similar in composition, although Spero (1988) has described a common formation process. Despite a comprehensive review by Robbins & Donachy (1991), they could not determine the composi-tion of the POM, OOL, and IOL. They proposed two alternative hypotheses: (i) Each layer is composed of differ-ent proteins with specific mineralization regulatory func-tions and (ii) these organic matrices are composed of similar proteins, which control calcification phases based on their structural configuration. Organic matrices in other biocalcifying organisms are composed of proteins, glyco-proteins, or polysaccharides (Weiner & Addadi, 1997) that intimately control the shape and properties of the growing inorganic-organic composite materials.
Our current knowledge of the macromolecular compo-nents (e.g., proteins, sugars, and lipids) in the organic matrix of foraminiferal shells is very poor (Hedley, 1963; Banner
et al., 1973; Weiner & Erez, 1984). Previous research has focused mainly on protein components in terms of amino acid composition (King & Hare, 1972a; Weiner & Erez, 1984; Haugen et al., 1989; Robbins & Donachy, 1991; Robbins & Healy-Williams, 1991; Stathoplos & Tuross, 1994). Very few studies have tried to identify the amino acid sequences of matrix shell proteins in foraminifera (Robbins
et al., 1993). In addition, very few taxa and species have been examined. For example, most studies investigated fossil planktonic foraminifera (King & Hare, 1972a; Haugen
et al., 1989; Robbins & Brew, 1990; Robbins & Donachy, 1991; Robbins & Healy-Williams, 1991; Stathoplos & Tuross, 1994). Prior to this study, the only living tropical benthic foraminifer to be analyzed in this way was Heteroste-gina depressad’Orbigny, 1826 (Weiner & Erez, 1984).
In this study, we performed a biochemical and proteomic approach to investigate the macromolecular components (amino acids and sugars) of the organic matrix of the tests of the benthic foraminiferSchlumbergerella floresiana (Schlum-berger, 1896), a member of the family Calcarinidae. This spe-cies has the same double-layered primary wall as other canaliculated, perforated foraminifera (i.e., Rotaliidae), although the shell of species belonging to Calcarinidae is complex and the mode of construction is not well resolved (Hottinger & Dreher, 1974; Hottinger & Leutenegger, 1980; Hottinger, 1986). Like the so-called larger, symbiont-bearing foraminifera, they are abundant in the western Indo-Pacific, inhabiting shallow water in high-energy envi-ronments. With high population densities, they are important carbonate producers (up 80%) in coral reef environments (Hallock, 1981; Langer, 2008). For these reasons, they rep-resent an important model for studying calcification (Plate 1).
Elucidating the molecular mechanisms of biomineraliza-tion will depend on a detailed characterizabiomineraliza-tion of shell matrix proteins. We therefore identified amino acid sequences from shell matrix proteins in S. floresiana. We compared the amino acid sequences found in this study with the amino acid distribution of proteins from all taxa present in UNIPROTdatabase. We wanted to know whether proteins involved in foraminiferal calcitic biomineralization contain sequences that are unique to these unicellular micro-organisms or whether they share some biochemical features with biocalcifying metazoan organisms. The fora-minifer data were also compared to mollusks, taking advan-tage of the variety of shell structures and compositions present in that group, as revealed by comprehensive bio-chemical, genetic, and proteomic studies.
MATERIALS AND METHODS
Sample collection and shell material preparation
Large specimens ofS. floresiana(about 2 mm in diameter) were collected at Amed, on the north coast of Bali, in Febru-ary 2011. Megalospheric tests were gently scraped off the top surfaces of reef rubble in five to seven meters of water. Speci-mens were transported to the laboratory, alive in seawater. They were maintained alive in a glass Petri dish under a light microscope for 2 days and observed to verify the presence of cytoplasm. Living specimens were transferred to new dishes with filtered seawater, and the outer surface of the shells was carefully cleaned with a small brush. Sediment and extraneous material adhering to it was gently scraped off, and the tests were rinsed clean. Specimens were immersed in 2.5% NaOCl for 10 min and agitated gently to remove all superficial contami-nants and traces of cytoplasm from the inner and the outer sur-face of the test. The shells were then thoroughly rinsed in pure fresh water and preserved in 70% ethanol until analysis.
To image the shell ultrastructural features and localize the organic layer(s), scanning electron micrographs ofS. floresi-ana were generated using a ZEISS Supra 55 SEM (Carl Zeiss, Oberkochen, Germany) at the laboratory Magie of University UPMC-Paris VI (Plate 1). Some sections of shell were etched with EDTA 0.5M for 2 min, to better reveal
the morphological details and the calcitic lamellas in the walls (Plate 1). Note that after the NaOCl blanching, the thick IOL is still present and visible in the shell (Plate 1).
EXPERIMENTAL PROCEDURES
Organic matrix extraction procedure
The shells of S. floresiana were crushed to a fine powder (4 g) using a mortar and pestle; 4Mguanidine thiocyanate
material, derived from the foraminifera and xenobiotic organisms. The solution was shocked for 10 min at room temperature (1 vortex 10 s/min for 10 min). The sample was incubated with guanidine thiocyanate until green pig-ments were eliminated, and several extractions of 10 min were executed. The supernatant, corresponding to the gua-nidine thiocyanate-soluble matrix (GT-SM), was then dia-lyzed against 50 mM ammonium bicarbonate through a membrane with a cutoff of 500 Da (Spectra/Por) at 4°C.
The process took 2 days and required several water changes. Finally, the samples were freeze-dried and weighed. The guanidine thiocyanate-insoluble fraction was decalcified in cold 6.5Macetic acid with intermittent
shak-ing until cessation of CO2release. The solution was centri-fuged at 30009g for 5 min. The resulting pellet corresponds to the acid-insoluble matrix (A-IM). It was rinsed twice with milliQ water and centrifuged again at 30009g for 5 min. After the second wash, the pellet was freeze-dried and weighed. The supernatant, which was saved after the demineralization of the guanidine
thiocya-nate-insoluble fractions, yielded the acid-soluble matrix (A-SM) after centrifugation at 30009g for 5 min. It was extensively dialyzed against 50 mMammonium bicarbonate
with a molecular weight cutoff of 500 Da (Spectra/Por) at 4°C. The dialysis took 2 days and three water changes.
The resulting samples were freeze-dried and weighed. We obtained a series of progressively cleaner organic matrices. The GT-SM obtained from the guanidine thiocyanate probably contained contaminants and some proteins (or macromolecules) from the foraminiferal shell. The A-SM fraction would be characterized by more typical shell pro-teins (or macromolecules). Finally, the AI-SM fraction likely contained proteins and/or other organic materials entrapped in shell calcium carbonate.
Neutral sugar assay
withD-glucose as standard (Monsignyet al., 1988). The
pro-tein content of acid-soluble (GT-SM, A-SM) and acid-insolu-ble (A-IM) fractions was derived from the amino acid composition analysis described in the next paragraph.
Protein and amino acid composition of GT-SM, A-SM and A-IM
Crude extracts of the GT-SM and A-SM (between 200 and 300lg) and the A-IM extract (300lg) were hydro-lyzed using 1% phenol 6N HCl for 20 h at 110°C. We
used a known amount of norleucine as an internal stan-dard. After the HCl evaporated, the protein samples were analyzed in a L-8800 Hitachi amino acids analyser (post-column derivatization with ninhydrin after ion-exchange chromatography separation). All analyses were performed at the Institut Pasteur (Paris, France).
GT-SM, A-SM and A-IM analysis on monodimensional SDS-PAGE
SDS-PAGE analysis of protein extracts was performed on a mini-Protean II device (BIO-RAD, Hercules, CA, USA; Pharmacia, New York, NY, USA) according to Laemmli (1970) using 12% polyacrylamide gels in reducing and denaturing conditions. Briefly, 100lg of GT-SM and 10lg of A-SM extracts were loaded on gels. Soluble pro-teins from the A-IM extract were recovered after boiling (5 min) approximately 1 mg of extract in 100lL of Lae-mmli buffer. Ten microliters of this extract was used for electrophoresis. Proteins were stained with silver nitrate after two fixation steps of 1 h each: the first in 50% ethanol, 13% acetic acid, and 18% formaldehyde and the second in 50% ethanol, 13% acetic acid, and 10% glutaral-dehyde. Then, gels were washed with 50% ethanol for 30 min and with water for another 1 h. Protein oxidation was carried out using 32lM dithiothreitol (DTT) for 1 h
followed by incubation with 0.2% AgNO3 for 1 h. The color was developed in 3% (w/v) sodium carbonate solu-tion containing 0.05% formaldehyde (Gotlivet al., 2003).
Proteomic analysis of GT-SM, A-SM and A-IM, and data treatment
Five hundred micrograms of lyophilized soluble extracts (GT-SM and A-SM) was dissolved in 50 mM ammonium
bicarbonate and reduced at 60°C for 20 min with 1 mM
dithiothreitol (DTT). After being cooled to room tempera-ture, alkylation was performed for 1 h in the dark with 5 mM
iodoacetamide under stirring. Then, the samples were ten-fold diluted in ammonium bicarbonate containing 5% aceto-nitrile before addition of 5lg of trypsin (Sigma, Proteomics Grade, St. Louis, MO, USA). About 34 mg of insoluble (A-IM) fraction was suspended in 50 mMammonium
bicarbon-ate and reduced under stirring at 60°C with 1 mMDTT.
After cooling to room temperature, alkylation was per-formed in the dark with 5 mMiodoacetamide. After
centrifu-gation, the obtained pellet was suspended in 50 mM
ammonium bicarbonate, 5% acetonitrile, and 20lg trypsin. Trypsin digestion of soluble and insoluble extracts was car-ried out for 18 h at 37°C. The peptide digest from the
organic matrices was separated on a C18 column (1509 1 mm, Cluzeau, France) at a flow rate of 40lL min 1with 0.1% formic acid (solvent A) and acetonitrile (solvent B), using a gradient that varied from 3 to 70% of B in 60 min. The eluted peptides were analyzed in an ESI-QqTOF mass spectrometer (pulsar Applied Biosystems, Ontario, Canada), using information-dependent acquisition mode. This mode allows switching between mass spectrometry (MS) and MS/ MS scans. A 2-sec MS scan was followed by 2-sec MS/MS acquisition using two most intense multiply charged precur-sor peptide ions (+2 to+4). The fragmented precursor ions
were excluded for 60 min to avoid reanalysis. Minimum ion intensity for MS/MS experiments was set to 10 counts, and the collision energy for the peptide ions was determined automatically by the acquisition software. Data acquisition and analyses were carried out with Analyst QS software (version 1.1, Applied Biosystems, Ontario, Canada).
Database search was carried out with an in-house version of MASCOT (version 2.2; Matrix Science Ltd.,
London, UK) and also PEAKS DB (Peaks studio 5.3; Bioinformatics Solutions Inc., Ontario, Canada) using NCBI nr database (downloaded on February 2012). Parameters used for database search were as follows: Mass tolerance for MS and MS/MS were 0.5 and 0.6 Da, respectively; carbamido-methylation as fixed modification for cysteine and methionine oxidation as variable modifi-cation; and the number of missed cleavages was set to 1. The quality of the fragmentation mass spectra and pep-tide-spectrum match were manually verified to confirm the validity of the results. Results from de novo sequenc-ing (Peaks studio) were filtered by settsequenc-ing total local con-fidence score (TLC) to three, average local concon-fidence to 50, and mass confidence tag to 35%. Finally, only pep-tides with at least 10 amino acids were considered as valid sequences. BLAST (http://blast.ncbi.nlm.nih.gov) was
carried out using the de novo-sequenced peptides to find homologous peptides or proteins present in the database.
Monosaccharide analysis of GT-SM and A-SM
Analyses were performed at GLYcoDIAG, Applied Glycomics (Universite d’Orleans, France). Neutral monosaccharide con-tent was determined after hydrolysis with trifluoroacetic acid (TFA) conducted as follows. Four hundred micrograms of dry extracts (guanidine thiocyanate-soluble and A-SM) obtained from S. floresiana was treated with 2M TFA in
capped tubes. Reaction mixture was then chilled at 4°C for
30 min and dried with speedVac evaporator. High-perfor-mance anionic exchange chromatographic (HPAEC) analysis was performed on a Dionex chromatography system equipped with a PA1 (49250 mm) column and a pulsed amperometric detector (PAD). Monosaccharide amounts were calculated by comparison with a range of known mono-saccharide standards (fucose, galactosamine, galactose, glu-cose, glucosamine, and mannose). Samples (100lg per 100lL water) were injected and eluted isocratically: 98% elu-ent A (water) and 2% eluelu-ent B (50 mM NaOH, 1.5 mM
sodium acetate; flow rate 1 mL min 1for 30 min).
N-linked glycan analysis using the matrix-assisted laser desorption ionization-time of flight mass spectrometry
Analyses of N-glycans from guanidine thiocyanate-soluble (GT-SM) and acid-soluble matrices (A-SM) ofS. floresiana
were performed at GLYcoDIAG, Applied Glycomics (Universite d’Orleans, France). Two milligrams of samples (GT-SM and A-SM) in 200lL of phosphate buffer pH 7.6 was used. Proteins were denaturated with SDS and deglycosylated using 20 units Peptide N-Glycosidase F (PNGase F, Roche 11365 193 001 lot #12724921) at 37°C, and released N-glycans were permethylated
accord-ing to Morelle & Michalski (2007).
Mass spectra of permethylated N-glycans resuspended in 4lL of 50% methanol/water were acquired in the positive reflector mode (m/z range 1100–4000 Da; voltage 20 kV; grid 75%; guide wire 0.002%; delay time 175 ns) on a MALDI-TOF DE pro (AB SCIEX ex Applied BioSystems, Inc., Framingham, MA, USA) with DHB as matrix (10 mg/ mL, ratio 1:1). External calibration of spectra permitted a mass accuracy of 20 ppm. Matrix-assisted laser desorption ionization-post-source decay (MALDI-PSD) fragmentation was performed on selected ions to confirm structures.
RESULTS
Yields from shell organic matrix extractions
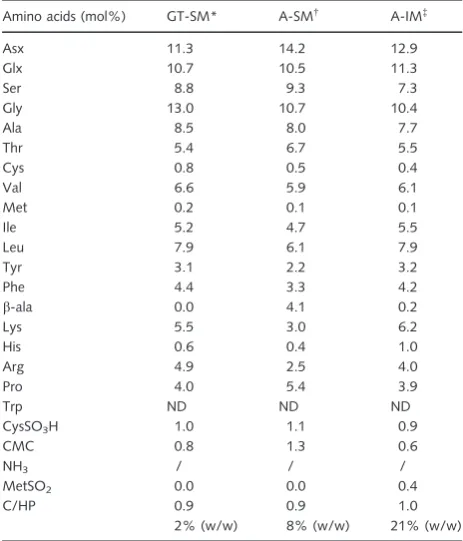
Using guanidine thiocyanate, we isolated a dry extract, which represented 0.14% (w/w) of the foraminiferal tests (Table 1). The A-SM represented 0.13% of the tests (Table 1), while the A-IM represented 3.1% of the tests. Most of the organic components of the shell were insolu-ble in guanidine thiocyanate and acetic acid.
Amino acid analysis, after hydrolysis, revealed that the A-IM was 21% (w/w) peptides while the acid-soluble matrices (GT-SM and A-SM) were less protein rich, 2% (w/w) and 8% (w/w), respectively (Tables 1 and 2). The protein con-tent of the soluble matrices (GT-SM and A-SM) was rela-tively low and constituted approximately 0.003–0.65% by weight of the foraminiferal shell (Table 1).
The guanidine thiocyanate-soluble matrix (GT-SM) con-tained more sugars (33%) than the A-SM (10%). The total amount of sugars in the soluble matrices (GT-SM and A-Table 1Yields of the organic matrix extracts from Schlumbergerella floresiana
Organic matrix components GT-SM A-SM A-IM
Dry weight (%)* 0.14 0.13 3.10
Protein content (%)†
2 8 21
Neutral sugar content (%)‡
33 10 ND
Protein content in shell (%) 0.003 0.01 0.65 Neutral sugar content in shell (%) 0.05 0.013 ND
GT-SM, guanidine thiocyanate-soluble matrix; A-SM, acid-soluble matrix; A-IM, acid-insoluble matrix, ND, not determined. *Percentage of organic matrix components relative to the powdered shell weight.†
Percentage of proteins according to the amino acid composition analysis.‡
Percentage of neutral sugars measured according to resorcinol/sulfuric acid assay in the organic matrix components.
Table 2Amino acid composition of the GT-SM, A-SM and A-IM of
Schlumbergerella floresiana
GT-SM, guanidine thiocyanate-soluble matrix; A-SM, acid-soluble matrix; A-IM, acid-insoluble matrix. Data are presented as molar percentages of total amino acids for each matrix. Asx (aspartate)=Asp (aspartic acid)+Asn (asparagine) and Glx (glutamate)=Glu (glutamic acid)+Gln (glutamine). Cysteine residues were quantified after oxidation. Tryptophan residues were not detected (ND) due to the hydrolysis conditions. The C/HP values correspond to the ratio between charged (Asx, Glx, His, Arg, Lys) and hydrophobic amino acids (Ala, Val, Phe, Pro, Met, Ile, Leu). In this case, the percentage of proteins among the organic matrices was measured according phenol/HCL hydrolysis and norleucine assay.*4.650lg proteins per 300lg. †
16.184lg proteins per 200lg. ‡
SM) represented approximately 0.05–0.013% by weight of theS. floresianashells (Table 1).
Amino acid composition of the GT-SM, A-SM and A-IM
The amino acid compositions of the three matrices were recorded in Table 2. Six amino acid residues dominated all matrices: aspartate (varying from 11 to 14%), glycine
(10–13%), glutamate (10–11%), serine (7–9%), alanine (7– 8%), and leucine (6–8%; Fig. 2). The hydrophobic amino acids (alanine, valine, leucine, isoleucine, phenylalanine, pro-line, and methionine) comprised 37% of the GT-SM, 34% of the A-IM, and 33% of the A-SM. Charged and polar amino acids (aspartate, glutamate, histidine, arginine, and lysine) and glycine were between~13% in the GT-SM and~10% in
the A-SM and A-IM. The C/HP values corresponded to the ratio between charged and hydrophobic amino acids; they were around one for all organic matrix components.
Analysis of shell organic matrices on SDS-PAGE
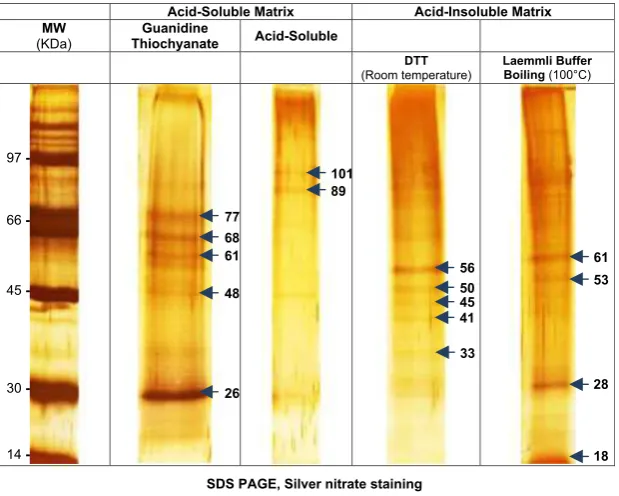
Gel electrophoresis and silver nitrate staining revealed the basic properties of the polypeptides that comprised the organic matrices of S. floresiana (Fig. 3). The guanidine thiocyanate-soluble matrix (GT-SM) was characterized by one major band of ~ 26 kDa and multiple faint bands of
higher and lower molecular weight producing a ladder-like pattern. The gel image of the A-IM showed a smear of macromolecules with molecular weight varying from ~77
to ~48 kDa. The A-SM contained a range of molecular
sizes. Within the smear, two bands were more evident, migrating at~101 and~89 kDa.
Treating the A-IM with DTT during the reduction step induced the release of one main protein at 56 kDa and several proteins of lower molecular weight. Boiling the A-IM in the Laemmli buffer before electrophoresis released
pro-0 4 8 12 16 20
Asx Glx Ser Gly Ala Thr Cys Val Met Ile Leu Tyr Phe β-ala Lys His Arg Pro
GT-SM A-SM
A-IM
%
Fig. 2Amino acid composition of the three fractions from
Schlumbergerel-la floresiana. The guanidine thiocyanate-soluble matrix (GT-SM) is
repre-sented by filled diamonds, the soluble mineral-associated matrix (A-SM) by filled squares, and the insoluble matrix (A-IM) by filled triangles. Differ-ences in amino acid composition between the three organic matrices are minor by comparison, and the one-wayANOVAtest carried out to ascertain these differences is not significant. Asx, aspartate; Glx, glutamate; Ser, ser-ine; Gly, glycser-ine; Ala, alanser-ine; Thr, threonser-ine; Cys, cysteser-ine; Val, valser-ine; Met, methionine; Ile, isoleucine; Leu, leucine; Tyr, tyrosine; Phe, phenylala-nine;b-ala,b-alanine; Lys, lysine; His, histidine; Arg, arginine; Pro, proline.
Acid-Soluble Matrix Acid-Insoluble Matrix MW
(KDa)
Guanidine
Thiochyanate Acid-Soluble
DTT (Room temperature)
Laemmli Buffer Boiling (100°C)
SDS PAGE, Silver nitrate staining
66
45
14 30 97
77 68 61
48
26
56 50 45 41
33
61
28 101
89
53
18
teins that were close to proteins released during the alkylation step with DTT. However, the extract was enriched with one protein of~28 kDa (Fig. 3). In the presence of DTT or
mer-captoethanol, the released proteins suggested that disulfide bridges were present between proteins in the A-IM.
Peptide sequences of the shell organic matrices
Liquid chromatography with tandem mass spectrometric (LC/MS-MS) analysis of the soluble matrices (GT-SM, A-SM) and A-IM was performed after hydrolysis with trypsin. We queried our data against theMASCOTdatabase (http://
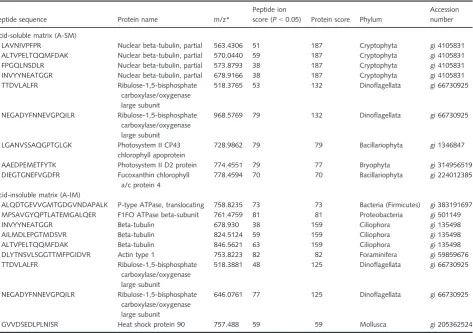
www.matrixscience.com/) to identify sequences. The search identified five proteins for the A-SM and six proteins for the A-IM. No proteins were identified from the guanidine thio-cyanate-soluble matrix (GT-SM; Table 3). In the A-SM, most of the sequences were related to chlorophyll proteins. Cytoplasm-localized proteins, including tubulin, actin, and a type of heat shock protein, were present in the A–IM. P-type ATPases from A-IM were found in bacteria.
Genomic resources forS. floresianaare limited to the com-plete small subunit ribosomal DNA gene sequence (http://
forambarcoding.unige.ch/). For other foraminiferal taxa, data are also available on small and large subunits of rDNA genes and sequences for a- and b-tubulin and actin genes (Bowser et al., 2006; Pawlowski et al., 2012). Recently, Haburaet al. (2011) provided the first consistent body of sequence information about the non-coding regions of the genome of a giant unilocular agglutinated foraminifer. How-ever, genomic resources are not yet available forS. floresiana
or other calcitic foraminifera. As a result, we interpretedde novosequences from the MS/MS spectra. We observed 33 peptide sequences for the guanidine thiocyanate-soluble matrix (GT-SM). Each contained 10 to 19 amino acids. In addition, 47 peptide sequences with 10 to 16 amino acid resi-dues were associated with the A-SM. For the A-IM, 76 pep-tide sequences were identified, and each contained 10–18 amino acids (see Supporting Information Table S1 for thede novo-sequenced peptides). In total, 156 peptide sequences were identified from the protein extracts that we isolated from
S. floresiana shells. These observations showed that only a few peptides with the same mass were present in the three organic matrices (see Supporting Information Table S1 for thede novo-sequenced peptides).
Table 3Results of theMASCOTsearch for the identification of the sequenced peptides fromSchlumbergerella floresiana
Peptide sequence Protein name m/z*
Peptide ion
score (P<0.05) Protein score Phylum
Accession number
Acid-soluble matrix (A-SM)
LAVNIVPFPR Nuclear beta-tubulin, partial 563.4306 51 187 Cryptophyta gi 4105831
ALTVPELTQQMFDAK Nuclear beta-tubulin, partial 570.0440 59 187 Cryptophyta gi 4105831
FPGQLNSDLR Nuclear beta-tubulin, partial 573.8793 38 187 Cryptophyta gi 4105831
INVYYNEATGGR Nuclear beta-tubulin, partial 678.9166 38 187 Cryptophyta gi 4105831
TTDVLALFR Ribulose-1,5-bisphosphate
carboxylase/oxygenase large subunit
518.3765 53 132 Dinoflagellata gi 66730925
NEGADYFNNEVGPQILR Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit
968.5769 79 132 Dinoflagellata gi 66730925
LGANVSSAQGPTGLGK Photosystem II CP43 chlorophyll apoprotein
728.9862 79 79 Bacillariophyta gi 1346847
AAEDPEMETFYTK Photosystem II D2 protein 774.4551 79 77 Bryophyta gi 314956519
DIEGTGNEFVGDFR Fucoxanthin chlorophyll a/c protein 4
778.4594 70 70 Bacillariophyta gi 224012385
Acid-insoluble matrix (A-IM)
ALQDTGEVVGMTGDGVNDAPALK P-type ATPase, translocating 758.8235 73 73 Bacteria (Firmicutes) gi 383191697 MPSAVGYQPTLATEMGALQER F1FO ATPase beta-subunit 761.4759 81 81 Proteobacteria gi 501149
INVYYNEATGGR Beta-tubulin 678.930 38 159 Ciliophora gi 135498
AILMDLEPGTMDSVR Beta-tubulin 824.5124 59 159 Ciliophora gi 135498
ALTVPELTQQMFDAK Beta-tubulin 846.5621 63 159 Ciliophora gi 135498
DLYTNSVLSGGTTMFPGIDVR Actin type 1 753.8223 82 82 Foraminifera gi 59859676
TTDVLALFR Ribulose-1,5-bisphosphate
carboxylase/oxygenase large subunit
518.3881 48 125 Dinoflagellata gi 66730925
NEGADYFNNEVGPQILR Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit
646.0761 77 125 Dinoflagellata gi 66730925
GVVDSEDLPLNISR Heat shock protein 90 757.488 59 59 Mollusca gi 205362524
MS BLASTsearch
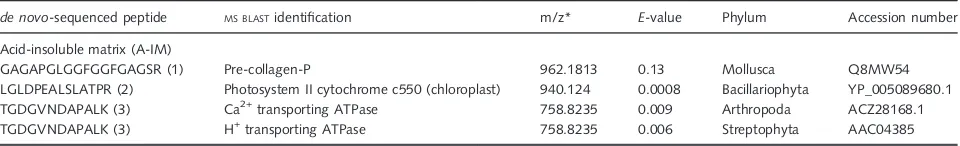
The de novo-generated sequences from the foraminiferal organic matrices were submitted to theMS BLASTdatabase to
identify proteins by sequence similarity searches using peptide sequences produced by the interpretation of tandem mass spectra (Shevchenkoet al., 2001). We identified three pep-tides from the A-IM with similar amino acid sequences. The first was related to the pre-collagen-P from the mollusk, My-tilus galloprovincialis. This peptide was a glycine-rich pep-tide containing Gly-X-Y repeats. X and Y represented proline and hydroxyproline, respectively. The second peptide blasted to sequences associated with chloroplast genes that coded for Photosystem II cytochrome c550 of Bacilla-riophyta. These hits can likely be attributed to the diatom symbiont associated withS. floresiana(Table 4). The third peptide was related to several ATPase families: calcium-transporting ATPase in an arthropod (Simulium nigrima-num), the plasma membrane hydrogen-transporting ATPase from Streptophyta (Upland Cotton), and a P-type ATPase from a rare enteric gram-negative bacterium (Rahnella aquatilis).
Monosaccharide composition of the GT-SM and A-SM
Neutral sugar analysis indicated that the guanidine thiocya-nate-soluble matrix contained 29% (w/w) neutral sugars. The GT-SM matrix contained traces of galactose (0.9%) and mannose (1.5%). The A-SM was represented by 9% (w/w) of neutral monosaccharide. Both matrices were rich in
D-glucose; they contained 98% and 82% total sugars,
respec-tively. They also contained galactose (9%), mannose (5%), and glucosamine (3%) as minor components (Table 5).
GT-SM and A-SM characterization of the N-glycans
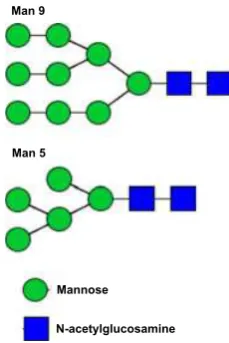
We identified N-linked glycans after digestion of the GT-SM and A-SM with PNGase F. Upon permethylation, the N-glycan profiles determined by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) analyses identified oligomannoses con-taining 5 to 9 mannoses in both extracts (Table 6; see Supporting Information Fig. S1 for MALDI-TOF-MS spectra analysis). To confirm the presence of particular
N-glycans (Man5 GlcNAc2 and Man9 GlcNAc2) in the guanidine thiocyanate-soluble matrix (GT-SM), the ions at m/z 1579 and 2396 were fragmented by MALDI-PSD (see Supporting Information Fig. S2 for MALDI-PSD spec-trum analysis). Fragmentation indicated that the core of the freed N-glycans was composed of N-acetyl-chitobiose, a dimer of b-1,4-linked glucosamine units. This represented the canonical signature of N-glycans. The fragmentation analysis allowed us to propose a chain structure for the two N-glycans (Fig. 4). After digestion of the extracts with the highly specific enzyme PNGase F, release of glycans indi-cated the presence of N-glycoproteins in the shell organic matrices ofS. floresiana.
GT-SM and A-SM characterization of hexose polymers
In the mass spectra of permethylated N-glycans obtained from the MALDI-TOF-MS analyses, we also noticed the presence of hexose polymers in the acid-soluble matrices (GT-SM and A-SM; Table 7; see Supporting Information Figs S1 and S2 for MALDI-TOF-MS spectra analysis). These polysaccharide chains contained 6–14 hexoses. They were likely composed of glucose according to the neutral sugar composition of both extracts.
DISCUSSION
The organic matrix plays an important role in biomineral formation. However, the components of these matrices in foraminifera remain unknown. Until now, only a few fora-miniferal matrix components have been identified and characterized at the protein level (Weiner & Erez, 1984; Robbins & Donachy, 1991; Robbins & Healy-Williams, 1991). However, these characterizations will become more common as transcriptomes and other genomic resources are developed for foraminifera (Burkiet al., 2006; Habura
et al., 2011; Pawlowskiet al., 2012).
Does organic matrix extraction correspond to morphological compartments?
We collected three organic matrix extracts containing proteins (GT-SM, A-SM, and A-IM) by employing our
Table 4Results of theMS BLASTsearch for the identification ofde novo-sequenced peptides fromSchlumbergerella floresiana
de novo-sequenced peptide MS BLASTidentification m/z* E-value Phylum Accession number
Acid-insoluble matrix (A-IM)
GAGAPGLGGFGGFGAGSR (1) Pre-collagen-P 962.1813 0.13 Mollusca Q8MW54
LGLDPEALSLATPR (2) Photosystem II cytochrome c550 (chloroplast) 940.124 0.0008 Bacillariophyta YP_005089680.1
TGDGVNDAPALK (3) Ca2+transporting ATPase 758.8235 0.009 Arthropoda ACZ28168.1
TGDGVNDAPALK (3) H+transporting ATPase 758.8235 0.006 Streptophyta AAC04385
extraction protocol (Table 1). All three had hydrolyzable amino acids, and they all showed staining upon gel electro-phoresis (Table 2; Figs 2 and 3).
Scanning electron micrographs of complete shells showed that all ectoplasm was likely removed by first washing with 2.5% NaOCl (Plate 1). Inside the shell, most of the IOL and diatom symbionts were still visible. There-fore, we cannot exclude the alternative that some residual cytoplasm (endoplasm) or digested remains of food organ-isms were still present (Langer & Bell, 1995). We used this washing protocol because it has been tested on other living benthic foraminifera (Borrelli et al., 2011) and NaOCl is often used in the preparation of mollusk shells (Marie et al., 2007, 2011, 2013; Ramos-Silva et al.,
2013). To avoid contamination by cellular debris, the organic film present on the outer surface (i.e., bacterial biofilm) and symbionts, the first extraction step was per-formed using denaturing guanidine thiocyanate HCl. This step was developed to wash away all non-mineral-associ-ated soluble biomolecules from the remaining mineralized tissue in vertebrates (Termine et al., 1980). The same approach was used by Stathoplos & Tuross (1994) in three planktonic foraminiferal species. They verified that the resulting matrix appeared to be free of ectoplasm and adherent organic debris as well. The first extraction was considered an effective cleaning step capable of solubiliz-ing the rest of the cytoplasm, foreign organic film and the diatom symbionts, because the resulting solution was a green/brownish color, corresponding to the guanidine thiocyanate-soluble matrix (GT-SM). However, it is possi-ble that some of the proteins present in the different organic layers might be removed through the GT-SM washing process. That fraction might have contained
con-Table 7Hexose polymer characterization of the acid-soluble matrices (GT-SM and A-(GT-SM) ofSchlumbergerella floresiana
Hexose polymers
m/z Interpretation
1293 Hex6
1497 Hex7
1701 Hex8
1906 Hex9
2110 Hex10
2314 Hex11
2518 Hex12
2723 Hex13
2927 Hex14
Hexose polymers present in GT-SM and A-SM were identified after MALDI-TOF analysis of two samples.
Table 5Monosaccharide composition of the acid-soluble matrices of
Schlumbergerella floresiana
Monosaccharides
GT-SM* (lg per 400lg of matrix)
GT-SM (% of the total)
A-SM†
(lg per 400lg of matrix)
A-SM (% of the total)
Fucose ND ND 0.06 0.2
Galactosamine ND ND 0.0980.03 0.3
Galactose 1.0470.00 0.9 3.150.07 9.0
Glucosamine ND ND 1.0730.27 3.1
Glucose 111.51.43 97.6 28.95.88 82.4
Mannose 1.7160.01 1.5 1.790.31 5.1
Total 114.26 35.14
29% (w/w) 9% (w/w)
GT-SM, guanidine thiocyanate-soluble matrix; A-SM, acid-soluble matrix. Neutral sugar compositions were obtained by high-performance anion exchange-pulsed amperometric detection (HPAE-PAD) after acid hydrolysis. Data are presented inlg per 400lg of the total matrix and in percentage of the total identified carbohydrate compounds. Averagestandard devia-tion (SD) is reported. ND=not detected, the signal is under the detection limit (0.01 ppm or 4 ng per 400lg of sample). *114.26lg neutral sugars per 400lg.†35.14
lg neutral sugars per 400lg.
Table 6N-glycan characterization of the acid-soluble matrices of Schlum-bergerella floresiana
[M+Na]+Permeth (mass m/z) Relative% Interpretation
Guanidine thiocyanate-soluble matrix (GT-SM)
1375.7 10.4 Man4GlcNAc2 Man4
1579.8 20.8 Man5GlcNAc2 Man5
1783.9 18.0 Man6GlcNAc2 Man6
1988.0 18.7 Man7GlcNAc2 Man7
2192.1 13.9 Man8GlcNAc2 Man8
2396.2 18.1 Man9GlcNAc2 Man9
Acid-soluble matrix (A-SM)
1375.7 3.7 Man4GlcNAc2 Man4
1579.8 13.1 Man5GlcNAc2 Man5
1783.9 17.3 Man6GlcNAc2 Man6
1988.0 26.6 Man7GlcNAc2 Man7
2192.1 21.4 Man8GlcNAc2 Man8
2396.2 17.9 Man9GlcNAc2 Man9
GT-SM, guanidine thiocyanate-soluble matrix; A-SM, acid-soluble matrix.
Man 9
Man 5
Mannose
N-acetylglucosamine
taminants and also proteins (or macromolecules) associated with the foraminiferal shell. During the demineralization with cold 6.5M acetic acid, the calcite test powder
dis-solved and exposed the organic layer(s) that were previ-ously protected by the mineral (King & Hare, 1972a; Weiner & Erez, 1984; Haugen et al., 1989; Robbins & Healy-Williams, 1991; Stathoplos & Tuross, 1994). Here, the acid-soluble proteins were extracted (A-SM), and the A-IM contained the remaining insoluble components. The fractions (GT-SM, A-SM, and A-IS) were the result of a progressive cleaning to separate contaminants from shell proteins potentially involved in the foraminiferal biominer-alization mechanism. The amino acid composition of all matrices of S. floresiana (GT-SM, A-SM, and A-IM) was similar (Table 2; Figs 2 and 5a). This might indicate that the shell organic layers (i.e., POM, OOL, IOL) are com-posed of proteins with comparable physicochemical prop-erties. Alternatively, the similarity in protein composition could be explained by the presence of only one kind of layer within the organic matrix. Despite numerous scan-ning electron microscopic observations of S. floresiana
shell fragments, no POM was clearly observed; however, a thick IOL was always distinguishable (Plate 1).
These results differ from those of Stathoplos & Tuross (1994). They reported that the mineral-associated organic matrix in the planktonic foraminifer Globigerinoides ruber
was significantly richer in aspartate (~30%) than the other
fractions (guanidine-soluble and guanidine-insoluble resi-dues) (~15%). They suggested that different proteins were
concentrated in the A-SM. The soluble fraction of the organic matrix in a larger tropical benthic foraminifer,
H. depressa, was rich in aspartate (~26%), glycine (~12%),
serine (~12%), glutamate (~11%), and alanine (~9%)
resi-dues, while the insoluble fraction was dominated by aspar-tate (~26%) and double the glycine (~24%; Weiner &
Erez, 1984). At the same time, the hydrophobic amino acids represented ~17% of the insoluble fractions, and
the values were doubled in the soluble fraction (~34%).
Our results (Table 1) differed quantitatively from those described by Weiner & Erez (1984). For example in
H. depressa, the soluble fraction contained almost all the proteins present in the shell; conversely, for S. floresiana,
most of the proteins were concentrated in the insoluble fraction. These three foraminiferal species may have evolved to partition protein composition differently. Alter-natively, discrepancies between the different datasets could be related to different extraction methodologies and/or could be related to a species-specific feature (King & Hare, 1972b).
Aspartate was an abundant amino acid in all matrices extracted fromS. floresiana(11–14% and Fig. 2). This evi-dence suggested that proteins capable of binding calcium ions were present and probably involved in the crystal formation. The high proportion of glycine and alanine in
the organic matrix composition suggested the presence of hydrophobic and fibrous structural proteins. These characteristics are common among matrix proteins isolated from diverse biomineralizing taxa using both CaCO3 and CaPO4 (Corstjens et al., 1998): corals (Watanabe et al., 2003; Puverel et al., 2005; Cusack & Freer, 2008), echinoderms (Weiner, 1985; Benson et al., 1986, 1987; Sucov et al., 1987; Treccani et al., 2005; Killian & Wilt, 2008), mollusks (Weiner, 1979, 1983; Wheeler & Sikes, 1989; Bedouet et al., 2001; Wilt et al., 2003; Marin & Luquet, 2004; Marin et al., 2008; Suzuki et al., 2009;
0%
Fig. 5 Spider chart distribution of amino acid composition of thede novo -sequenced peptides from the organic matrices (GT-SM, A-SM, and A-IM) of
Marie et al., 2011), and mammals (Butler et al., 1983; Wasi et al., 1983; Oldberg et al., 1988; Goldberg et al., 2001).
Polypeptides
A heterogeneous mixture of polypeptides was found in all organic matrices of the S. floresiana shell samples. Unre-solved smears ranged from ~26 to ~100 kDa; however,
more discrete bands were visible at~26 kDa and~28 kDa
in the guanidine thiocyanate-soluble matrix (GT-SM) and in the A-IM (Fig. 3). Electrophoresis analysis is a low-reso-lution method, as emphasized by Pereira-Mouries et al.
(2002) and Bedouetet al.(2007) in their analysis of shell matrix proteins in mollusks. Stathoplos & Tuross (1994) described a similar situation in the planktonic foraminifer
G. ruber, where extracts from the A-SM yielded only unre-solved smears of products. For another planktonic species (Globorotalia menardii), the same authors found a mini-mum of 10–15 discrete products for the acid-soluble extracts with apparent molecular weight ranging from 15 to 65 kDa. Using gel electrophoresis to analyze the soluble matrix from fossil planktonic foraminifera (Orbulina universa and Pulleniatina obliquiloculata), Robbins & Donachy (1991) observed a band of 65 kDa, noted as FP1. In a later study, Robbinset al.(1993) identified pro-teins in the range of 18 and 109 kDa in livingO. universa. Together, these studies suggest that there are many yet unresolved proteins involved in the biomineralization of foraminiferal tests.
So far, only one study (Stathoplos & Tuross, 1994) reported separation of the proteins in the A-IM. They rec-ognized a discrete band of 43 kDa corresponding to actin in two living planktonic foraminiferal species.
The present study has provided only the first preliminary data on gel electrophoresis products of living benthic fora-minifera. These data cannot yet be compared to the data from planktonic foraminifera, but future studies will identify the electrophoresis products and follow up with sequence data.
Identification of peptides in shell organic matrices
Here, we present the first tentative interpretation of thede novo-generated sequences of shell organic matrices of
S. floresiana. These sequences were obtained from shell peptides after trypsin digestion of the extracts. Our proteo-mic strategy revealed 156 peptides containing 10–19 amino acid residues present in all matrices.MS BLAST
analy-ses identified three homologous proteins containing amino acid sequences that were similar to the de novo peptides. They all were from the acid-insoluble matrix (Table 4). Minimal putative homologies indicated that these peptides were derived from sequences of unknown origin and may
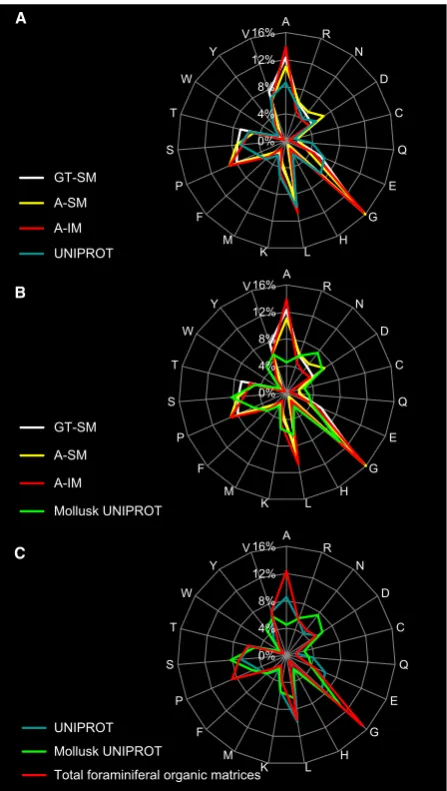
be unique to foraminiferal organic matrices. Unfortunately, without homologous sequences in existing databases, we could not propose hypotheses regarding the structure or function of these peptides. However, their amino acid distribution, viewed in the spider chart (Fig. 5a,b), showed that the three organic extracts (GT-SM, A-SM, and A-IM) shared a similar amino acid composition. This evidence showed that none of the extraction steps in the protocol enriched for a particular class of peptide, although the pep-tide sequences found in the three organic matrices were different. This implied that different reagents extract simi-lar proteins, and localization or modification of proteins should not be defined solely based on solubility parameters (Weiner & Erez, 1984; Stathoplos & Tuross, 1994).
The amino acid distribution for proteins involved in mollusk biomineralization was compared to all taxa present in the UNIPROT database (http://www.ebi.ac.uk/uniprot/
TrEMBLstats/; Fig. 5c). We found that the mollusk pro-teins were enriched with glycine, asparagine, and serine rel-ative to other proteins in the database.
Interestingly, in foraminifera (S. floresiana), the amino acid distribution of all the peptides from the three organic matrices showed higher percentages of glycine, alanine, and proline. The percentages of the three amino acids in the foraminiferal proteins were higher than in any other sequences in the UNIPROT database, including mollusks
(Fig. 5c). In addition, the charge/hydrophobicity (C/HP) ratio for all three organic matrices (GT-SM, A-SM, and A-IM) was 0.48. However, in case of mollusk proteins, the sequences were enriched with hydrophobic amino acids (alanine and leucine) and the C/HP ratio was much higher (0.84) than the foraminiferal organic matrix (0.48). The ratio between hydrogen-bonding and hydrophobic residues is relevant to aggregative properties of the matrix proteins such as the molecular interactions leading to carbonate assembling and its stabilization as biomineral. Difference in C/HP values between foraminifera and mollusks might indicate specificity in shell matrix protein patterning.
bys-sus, squid skin, and abalone muscle structures (Gosline & Shadwick, 1983; Kimura & Tanaka, 1983; Olaecheaet al., 1993; Qin & Waite, 1995; Deming, 1999; Yonedaet al., 1999).
Another peptide blasted to the Photosystem II cyto-chrome c550 (chloroplast) of Bacillariophyta and was likely a signature of contaminants resulting from diatom endos-ymbionts, despite the blanching and denaturing guanidine thiocyanate HCl treatments (Table 4; Plate 1). Finally, one peptide was related to several ATPase families: calcium-transporting ATPase of an arthropod (Simulium nigrima-num), or the plasma membrane hydrogen-transporting ATPase from Streptophyta (Upland Cotton) or a P-type ATPase from a rare enteric gram-negative bacterium (Rahnella aquatilis).
The P-type ATPases are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. Some of these, such as Ca2+ AT-Pase, serve to regulate the amount of Ca2+ within cells and are required for biomineralization. Other categories of transmembrane ATPase include exchangers such as vesicular H+-ATPases that increase the pH inside vesicles and decrease the pH of the cytoplasm. Large alkaline vac-uoles were involved in the foraminiferal biomineralization model proposed by Bentov et al. (2009) and de Nooijer
et al. (2009). They concentrated inorganic carbon at the calcification site. In particular, within the cell, endo-cytosis forms seawater vacuoles, and various pump and channels likely work to increase the pH, Ca2+, and inorganic carbon pool in these vacuoles. Recently, Taylor
et al. (2011) demonstrated that the presence of a volt-age-gated H+ channel in coccolithophores played an important role in pH homeostasis for these calcifying unicellular algae.
N-glycoproteins
This study also identified N-glycoproteins in theS. floresi-ana shell. Confusingly, glycoprotein and glycosaminogly-can or mucopolysaccharide are often used as synonyms to indicate the same class of composites (Hedley, 1963; Langer, 1992; Langer & Gehring, 1994). Hedley (1963) speculated that the inner layer of eleven species of arenaceous foraminifera was a glycoprotein complex, in the sense of an acid mucopolysaccharide combined with proteins, which he called ‘tectin’. Angell (1967a,b) showed that between the IOL and the basal calcareous lamella, there was a fibrous ‘basal membrane’ composed of a protein–polysaccharide complex. The complex pene-trated each pore and fused to each interlamellar mem-brane of the succeeding lamellar of the calcareous wall, forming the honeycomb structure of organic membranes described by Lee et al. (1963) as the ‘protein– carbohy-drate complex’.
Our results indicated that the acid-soluble (GT-SM and A-SM) matrices contained glycoproteins. In particular, the presence of aspartate (14%) in the mineral-associated matri-ces (A-SM) and the monosaccharide glucosamine (3%) allowed us to infer that the foraminiferal shell matrix con-tained some N-glycoproteins. This explanation was sup-ported by reports showing that N-glycoproteins are common components of shell organic matrices in echino-derms (Berman et al., 1988, 1990; George et al., 1991; Mann et al., 2008) and mollusks (Bedouet et al., 2001; Levi-Kalisman et al., 2001; Sch€affer et al., 2001; Samata, 2004; Marin et al., 2005; Marie et al., 2009, 2011). The gene SM30 controls the production of intraspicular pro-teins in echinoderms; it is a 30-kDa acidic N-glycoprotein (George et al., 1991; Mann et al., 2008). Leaf et al.
(1987) identified a gene related to echinoderm biomineral-ization as a specific cell surface glycoprotein. Experimental evidence suggested that it is involved in Ca2+ uptake (Chow & Benson, 1979). Bedouetet al. (2001) detected three glycoproteins in the soluble matrix ofHaliotis tuber-culata, and they suggested that the addition of glycans to proteins may be favorable for the biomineralization pro-cess. Eukaryotic glycoproteins usually carry sialic acid, which would make additional negative charges available for calcium binding. Marin et al. (2005) suggested that cal-prismin might be a glycoprotein of the shell calcitic prisms of Pinna nobilis involved in matrix–cell interaction. Marie
et al. (2011) identified an acid-soluble acidic shell matrix glycoprotein that is specific to the nacreous layer of the cephalopod Nautilus macromphalus, and they demon-strated that it was able to bind chitinin vitro.
Profiles determined by MALDI-TOF mass spectrometry combined with the carbohydrate composition analysis indi-cated that the hypothetical foraminiferal N-glycan structure contained the ubiquitous N-glycan core with 5–9 mannos-es (Fig. 4). Based on thmannos-ese data, we proposed that the acid-soluble proteins of the foraminiferal skeleton organic matrix contain N-glycoproteins-bearing sugar moieties with N-linked high-mannose-type oligosaccharide. The major soluble 19.6-kDa protein of the organic shell matrix of the freshwater snail Biomphalaria glabrata was also an N-glycosylated dermatopontin (Samata, 2004). Dermato-pontin is widespread in mammalian extracellular matrices (i.e., in the matrix where bone and teeth form). It may function in the acceleration of collagen fibril formation
These observations supported the hypothesis that N-glyco-proteins in the acid-soluble matrices may be associated with the biomineralization process inS. floresiana. Banneret al.
(1973) proposed that the biomineralization of the foraminif-eral exoskeleton was enhanced by glycoprotein (‘tectinous’) intralamellar membranes and that the membranes operated as templates for epitaxial growth.
Carbohydrates
Proteins guide nucleation and growth of the mineral phase, and together with polysaccharides, they account for the mechanical properties of most of the polymer compos-ites (Kuo et al., 1996; Treccani et al., 2005). Our study also characterized sugar components of the foraminiferal organic matrices (Tables 5 and 7), and we identified the nature of S. floresiana carbohydrates. Weiner & Erez (1984) used the infrared spectrometry method and sug-gested that polysaccharides were more abundant in the insoluble fraction. Polysaccharides were also reported by other authors (Hedley, 1963; Angell, 1967a,b; Towe & Cifelli, 1967; Feizi, 1990; MacBeath et al., 1993; Marxen
et al., 2003); however, nothing is known about the components.
We collected data on monosaccharide composition for acid-soluble matrices (GT-SM and A-SM), but we assumed that monosaccharides were present in the A-IM as well. Neutral sugar analysis indicated that the acid-soluble matri-ces were rich in monosaccharides (represented for 82–98% by theD-glucose). The high content of the D-glucose and
the presence of hexose polymers (i.e., cellulose, starch) might be associated with the diatom endosymbionts that inhabit the foraminifer S. floresiana (Table 7). These hex-ose polymers might represent excretion products trapped in the shell organic matrices (Banneret al., 1973).
Geobiological implications of the larger symbiont-bearing foraminifera calcification
Our results represent a first step toward understanding the functioning mechanism behind the molecules involved in the foraminiferal biomineralization. This line of research goes beyond the scope of biology, and it will have environ-mental as well as geological implications.
Larger symbiont-bearing foraminifera (including the calcarinid S. floresiana studied here), together with corals and coralline algae, are the most important carbonate pro-ducers in the coral reef ecosystems (Langer et al., 1997; Langer, 2008). Larger symbiont-bearing foraminifera produce approximately 130 million tons of carbonate per year, thereby contributing approximately 4.8% of the total reef carbonate budget (Langer, 2008). On the global scale, all calcareous foraminifera (planktonic and benthic) gener-ate 1.4 billion tons of carbongener-ate per year, which represents
one-quarter of the global calcium carbonate produced every year (Langer, 2008).
In some places, larger symbiont-bearing foraminifera can produce more carbonate per square meter than corals (Yamano et al., 2000, Harney & Fletcher III, 2003, Hart & Kench, 2007). In Indonesia, we observed thatS. floresi-ana dominates many reefs and certainly generates more carbonate than corals or algae, especially in high-energy wave zones and lagoon environments. Moreover, in many Indo-Pacific reef islands, calcarinids represent a critical source of beach sand because of their turnover rate. They prevent beach erosion and play a fundamental role in reef resilience (Hallock, 1981; Hohenegger, 2006; Dawson
et al., 2012; Dooet al., 2012).
As carbonate producers, these protists are vulnerable to climate-driven ocean warming and ocean acidification (Uthicke et al., 2010; Schmidt et al., 2011, Doo et al., 2012). Larger symbiont-bearing foraminifera secreting high-Mg calcite, including calcarinids, are particularly sen-sitive (Blackmon & Todd, 1959; Bentov & Erez, 2006). They will be more affected by ocean acidification than corals with aragonite skeletons or foraminifera secreting low-Mg calcite (Uthickeet al., 2010). However, tolerance levels to thermal stress and ocean acidification may vary among living larger benthic foraminiferal species and algal symbionts (Fujita et al., 2011, Hikami et al., 2011). To protect reef ecosystems, we need a better understanding of the physiology behind the calcification mechanism of these benthic foraminifera.
On geologic timescales, extensive limestone deposits from the late Paleozoic were produced by larger benthic foraminifera, contributing to long-term storage of atmo-spheric CO2. Since the Cretaceous, planktonic foraminifera also largely contributed to this sink. Those calcifying foraminifera were important actors in past ecosystems and continue to play an important role by participating in modern geochemical cycles. They constitute an important tool used for paleoenvironmental reconstructions because of their nearly continuous fossil record and abundance. Many studies focus on their biomineralization process using a geochemical perspective to record environmental and climate changes from shell isotopic and trace element compositions.
and reservoirs as well as future modeling efforts, and (iii) improve the use of foraminifera as paleoclimatological and paleoceanographical tools.
Our results provided a first look at the molecular under-pinnings of biomineralization in these carbonate produc-ers.
CONCLUDING REMARKS
It is worth-studying biomineralization in S. floresiana
because larger symbiont-bearing foraminifera play an essential role in carbonate production on reefs and in the global carbon budget. The organic layers associated with the shell ofS. floresiana all yielded a similar suite of mac-romolecules. Alternatively, these organisms may have only a single layer within the organic matrix. The molecules identified included acid-soluble N-glycoproteins and sugar moieties represented by high-mannose-type glycans and carbohydrates. Preliminary results highlighted the fact that very few proteins are related to existing sequences in the proteomic database. This suggested that the forami-niferal organic matrix peptides are derived from as yet unknown precursors or that related sequences have not yet been uploaded onto proteomic databases. Other than sequences similar to collagen or with calcium-binding properties, these results did not allow much inference about structure or function of shell proteins. However, when compared to the UNIPROT databases, the
distribu-tion of amino acids in these peptides indicated that the biomineralization proteins may contain sequences that are unique to foraminifera.
The structure and composition of the organic framework evolved to perform essential regulating and/or organizing functions and led to the formation of composite biominer-als. We hope that our biochemical characterization of fora-miniferal shell matrices, and the de novo sequencing of peptides from the organic matrices, will lead to additional studies of the biomineralization process in these unique organisms. Our work represents preliminary progress toward understanding the molecular and cellular machinery of biocalcification based on a protozoan unicellular model.
Finally, we conclude that coupling geochemical and bio-logical perspectives on foraminifera will enhance interpreta-tion of the proxies used for environmental and climatic reconstructions and improve future modeling efforts in the field of paleoclimatology.
ACKNOWLEDGMENTS
We acknowledge the Animal Biomedical and Molecular Biology Laboratory (Indonesia) because it allowed the trans-fer of living foraminitrans-feral specimens ofSchlumbergerella to the Museum National d’Histoire Naturelle (MNHN) of Paris, thanks to the MTA agreement, number
DOC060613-06062013084816, between the two institutions. This study was largely supported by the ATM Biomineralization pro-gram of the Museum National d’Histoire Naturelle of Paris (project granted to A. Bartolini). The authors gratefully acknowledge Andrew J Gooday (National Oceanography Centre, Southampton), Alessandra Negri, (Polytechnic Uni-versity of Marche, Ancona), Maria Pia Nardelli (Universite d’Angers), and Caterina Morigi (University of Pisa) for con-structive discussions and for critical assessment of the manu-script. The authors thank the numerous technicians involved in laboratory analyses of biochemical samples over the years. Particular thanks go to the Proteodynamics team for their contribution to the MALDI-TOF results and inter-pretation, Omar Boudouma (University UPMC-Paris VI) who made numerous scanning electron micrographs of
S. floresiana, and Alexandre Lethiers (University UPMC-Paris VI) who helped with graphical construction of the plate and Fig. 1. Authors are grateful to the editor, Mar-tin Langer and other two anonymous reviewers for their constructive comments and suggestions, which helped us to improve the manuscript.
REFERENCES
Angell RW (1967a) The test structure and composition of the foraminiferRosalina floridana.Journal of Protozoology14, 299–307.
Angell RW (1967b) The process of chamber formation in the foraminiferRosalina floridana.Journal of Protozoology14, 566–574.
Angell RW (1979) Calcification during chamber development in Rosalina floridana.Journal of Foraminiferal Research9, 341–353.
Angell RW (1980) Test morphogenesis (chamber formation) in the ForaminiferSpiroloculina hyalinaSchulze.Journal of Foraminiferal Research10, 89–101.
Banner FT, Sheehan R, Williams E (1973) The organic skeletons of rotaline foraminifera: a review.Journal of Foraminiferal Research3, 30–42.
Be AWH, Hemleben C, Anderson OR, Splinder M (1979) Chamber formation in planktonic foraminifera. Micropaleontology25, 294–307.
Bedouet L, Schuller MJ, Marin F, Milet C, Lopez E, Giraud M (2001) Soluble proteins of the nacre of the giant oyster Pinctada maximaand of the abaloneHaliotis tuberculata: extraction and partial analysis of nacre proteins.Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology128, 389–400.
Be´douet L, Marie A, Dubost L, Pe´duzzi J, Duplat D, Berland S, Puisse´gur M, Boulzaguet H, Rousseau M, Milet C, Lopez E (2007) Proteomic analysis of the nacre soluble and insoluble proteins from the oyster Pinctada margaritifera.Marine Biotechnology9, 638–649.
Benson SC, Benson N, Wilt FJ (1986) The organic matrix of the skeletal spicule of sea urchin embryos.The Journal of Cell Biology102, 1878–1886.