Short communication
Dissipation of herbicides in soil and grapes in a
South Australian vineyard
Guang-Guo Ying, Brian Williams
∗Department of Environmental Science and Management, The University of Adelaide, Roseworthy Campus, Roseworthy, SA 5371, Australia
Received 8 February 1999; received in revised form 12 June 1999; accepted 16 September 1999
Abstract
The persistence of herbicides applied in vineyards has become a concern in recent years due to their wide use. Investigations into the fate of herbicides in a vineyard in the Barossa Valley, South Australia, have been directed towards the dissipation of herbicides in soil and on grapes. Concentrations of the herbicides, norflurazon, oxadiazon, and oxyfluorfen in soil were monitored following their application in the vineyard. With the exception of oxyfluorfen, dissipation of these herbicides in the vineyard soil was characterised by fast initial loss followed by slow degradation. For oxyfluorfen the dissipation was slow throughout the period of the study. The half-life for norflurazon in surface soils varied from 50 days in 1996 to 22 days in 1997 while that for trifluralin was 27 days in 1996 and 30 days in 1997. Oxyfluorfen had a very low dissipation rate with a half-life of 119 days. Oxadiazon had a relatively short half-life of 14 days. Dissipation of the herbicide residues on grapes in the Roseworthy campus vineyard showed that trifluralin and oxyfluorfen were not detected 4 days after treatment, while norflurazon and oxadiazon remained on grapes 1 month after treatment. This study showed that the dissipation of herbicides in soil and on grapes was dependent on the physicochemical properties of the herbicides and environmental conditions. The use of these relatively persistent herbicides in vineyards has the potential to harm vines and to contaminate grapes and the wine made from them. ©2000 Elsevier Science B.V. All rights reserved.
Keywords: Dissipation; Herbicide; Soil; Grape; Vineyard
1. Introduction
South Australia, especially the Barossa Valley, is Australia’s largest and most famous viticultural and wine-producing area. It has a unique environment with a Mediterranean type climate and mainly sandy soil features. Herbicides play an important role in the grape production of Australia, but their residues may
∗Corresponding author. Tel.:+6183037840; fax:+6183037956.
E-mail address: [email protected] (B. Williams).
cause environmental problems. The use of herbicides may contaminate surface-water and ground-water through leaching and run-off, and may also contam-inate grapes through spray drift during application. On the other hand, herbicide residues in soils may also injure vines and/or reduce the yield of grapes, and thus they might potentially affect wine quality and enter the food chain.
Norflurazon, oxadiazon, trifluralin and oxyfluorfen are some common soil-applied residual herbicides reg-istered for use in Australian vineyards. They belong to
four different chemical classes: norflurazon — a pyri-dazinone, oxadiazon — an oxadiazolone, trifluralin — a dinitroaniline and oxyfluorfen — a diphenyl ether, respectively.
Some studies have examined the persistence of the above in the environment. Braunschweiler (1992) studied the persistence of several pesticides including trifluralin, in cultivated clay, fine sand and organic soils at Jokioinen, southern Finland in 1985–1988 and found that trifluralin was the most persistent. Residues of trifluralin on the surface of peat soil were still high 1.5 years after treatment (Braunschweiler, 1992). Eleftherohorinos and Kotoula-Syka (1990) also found that trifluralin was persistent in soils planted to cotton in Greece. Their 3-year study showed that all the herbicides studied persisted longer in soils with high organic matter and clay content. Jolley and John-stone (1994) studied trifluralin degradation in three Victorian soils under field and laboratory conditions and found that trifluralin degradation increased with both increasing moisture and increasing temperature. All of these studies showed that trifluralin is a very persistent herbicide.
There are few reports on the persistence of norflura-zon, oxadiazon and oxyfluorfen on soil or grapes (e.g. Hubbs and Lavy, 1990; Ambrosi et al., 1977; Frank et al., 1991). Schroeder and Banks (1986a, b) evalu-ated the persistence of norflurazon in five Georgia soils and found that cool and/or dry environmental condi-tions combined with high soil organic matter content caused slower herbicide loss. This result is similar to that of Jolley and Johnstone’s (1994) study. Oxadia-zon also degraded slowly in soils and was postulated to adsorb strongly on soil organic matter (Carringer et al., 1975; Ambrosi et al., 1977).
Pesticide residues have been detected in the grapes and wines of many wine-producing countries (Cabras et al., 1987, 1995; Watterson, 1991), although most of them were widely used fungicides and insecticides, herbicide residues were found. Therefore, the use of persistent herbicides requires a thorough understand-ing of their dissipation and movement in vineyards. Unfortunately, little research has been undertaken on the behaviour of herbicides in Australian vineyards or in Australian horticulture generally (Ying and Williams, 1999a,b). This paper reports the dissipation of some herbicides in soil and on grapes in a South Australian vineyard.
2. Materials and methods
2.1. Herbicide application on soil
The field study was conducted on two plots in the Mountadam Vineyard of the Barossa Region in South Australia. The experimental site is on the down slope of a vineyard adjacent to a dam. The soil within the study area is classified at the great group level as a Haploxeralf (Soil Survey Staff, 1975). The surface soil has an organic carbon content of 8.6 g kg−1.
In 1996 and 1997, herbicides were applied at rec-ommended rates, without incorporation into soil, onto plots A and B to investigate their dissipation in soil. The application of different herbicides as mixtures or sequentially is a common weed management prac-tice in South Australia. The application rates were 3.3 kg ha−1 for norflurazon (Solicam® with active
constituent of 800 g kg−1), 4 kg ha−1 for oxadiazon
(Ronstar® with active constituent of 20 g kg−1),
1.7 l ha−1 for trifluralin (Treflan® with active
con-stituent of 400 g l−1) and 10 l ha−1 for oxyfluorfen
(Goal® with active constituent of 240 g l−1).
Norflu-razon and trifluralin were sprayed on Plot A on 19 August, 1996 while oxyfluorfen was sprayed on the plot on 2 September, 1996. Oxadiazon was sprayed on Plot A on 27 May, 1997. Norflurazon and tri-fluralin were applied on Plot B on 27 May, 1997. Following application, surface soils (0–5 cm) were sampled using a hand auger at specified intervals. More than 10 surface soil samples (0–5 cm) were randomly taken at each sampling occasion from each plot, mixed and analysed as one composite sample.
2.2. Herbicide treatment on grapes
(Doradillo) and red (Tarrango) grapes in the Rose-worthy campus vineyard were dipped in the herbicide solution of 100mg cm−3 of each herbicide
(norflu-razon, oxadiazon, trifluralin and oxyfluorfen) on 9 February, 1998. Grape samples were randomly picked from bunches and mixed at 1 h, 1 day, 2 days, 4 days, 7 days, 14 days, 21 days and 28 days after treatment.
2.3. Extraction
Soil samples were mixed with methanol under sonication and filtered through Millipore filter pa-pers. The filtrates were diluted with distilled water and the mixtures were extracted by C18 cartridges
pre-conditioned with methanol and water. The car-tridges were dried by drawing air through them for half an hour. Then the adsorbed herbicides were eluted with 5 ml of methanol and analysed as described below.
Grapes (about 100 g) were crushed and mixed us-ing a Ronson mixer, then extracted with 50 ml of acetonitrile. The mixture was centrifuged at 6000 rpm for 15 min. The upper liquid phase was filtered se-quentially through glass fibre and millipore filter papers. The filtrate was diluted with distilled water and passed through a C18 cartridge (6 ml) which was
pre-conditioned by using acetonitrile followed by 20% ethanol in water. The herbicide residues in the cartridge were eluted with ethyl acetate.
2.4. Herbicide analysis
The GC analyses were performed using a Hewlett Packard 5890A gas chromatograph, equipped with a nitrogen-phosphorus detector (GC-NPD). The column used in this study was an HP fused-silica capillary column coated with cross-linked methyl silicone (col-umn length 25 m, col(col-umn ID 0.31 mm, film thickness 0.52mm). Nitrogen (N2) was used as both the
car-rier and make-up gas at a flow rate of 30 ml min−1
for the NPD. Hydrogen was used at a flow rate of 3.5 ml min−1and air at 110 ml min−1. The oven
tem-perature was programmed from 200◦C (1 min) to
300◦C (5 min) at a rate of 10◦C min−1, with injector
temperature at 250◦C and detector temperature at
300◦C.
Table 1
The regression equations of herbicide dissipation in soil
Herbicide Regression equationa
1996 1997
Norflurazon y= −0.006x+1.4794 y= −0.0137x+2.0172 R2=0.8768 R2=0.9630
Oxadiazon y= −0.0215x+1.3974
R2=0.8672 Oxyfluorfen y= −0.0026x+1.8638
R2=0.7699
Trifluralin y= −0.011x+1.0396 y= −0.0101x+0.8488 R2=0.9508 R2=0.9526
ay is the log of herbicide concentrations (
mg g−1) in soil, and x is the time after treatment (days).
3. Results and discussion
3.1. Dissipation of herbicides in soil
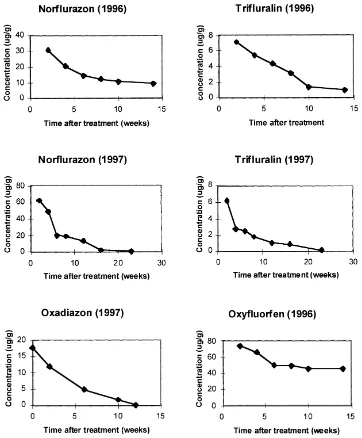
The measurement of herbicides in Plot A in 1996 and in Plot B in 1997 shows that the dissipation of norflurazon, oxadiazon and trifluralin in sandy sur-face soil was characterised by fast initial loss followed by slow subsequent degradation (Fig. 1). Oxyfluorfen degraded more slowly. Herbicides that had been ap-plied in 1996 to Plot A were detected in May 1997, some 9 months after application, at concentrations of 2.29mg g−1for norflurazon, 0.56mg g−1for trifluralin
and 13.04mg g−1 for oxyfluorfen in the surface soil.
The dissipation of these herbicides in the surface soils was calculated on the basis of first order kinetics for degradation. The regression equations and half-lives are shown in Tables 1 and 2. Oxyfluorfen had the longest dissipation half-life of 119 days while that for oxadiazon was only 14 days. The dissipation half-life for trifluralin in surface soil was 27 days in 1996 and 30 days in 1997, but that of norflurazon varied from about 50 days in 1996 to 21 days in 1997.
Table 2
The dissipation half lives and kinetic rate constants of herbicides in soil
Herbicide Rate constant (k) Half-life (da (days)
1996 1997 1996 1997
Norflurazon 0.0138 0.0316 50 22
Oxadiazon – 0.0495 – 14
Oxyfluorfen 0.0059 – 119 –
Fig. 1. Dissipation of herbicides in soils in 1996 and 1997. See Table 1 for regression equations.
The persistence or dissipation of a chemical is mainly controlled by its physico-chemical properties, management practices and environmental conditions including climate, soil physico-chemistry and mi-crobial activity in the soil. Studies on norflurazon (Schroeder and Banks (1986a, b) and trifluralin (Jol-ley and Johnstone, 1994) suggested that cool and/or dry environmental conditions lead to increased per-sistence in soil. However, Barrett and Lavy (1984)
found that changes in soil water content above some minimum level will not affect the sum of the pro-cesses responsible for oxadiazon dissipation. Jolley and Johnstone (1994) studied the effect of moisture content on the half life for trifluralin in three soils at 20◦C in the laboratory and the data obtained showed
field studies reported herein were undertaken during winter, so the dissipation rates were expected to be affected by the cool and wet climate.
The reported half-life values for norflurazon, ox-adaizon, trifluralin and oxyfluorfen differ from those found in this study. Schroeder and Banks (1986b) showed half-lives of about 20–35 days for norflura-zon in five Georgia soils at 20–30◦C in a greenhouse.
In a laboratory study, Rahn and Zimdahl (1973) ob-served a half-life of 70 days for norflurazon in a Col-orado sandy loam at 35◦C. Southwick et al. (1993)
found that the DT50(time to 50% disappearance) for
norflurazon in the top 15 cm of Mississippi River al-luvial soil was about 40 days in the 1988–1989 sea-son, and about 120 days in the 1989–1990 season. The authors did not explain why there was such a large difference between the two seasons. In fact, the rain-fall was higher in 1989–1990 than in 1988–1989. The present field study also found quite different half-lives for norflurazon: about 50 days in 1996 and about 22 days in 1997. In 1996, a slight rain after herbicide ap-plication may have helped the herbicide incorporate into soil due to its high mobility and, therefore, may have led to lower loss of the herbicide from the soil surface by volatilization. On the other hand, the leach-ing process contributed only partly to the dissipation of norflurazon in soil. Due to their low mobility, most of the trifluralin, oxadiazon and oxyfluorfen remained on the top of the soil where their dissipation would be affected by microbial and chemical degradation and volatilization losses.
Jolley and Johnstone (1994) found that half lives for trifluralin in three Victorian soils during 1985–1987 varied from 100 days to 214 days under field condi-tions. The half-lives of trifluralin after 9 or 10 years of continued use were 8.7–10.1 months in a Dundee silt loam and 15–11 months in a Sharkey soil. So tri-fluralin dissipated very slowly in soil after long-term use. The field half-lives determined by Wauchope et al. (1991), ranged from 60 to 132 days in several soils. The half-life from this field study was about 27–30 days, which is lower than the above data. The rea-son for this short dissipation half-life is believed to be due to microbial degradation and/or volatilization loss. Trifluralin is subject to biodegradation in soil (Golab et al., 1979). Trifluralin was not incorporated into soil after application and stayed mainly on the soil sur-face because of its low mobility in soil. It would be
expected to volatilize from the surface due to its high vapour pressure.
The half lives for oxadiazon in the literature var-ied greatly from 15 days to 180 days (Wauchope et al., 1991). Its dissipation is largely influenced by the climate or soil moisture (Barrett and Lavy, 1984). Ambrosi et al. (1977) found that less than 25% of oxadiazon was degraded after 175 days under both moist (75% field capacity) and flooded conditions. Barrett and Lavy (1984) found that, in the field, 50% of the surface-applied oxadiazon dissipated from the soil within 6–11 days when soil was flush irrigated and then flooded, compared to 15–17 days when the soil was irrigated but not flooded in two rice man-agement systems. Similarly, in this present study, in 1997 a half-life of 14 days for oxadiazon was found. The short field half-life in the vineyard was attributed to the fact that there was no incorporation of the her-bicide into soil coupled with its rapid degradation in moist soil.
According to the Herbicide Handbook (Humburg et al., 1989), the half life for oxyfluorfen is about 30–40 days. In this study, in 1996, a half-life of 119 days for oxyfluorfen in the Mountadam vineyard soil was determined. In soils, oxyfluorfen is not subject to microbial degradation and is not subject to hydrolysis at pH 5–9 (Tomlin, 1994). It is, therefore, highly re-sistant to degradation in soil. The sorption coefficient of oxyfluorfen indicates that oxyfluorfen can strongly adsorb to soil particles. So the dissipation of oxyflu-orfen was most likely due to volatilisation from the soil surface.
Therefore, the different half lives for the herbicides are controlled by many factors including their own physico-chemical properties and environmental con-ditions such as soil moisture, temperature and organic matter. As Aylmore et al. (1995) has pointed out, the order of ranking in dissipation may also change given a different set of environmental conditions, as each chemical may respond differently to dissipation in the new environment.
3.2. Dissipation of herbicides on grapes
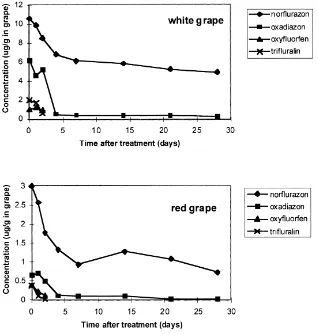
sur-Fig. 2. Residues of herbicides in grapes from the Roseworthy campus vineyard.
faces (Fig. 2). Trifluralin and oxyfluorfen were not detected in grapes from the Roseworthy campus vine-yard 4 days after treatment. However, norflurazon and oxadiazon residues remained in grapes for as long as one month following treatment. So the dissipation of the four herbicides was different on grapes.
The dissipation rate of a chemical on grape surfaces depends on many factors such as the inherent abil-ity of the chemical to adhere to the grape surface, the physico-chemical properties of the chemical, the tex-ture of the grape surface including surface waxes and the climatic conditions. During the experiments in the Roseworthy campus vineyard there were no rainfall events, so the environmental (climatic) factors operat-ing in this study were wind and sunlight. In the Rose-worthy campus vineyard, the grapevines were well pruned and canopy thinned and hence grapes were
di-rectly exposed to sunlight. The decay rates of herbi-cides on grapes in the Roseworthy campus vineyard might be higher than those vines having abundant vine leaves, because the vine canopy protects grapes from exposure to sunlight.
4. Conclusions
This study shows that dissipation of herbicides in soil and grapes depends on the physico-chemical prop-erties of the herbicides and the environmental condi-tions. The field dissipation half-lives of herbicides in the vineyard soil varied from 14 days for oxadiazon to 112 days for oxyfluorfen. The dissipation of herbi-cides on grapes was different from that in soil. Nor-flurazon and oxadiazon persisted on grapes for more than 1 month, while trifluralin and oxyfluorfen disap-peared within several days after treatment.
The findings from this study emphasize the need for care when using persistent herbicides to control weeds in vineyards in order to minimise the impact on the environment, vines and grapes. To reduce possible residues in soil and vine damage, it is better to select short-residual herbicides and use the lowest available application rate in vineyards. Herbicide residues can occur on grapes if too much herbicide is applied to the vines, or if the herbicide is applied too close to harvest. It is also important to apply herbicides during optimum weather conditions in order to avoid spray drift of herbicides and movement of the herbicides caused by heavy rains following the application.
References
Ambrosi, D., Kearney, P.C., Macchia, J.A., 1977. Persistence and metabolism of oxadiazon in soils. J. Agric. Food Chem. 25, 868–872.
Aylmore, L.A.G., Kookana, R.S., Di, H.J., 1995. Pesticide leaching and model evaluation under field conditions. In: Heatwole, C. (Eds.), Water Quality Modeling. Proceedings of the International Symposium, American Society of Agricultural Engineers, pp. 128–136.
Barrett, M.R., Lavy, T., 1984. Effects of soil water content on oxadiazon dissipation. Weed Sci. 32, 697–701.
Braunschweiler, H., 1992. The fate of some pesticides in Finnish cultivated soils. Agric. Sci. Finland 1, 37–55.
Cabras, P., Garau, V.L., Melis, M., Pirisi, F.M., Spanedda, L., 1995. Pesticide residues in Italian wines. Ital. J. Food Sci. 2, 133–145.
Cabras, P., Meloni, M., Pirisi, F.M., 1987. Pesticide fate from vine to wine. Rev. Environ. Contam. Toxicol. 99, 84–117. Carringer, R.D., Weber, J.B., Monaco, T.J., 1975.
Absorption-desorption of selected pesticides by organic matter and montmorillonite. J. Agric. Food Chem. 23, 568–572. Eleftherohorinos, I.G., Kotoula-Syka, E., 1990. Field persistence
of dinitramine, trifluralin and pendimethalin in soils in Greece. Agricoltura-Mediterranea 120, 256–261.
Frank, R., Clegg, S., Ritcey, G., 1991. Disappearance of oxyfluorfen (Goal) from onions and organic soils. Bull. Environ. Contam. Toxicol. 46, 485–491.
Golab, T., Althaus, W.A., Wooten, H.L., 1979. Fate of 14C trifluralin in soil. J. Agric. Food Chem. 27, 163–179. Hubbs, C.W., Lavy, T.L., 1990. Dissipation of norflurazon and
other persistent herbicides in soil. Weed Sci. 38, 81–88. Humburg, N.E., Colby, S.R., Lym, R.G., Hill, E.R., McAvoy, W.J.,
Kitchen, L.M., Prasad, R., 1989. Herbicide Handbook, 6th ed. Weed Science Society of America, Illinois.
Jolley, A.V., Johnstone, P.K., 1994. Degradation of trifluralin in three Victorian soils under field and laboratory conditions. Austr. J. Exp. Agric. 34, 57–65.
Rahn, P.R., Zimdahl, R.L., 1973. Soil degradation of two phenyl pyridazinone herbicides. Weed Sci. 21, 314–317.
Schroeder, J., Banks, P.A., 1986a. Persistence of norflurazon in five Georgia soils. Weed Sci. 34, 595–599.
Schroeder, J., Banks, P.A., 1986b. Persistence and activity of norflurazon and fluridone in five Georgia soils under controlled conditions. Weed Sci. 34, 599–606.
Soil Survey Staff., 1975. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys. USDA Agric. Handbk. No. 436. Govt. Printer, Washington DC. Southwick, L.M., Willis, G.H., Bengtson, R.L., 1993. Runoff losses
of norflurazon: effect of runoff timing. J. Agric. Food Chem. 41, 1503–1506.
Tomlin, C.(Ed.), 1994. The Pesticide Manual — Incorporating the Agrochemicals Handbook 10th ed. Crop Protection Publications, pp. 740–741, 753–754, 764–765, 913–914, 1025–1026.
Watterson, A., 1991. Pesticides and Your Food. Green Print, London. pp. 65–99.
Wauchope, R.D., Buttler, T.M., Hornsby, A.G., Augustijn-Beckers, P.W.M., Burt, J.P., 1991. The SCS/ARS/CES pesticide properties database for environmental decision-making. Rev. Environ. Contam. Toxicol. 123, 1–164.
Ying, G.-G., Williams, B., 1999a. Photodegradation of norflurazon in water. Toxicol. Environ. Chem., in press.