L
Journal of Experimental Marine Biology and Ecology 242 (1999) 41–57
www.elsevier.nl / locate / jembe
Effects of contaminated sediments on particle size selection
by the polychaete Capitella sp. I
* Ching-Yi Horng, Gary L. Taghon
Rutgers University, Institute of Marine and Coastal Sciences, 71 Dudley Road, New Brunswick,
NJ08901-8521, USA
Received 4 January 1999; received in revised form 28 May 1999; accepted 28 June 1999
Abstract
Capitellid polychaetes are generally considered opportunistic species, characteristic of or-ganically enriched and disturbed habitats. The feeding behavior of capitellids dramatically affects physical properties of sediments by repackaging sediment particles into large fecal pellets that persist for years. Particle size selection by Capitella sp. I was quantified using two approaches. Organic-rich sediments from four locations subjected to varying degrees of anthropogenic disturbance were used in the first approach. Sediment contamination, measured as the con-centrations of selected polycyclic aromatic hydrocarbons (PAHs) varied from 0.08 to 31 ppm. The median size of mineral particle ingested was ¯4 mm for all sediments. Worms selectively ingested the smallest particles in the most contaminated sediment. Because of potential ambiguity in obtaining accurate measurements of in situ particle sizes of natural sediments, a second experiment used glass beads as tracers added to natural sediment. Treatments consisted of phenanthrene added at six concentrations, from 0.07 to 13 ppm. Worms preferentially fed on small beads (most preferred particle size 1764mm) in all treatments, and selection was unaffected by phenanthrene concentrations. Selective feeding on the finest sediment particles and their incorporation into long-lived fecal pellets may affect the persistence of organic contaminants in sediments. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Capitella; Particle selection; PAHs
1. Introduction
Important insights concerning the effects of benthic deposit-feeding macrofauna on
*Corresponding author. Tel.:11-732-932-6555 ext. 547; fax:11-732-932-8578.
E-mail address: [email protected] (G.L. Taghon)
sediment-bound organic contaminants can be gained by quantifying selection by the organism for different particle types. Contaminants associated with preferred particles will have a higher probability of being ingested, packaged into fecal material, and kept in the bioturbated zone of the sediment column (e.g. Jumars et al., 1981). Once contaminants are ingested by the deposit-feeder, they may be metabolized by the animal itself (McElroy, 1985; McElroy et al., 1990) or by bacteria present in the hind-gut of the animal (Plante et al., 1990). Particle-bound contaminants can also be passed out of the gut and incorporated in fecal pellets. The feces of invertebrates can be sites of elevated microbial activity (Hargrave, 1976); higher organic contents of feces relative to ambient sediment, resulting from selective feeding, is one possible cause.
Selective feeding has been documented in a wide variety of deposit feeders. Despite considerable variations among taxa in feeding mode and feeding appendages, deposit feeders generally preferentially ingest smaller, low-density particles (for review, see Taghon, 1989), although exceptions exist (e.g. Whitlatch and Weinberg, 1982). Further studies have shown that surface properties of particles, such as roughness and presence of an organic coating, strongly influence particle selection in species that use tentacles to collect particles (Self and Jumars, 1978, 1988; Jumars et al., 1982; Taghon, 1982). The effects of particle-bound contaminants on particle selection are unexplored. Con-centrations of organic contaminants such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) are often much greater in smaller size and
´
low-density fractions of sediments (Prahl and Carpenter, 1983; Pierard et al., 1996; Horng, 1998). Hence, knowledge of the selectivity of deposit feeders for different types of particles is important in predicting the long-term fate of contaminants in the marine environment.
The impact of fecal pellet production by polychaetes of the genus Capitella could be especially significant for the fate of organic contaminants. Capitella capitata, originally believed to be a single species, is now known to consist of a complex of species that differ in life history characteristics (Grassle and Grassle, 1976). Members of the
Capitella sibling species complex are often the dominant inhabitants of organically
There have been numerous studies of the effects of sediment chemical composition on the feeding and growth rates and reproductive output of Capitella spp. (Tenore, 1977;
´
Gremare et al., 1988; Marsh et al., 1989; Forbes and Lopez, 1990; Tsutsumi et al., 1990; Bridges et al., 1994). Only one previous study has quantitatively evaluated particle selectivity by Capitella spp., and that study used uncontaminated sediments (Self and Jumars, 1988). In this study, we used two different approaches to examine the influence of contaminants on particle selection by Capitella sp. I. The first approach tested whether particle selection on natural sediments varied with the degree of contamination. Organically enriched sediments from four different collection sites were used. Particle selection was examined by comparing the particle size distributions in fecal pellets with those in the sediments. This approach provided information about the sizes of mineral particles being ingested from natural sediments. From the perspective of testing particle selection, however, this method is relatively insensitive with respect to small particles, due to the very great abundance of small particles in natural sediments. The second approach was to determine the effect of a single PAH on particle selection. Phenan-threne-spiked sediments were used for this selection experiment. Glass beads were used as a tracer to evaluate size selection, because they offer the advantage of testing preference for different particle sizes with equal sensitivity (Self and Jumars, 1988).
2. Materials and methods
2.1. Particle selection on natural sediments
2.1.1. Sediment sources and properties
Relatively uncontaminated sediments were obtained from the intertidal zones of two
Spartina-dominated salt marshes. Sediment from Sippewissett Marsh, on Cape Cod,
Massachusetts, is used for laboratory culturing of Capitella sp. I (Grassle et al., 1992) and was a generous gift from Dr J.P. Grassle. Sediment was collected from Schooner Creek, a tidal creek in an extensive salt marsh surrounding the Rutgers University Marine Field Station in Tuckerton, New Jersey. Moderately contaminated sediment was obtained from a subtidal site in Barnegat Bay, New Jersey. The sampling spot was in a bottom depression (depth 6.7 m) where fine-grain sediments and organic matter accumulated. Anoxic conditions existed in the summer and no benthic infauna were present. During the winter, the water was oxygenated and Capitella spp. were the only macrofauna identified in the sediments. Since the site is bordered on two sides by marinas (serving commercial fishing boats, sail, and power pleasure boats), the major contaminant inputs are probably contributed by boating activities. The most heavily contaminated sediments were obtained from Piles Creek (40836.539N, 74813.69W), a tidal creek emptying into the Arthur Kill, the navigational channel between New Jersey and Staten Island, New York. Piles Creek is in the center of an industrial park, surrounded by petroleum refineries and oil storage tanks.
NA-1500 elemental analyzer. Sediment protein content was estimated using the method of Mayer et al. (1995), a biomimetic assay of digestible protein based on direct incubation of sediments with a proteolytic enzyme. The single-point method with a 6-h incubation was used. PAHs were analyzed by placing an aliquot of approximately 1 g dry weight sediment into a 50-ml Teflon centrifuge tube. Methylene chloride (15 ml, Burdick & Jackson, HPLC / GC grade) was added and the tube was placed into a 408C ultrasonic bath for 30 min. The extract was separated from sediment by centrifugation at 88203g for 15 min and decanted into a 50-ml evaporation flask. These steps were
repeated two more times and the three successive extracts were combined. Activated copper turnings (pre-oxidized with 1 N HCl and cleaned with methylene chloride) were added to the evaporation flask to remove elemental sulfur. This ultrasonic extraction technique had an average efficiency of 87% compared with a 60-cycle Soxhlet extraction (Horng, 1998). The extract was reduced in volume to |2 ml using a rotary evaporator
under a slight vacuum (250 kPa) at 358C. The extract was quantitatively transferred to an amber vial and further reduced in volume to |0.5 ml under a stream of high purity
nitrogen. The extract was then solvent-exchanged to acetonitrile by adding 2 ml of acetonitrile. The mixture was reduced again to |0.5 ml with a nitrogen stream and
moderate heating (608C). After volumetric quantification with a 1-ml microsyringe, the acetonitrile extract was transferred to an autosampler vial for HPLC analysis.
PAHs were separated and quantified with a Hewlett-Packard (Rockville, MD) 1050 series HPLC equipped with a binary gradient elution control, a diode array detector (set at 254 nm), a C18reversed phase column (Vydac, Hesperia, CA, USA; 25034.6 mm, 5
mm), and a guard column (Vydac, 2034.6 mm, 5 mm). The column was initially equilibrated with a 50% v / v acetonitrile / water mixture for at least 15 min. PAHs were separated using a gradient elution in which the solvent linearly increased to 100% acetonitrile after 50 min and remained isocratic for another 5 min. The flow rate was 1
21
ml min . Peaks were identified by comparing retention times and spectra of samples with those of 16 PAH standards (TCL PAH mix standard, Supelco, Bellefonte, PA). The PAHs were then quantified by calibration curves developed in advance using known concentrations of standards. Although a PAH standard mixture with 16 EPA priority compounds was used as the reference, only ten PAHs from the sediment samples are reported here. These ten (phenanthrene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, ben-zo[ ghi]perylene, and indeno[1,2,3-cd]pyrene) were selected because their quantification could be reasonably achieved (within 30% variation) upon analyzing a standard marine sediment (NIST SRM [1941a).
2.1.2. Selection experiments
test sediment (five replicates each) and allowed to feed for 7 days in a 158C environmental chamber. Fecal pellets were separated from residual, uneaten sediment by sieving with a 90-mm mesh. Pellets were completely disaggregated by treatment with 30% (w / v) hydrogen peroxide in an ultrasonic bath. Samples were taken from the sediment stocks used to feed the worms and treated similarly.
Size–frequency distributions of particles in pellets and ambient sediment were determined using a laser-based particle counter (Spectrex, Redwood City, CA), which analyzes the forward scattering interference patterns created by illuminating suspended particles with a laser beam. Particles were kept in suspension by a magnetic stirring bar in a 250-ml beaker. Calgon (Benckiser, Danbury, CT), a commercial detergent, was added to disperse particles in the suspension. Potential interference from background particles was prevented by the use of 0.2-mm filtered, ultra-pure deionized water and by background subtraction. The system was configured to detect particles between 1 and 100 mm in diameter, and a maximum count of 800 particles was set to reduce the probability of more than one particle simultaneously passing through the laser beam. Particle counts and sizes were within 5% of actual values, based on analysis of NIST calibration standards.
2.1.3. Data analysis
Data were analyzed following the methods of Petch (1986). The particle counter categorized particles into 33 size classes. Particle size–frequency distributions of fecal pellets and ambient sediment were compared by G-test. Size categories causing significant differences between size–frequency distributions were identified by compar-ing values of the adjusted residuals for each size category to 1.96, the value of the 5% standard normal deviate (Petch, 1986). The adjusted residual also provides information about the direction and strength of selection, because it is symmetrically distributed about zero.
2.2. Effect of phenanthrene on particle selection
2.2.1. Sediments and glass bead tracers
Schooner Creek sediment was used for this study. This sediment was relatively clean, in terms of PAHs, but was high in organic matter. Glass beads were used as a particle tracer. Glass beads from four stocks (1–20, 28–53, 53–74, and 88–105 mm, specific
23
gravity52.42 g cm , Cataphote Corporation, Jackson, MS) were sieved and re-combined to give roughly equal numbers of beads in consecutive 15-mm size intervals:
,15, 15–30, 30–45, 45–60, 60–75, 75–90, and 90–105 mm. The bead mixture was soaked in laboratory glassware detergent, rinsed thoroughly, boiled in one change of deionized water, rinsed twice with acetone, and finally rinsed with deionized water.
Approximately 1:1 (w / w) of sediment and beads were placed into six 250 ml glass flasks. The mixtures were equilibrated on an orbital shaker (100 rpm) with filtered seawater (1.2 mm glass microfibre filter, Whatman grade GF / C) for 24 h. Different concentrations of phenanthrene (Sigma, St. Louis, MO, .96% HPLC) were prepared in acetone to achieve nominal PAH concentrations of 0, 1, 5, 10, 50, and 100 mg (g
21
and shaken (100 rpm) for 24 h. To avoid interference of dissolved and particulate phenanthrene, sediment–bead mixtures were rinsed twice with filtered seawater follow-ing the equilibration. Samples were taken from each mixture for quantifyfollow-ing phenan-threne concentration and for estimating the size distribution of particles.
2.2.2. Selection experiment
Juvenile worms (4–5 weeks old) were used in this experiment. After measuring its size, each worm was randomly assigned to a glass vial (20 ml, 2.5 cm in diameter) containing 2 g (wet wt) test sediment and 10 ml seawater (32‰, 1-mm filtered). Five vials were prepared for each treatment. Vials were kept in a 158C environmental chamber for 7 days. Worms, pellets, and residual sediments were subsequently separated by sieving on a 90-mm mesh.
Particle sizes in ambient sediment and fecal pellets were measured using different procedures. Ambient sediment samples (|0.5 g) were placed into small test tubes with 2
ml 30% (w / v) hydrogen peroxide, then placed into an ultrasonic bath. Following disaggregation, each tube was vigorously mixed on a vortex mixer. A 50-ml aliquot was immediately withdrawn from the middle of the tube. The particle sample was then placed on a micro-slide with a few drops of 50% glycerol solution. Particles were spread evenly by camel-hair brush. Three slides were prepared for each sediment sample. For fecal pellet samples, ten pellets were haphazardly picked from the group of pellets produced by each individual worm (total of 50 pellets per treatment). Each pellet was transferred to a depression micro slide. After measuring the major and minor axes of the pellet under a dissecting microscope, the pellet was disaggregated by adding a few drops of 30% hydrogen peroxide and letting the reaction proceed for at least 24 h.
Glass beads were counted and measured using a compound microscope (Zeiss Axioplan) interfaced with a computer-based image analysis system (Sony DXC-750 image controller, Sony 3CCD camera, Macintosh IICi computer). On the microscope, a blue light filter, 2503 magnification, and phase-contrast optics were used. For the ambient sediment samples, at least ten fields and a minimum of 100 glass beads were measured on each slide. These ten fields were arbitrarily spread out to cover more area on the slide. For the pellet samples, the whole slide was scanned. The maximum and minimum diameters of glass beads were measured (ColorImage v1.36, National Institutes of Health) and used to calculate the mean diameters.
2.2.3. Data analysis
Particles were classified into seven size classes (from ,15 to 90–105 mm), each spanning a 15-mm interval. In addition to analyzing data with the G-test and adjusted residuals, the log of the odds ratio (LOR) was used as the selectivity index for estimating the strength of selection (Cock, 1978) and testing the treatment effect with a multiple regression of LOR against particle sizes (Self and Jumars, 1988). The LOR is a symmetric index, with positive values indicating preference for the particle and negative values indicating avoidance. A value of 1 indicates that a particle is taken ten times more frequently than would occur with no selection.
effect of phenanthrene concentration using a particle preference model modified after Self and Jumars (1988):
2
LOR5a1b log10D1c logs 10Dd 1d C
s
phenanthrened
1ewhere D is the mean test particle diameter, Cphenanthrene is the concentration of phenanthrene in sediment, and e is the residual error. Self and Jumars (1988) used geometric mean diameter within a size interval to represent the mean size of the particles within that size range. The use of the geometric mean presumes a lognormal size distribution, which is largely true for natural sediments. For this selectivity experiment, the glass bead mixture was made from four types of bead stocks, each distributed in a rather narrow range (about 20mm). The size distributions within every 15-mm wide size category may not be continuous and lognormal. Therefore, the mean size of particles for each size interval was estimated by pooling all size data collected from ambient sediments and fecal pellets, and then averaging every single particle within the size range for the mean. The mean diameters for,15, 15–30, 30–45, 45–60, 60–75, 75–90, and 90–105-mm intervals were 12.7, 20.7, 39.1, 51.3, 64.1, 86.6, and 94.2 mm, respectively. The method of least-squares was used to determine the values of the coefficients a, b, c, and d in the multiple curvilinear model. The most preferred particle size and its variance were estimated as in Self and Jumars (1988).
3. Results
3.1. Particle selection on natural sediments
All four sediment had high, similar concentrations of total C and total N (Table 1). Protein concentrations were also similar in the sediments from Sippewissett Marsh, Schooner Creek, and Barnegat Bay; Piles Creek sediment had lower protein. There were obvious differences in the concentrations of the measured PAHs, reflecting the expected patterns based on location of the sampling sites.
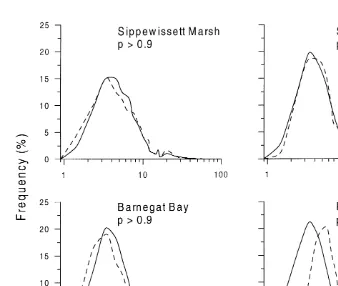
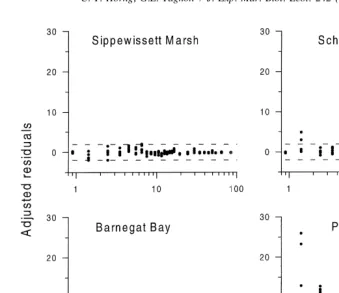
The size–frequency distributions of ingested particles and available particles were not significantly different ( p .0.9) for Sippewissett Marsh, Schooner Creek, and Barnegat Bay sediments, but were significantly different ( p,0.001) for the Piles Creek treatment (Fig. 1). The adjusted residuals in the Sippewissett Marsh, Schooner Creek, and Barnegat Bay treatments were mostly within the 5% deviation from zero and indicated
Table 1
a
Total C, total N, protein, and PAH concentrations in natural sediments
21 21 21 21
Sediment C (mg g ) N (mg g ) Protein (mg g ) PAH (mg g )
Sippewissett Marsh 50.1 (0.12) 4.78 (0.24) 2.68 (0.11) 0.08 (0.03)
Schooner Creek 45.7 (0.19) 4.48 (0.32) 2.67 (0.12) 0.83 (0.09)
Barnegat Bay 41.6 (0.22) 4.35 (0.07) 2.94 (0.20) 5.40 (0.56)
Piles Creek 44.6 (0.45) 3.32 (0.09) 1.61 (0.07) 31.1 (5.34)
a
Fig. 1. Size–frequency distributions of available (dashed lines) and ingested (solid lines) particles in selection experiment with four natural sediments. The p values indicate probability that the two distributions are equivalent (G-test).
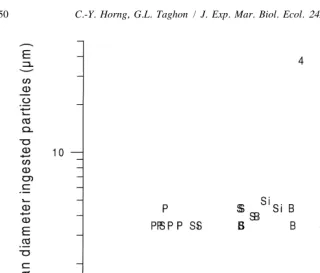
no selection for particles ,100mm (Fig. 2). The very smallest particles of Piles Creek sediment (a few micrometers in diameter) were selected for, and the larger particles were ingested indiscriminately. There was no evidence that the size of worms affected particle selection (Fig. 3).
3.2. Effect of phenanthrene on particle selection
Phenanthrene concentrations in the spiked sediments were only about 10% of the target concentrations (Table 2). Some phenanthrene was probably lost during the rinsing
21
procedure. Phenanthrene is relatively soluble in water (1290mg l ; Mackay and Shiu, 1977) and much of it could have been lost by desorption. The amounts of phenanthrene were, however, comparable to the levels in natural sediments from several sites (Table 2).
Fig. 2. Adjusted residuals for comparison of frequency of particles ingested to frequency of particles available in selection experiment with four natural sediments. The regions enclosed by dashed lines are the 5% deviates from no selection.
Multiple regression analysis indicated no significant contribution of phenanthrene
21
concentration (at levels up to 13 mg g ) to the quadratic model for particle selection (Table 3). The model was improved by a mere 0.1% with the addition of phenanthrene concentration. No significant difference in median size of ingested beads among treatments (ANOVA, F5,2451.47, p .0.2) also indicated that particle selection was independent of phenanthrene concentration over the range used in these experiments. Because the ANOVA (Table 3) indicated that the quadratic model could not explain the data completely (lack of fit, p,0.001), we can only conclude that there was insufficient evidence to reject the null hypothesis of no effect of phenanthrene on particle selection.
2
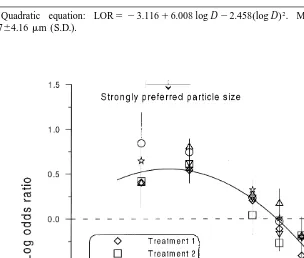
The coefficient of determination (r ) for the regression, on the other hand, indicated that the quadratic model accounted for a reasonable amount (78%) of the variability in the data (Fig. 6). Assuming this model was valid to describe the particle preference of
Fig. 3. Median diameter of ingested particles versus body size of Capitella sp. I in selection experiments with four natural sediments (Si, Sippewissett Marsh; S, Schooner Creek; B, Barnegat Bay; P, Piles Creek) and with glass bead–sediment mixtures (numbers refer to treatments).
Table 2
Concentration of phenanthrene in experimental sediments and field sediments collected from coastal sites in
a
New Jersey and New York
21
Sediment Concentration (mg g ) Target concentration
Particle selection experiment
Treatment 1 0.07 (0.03) 0
Treatment 2 0.15 (0.02) 1
Treatment 3 0.67 (0.03) 5
Treatment 4 1.16 (0.13) 10
Treatment 5 4.81 (0.04) 50
Treatment 6 13.0 (0.16) 100
Field sediments
Barnegat Bay 0.35 (0.05)
b
Arthur Kill (main channel) 0.65 (0.16)
Piles Creek 0.89 (0.18)
b
Newtown Creek, East River 9.33 (0.85)
a
Means (S.D.) based on three replicates.
b
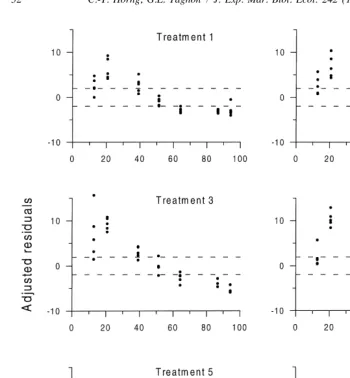
Fig. 4. Size distributions of available particles (dashed lines) and ingested particles (solid lines) in selection experiment with glass bead–sediment mixtures spiked with phenanthrene. The p values indicate probability that the two distributions are equivalent (G-test).
4. Discussion
Capitella spp. are subsurface deposit feeders and use an eversible, sac-like pharynx to
Fig. 5. Adjusted residuals for comparison of frequency of particles ingested to frequency of particles available in selection experiment with glass bead–sediment mixtures spiked with phenanthrene. The regions enclosed by dashed lines are the 5% deviates from no selection.
Table 3
ANOVA results for the multiple regression of LOR, the best fit quadratic equation, and the most preferred
a
particle size
2
Source SS df MS F ratio p Model parameter r (adjusted)
Estimate S.E.
Constant a5 23.116 0.6007
Model 49.81 3 16.60 237.96 ,0.001
Extra for linear 43.35 1 43.35 621.30 ,0.001 b56.008 0.8007 0.683 Extra for quadratic 6.29 1 6.29 90.21 ,0.001 c5 22.458 0.2587 0.781
Extra for phenanthrene 0.17 1 0.17 2.41 0.123 0.783
Residual 13.54 194 0.07
Lack of fit 6.64 38 0.17 3.96 ,0.001
Experimental error 6.90 156 0.04
Total 63.35 197
aQuadratic equation: LOR5 23.11616.008 log D22.458 log D . Most preferred particle sizes d2 5
16.764.16mm (S.D.).
Capitella sp. I preferentially ingested smaller particles from the most heavily
contaminated field sediment; particles in less contaminated sediments were ingested indiscriminately. No parallel result was seen when mixtures of glass beads and relatively clean sediment were spiked with varying concentrations of phenanthrene; smaller particles were consistently ingested preferentially. There are at least two possible explanations for these apparently disparate results. One is that the sizes of worms used here ingest particles only within a narrow size range. Morphological constraints, such as the geometry of the feeding apparatus, could be responsible. Supporting this explanation are the essentially identical size–frequency distributions of ingested particles from the four natural sediments (Fig. 1). These distributions were not significantly different from each other ( p.0.9, pairwise comparisons by G-test). The lack of significant selection when feeding on the uncontaminated (Sippewissett Marsh and Schooner Creek) and moderately contaminated (Barnegat Bay) sediments was therefore due to fortuitous similarities in the size–frequency distributions of available particles to those ingested by the worms (Fig. 1). The significant selection observed on Piles Creek sediment was a result of the slight shift in the size–frequency distribution of available particles to the right, rather than to contaminant–mucus interaction or behaviorally mediated shifts in particle selection.
An alternative explanation is that the size–frequency distribution of sonicated and peroxide-treated field sediments does not accurately reflect that of sediments in situ, especially for the Piles Creek sediment. High levels of PAHs (and other organic contaminants not measured here) in Piles Creek sediment could have caused hydro-phobic coagulation of the smallest particles into aggregates or onto larger grains, which were then ingested. Subsequent treatment for size analysis would break down these aggregates to the constituent grains.
The experiment using mixtures of glass beads and Schooner Creek sediment was conducted to help alleviate uncertainty over the actual sizes of ingested particles. Patterns of glass bead size selection were remarkably similar in the six sediment treatments (Fig. 4). There was no indication that phenanthrene concentration over the range 0.07–13 ppm affected particle selection. Worms always preferentially ingested smaller particles and avoided larger particles (Fig. 5). These results suggest that the more likely explanation for the results of the first experiment was consistent ingestion of a ‘fixed’ size range of particles, independent of contaminant concentration. The similarity with which Capitella sp. I processes glass beads over a range of phenanthrene concentration supports the use of beads to measure mixing rates of contaminated sediments by this species, as done recently by Madsen et al. (1997). The possibility of hydrophobic coagulation of small particles at high contaminant levels cannot be completely ruled out, however. Only a single PAH was present in the glass bead– sediment mixtures used in the second experiment, while the field sediments in the first experiment contained a mixture of contaminants. Piles Creek sediments, in particular, contain a variety of organic (e.g. PAHs, PCBs) and inorganic (e.g. Hg, Zn, Pb) contaminants (Weis et al., 1981) that could act synergistically. In addition, the total concentration of all these contaminants in the Piles Creek sediments was greater than the highest concentration of phenanthrene added to the glass bead–sediment mixture.
experiment using glass bead tracers indicated a median size nearer to 40 mm (Fig. 3). Although the sizes of worms used in the two experiments differed somewhat, the obvious break in median size of ingested particles suggested that body size was not the explanation. As previously discussed the actual sizes of particles that worms experienced while feeding on natural sediments cannot be known unambiguously, because of potential aggregation of small particles. The estimate of a median glass bead size of 40
mm and the occasional presence of beads larger than 90mm in fecal pellets showed that
Capitella sp. I can ingest large particles (Fig. 4). The prediction of the quadratic model
of maximum preference for particles in the size range 12–20mm with a peak at 17mm (Fig. 6) was similar to the result of Self and Jumars (1988). They derived a preference range of 2–20mm for Capitella capitata, the collective name used for the sibling species group of which Capitella sp. I is a member (Grassle and Grassle, 1976).
Body-size-dependent particle selection has been observed in within-species (Taghon, 1982; Shimeta, 1996; Hentschel, 1996) and among-species comparisons (Self and Jumars, 1988). Most observations have been on tentaculate feeders. Larger individuals usually feed on larger particles, with the exception of very small tentacle feeders, whose tentacle diameters approach the grain sizes ingested (Hentschel, 1996). There was little indication that body size affected particle selection by the Capitella sp. I used in our experiments (Fig. 3). Uncertainty about the actual sizes of particles experienced by worms when feeding on natural sediments may have affected this conclusion, however. Glass bead tracers allow better estimates of the size of particles actually experienced by worms. The size of ingested beads also showed no relationship with body size (Fig. 3). Body size may have less effect on particle selection by non-tentaculate deposit feeders, such as Capitella sp. I.
In conclusion, Capitella sp. I selectively ingested the smallest particles in sediments. Selectivity was not affected by the degree of contamination of the sediments. Therefore, the finest particles, potentially carrying the greatest mass-specific concentration of contaminants, will be packaged into long-lived fecal pellets. Release of hydrophobic organic contaminants from intact pellets of tubificid oligochaetes is greatly reduced (Karickhoff and Morris, 1985), but effects of other taxa are largely unknown. We are presently investigating the fate of PAHs incorporated into fecal pellets of Capitella sp. I.
Acknowledgements
Comments by P.A. Jumars and an anonymous reviewer substantially improved an earlier version of the manuscript. This research was supported by a University Research Initiative to Rutgers University from the Office of Naval Research (grant N00014-92-J-1888).
References
Cock, M.J.W., 1978. The assessment of preference. J. Anim. Ecol. 47, 805–816.
Drake, D.E., 1996. Fecal pellets in effluent-affected sediment on the Palos Verdes Margin. Trans. Am. Geophys. Union 76, 49.
Fauchald, K., Jumars, P.A., 1979. The diet of worms: a study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 17, 193–284.
Forbes, T.L., Lopez, G.R., 1990. The effect of food concentration, body size, and environmental oxygen tension on the growth of the deposit-feeding polychaete, Capitella species 1. Limnol. Oceanogr. 35, 1535–1544.
Gallagher, E.D., Keay, K.E., 1998. Organism-sediment-contaminant interactions in Boston Harbor. In: Stolzenbach, K.D., Adams, E.E. (Eds.), Contaminated Sediments in Boston Harbor, MIT Sea Grant College Program, Cambridge, MA, pp. 89–132.
Grassle, J.F., Grassle, J.P., 1974. Opportunistic life histories and genetic systems in marine benthic polychaetes. J. Mar. Res. 32, 253–284.
Grassle, J.P., Grassle, J.F., 1976. Sibling species in the marine pollution indicator Capitella (Polychaeta). Science 192, 567–569.
Grassle, J.P., Butman, C.A., Mills, S.W., 1992. Active habitat selection by Capitella sp. I larvae. II. Multiple-choice experiments in still water and flume flows. J. Mar. Res. 50, 717–743.
´
Gremare, A., Marsh, A.G., Tenore, K.R., 1988. Short-term reproductive responses of Capitella sp. I (Annelida: Polychaeta) fed on different diets. J. Exp. Mar. Biol. Ecol. 123, 147–162.
Hargrave, B.T., 1976. The central role of invertebrate faeces in sediment decomposition. In: Anderson, J.M., Macfadyen, A. (Eds.), The Role of Terrestrial and Aquatic Organisms in Decomposition Processes, Blackwell, Oxford, pp. 301–321.
Hentschel, B.T., 1996. Ontogenetic changes in particle-size selection by deposit-feeding spionid polychaetes: the influence of palp size on particle contact. J. Exp. Mar. Biol. Ecol. 206, 1–24.
Horng, C.-Y. 1998. Influence of the marine polychaete, Capitella sp. I, on the fate of sediment-bound polycyclic aromatic hydrocarbons: the role of feeding activity. PhD thesis, Rutgers University, New Brunswick.
Jumars, P.A., Nowell, A.R.M., Self, R.F.L., 1981. A simple model of flow-sediment-organism interaction. Mar. Geol. 42, 155–172.
Jumars, P.A., Self, R.F.L., Nowell, A.R.M., 1982. Mechanics of particle selection by tentaculate deposit feeders. J. Exp. Mar. Biol. Ecol. 64, 47–70.
Karickhoff, S.W., Morris, K.R., 1985. Impact of tubificid oligochaetes on pollutant transport in bottom sediments. Environ. Sci. Technol. 19, 51–56.
Lee, H.J., Drake, D.E., Edwards, B.D., Hamer, M.R., Hampton, M.A., Karl, H., Kayen, R.E., Wong, F.W., Murray, C.J., 1996. Contaminated, effluent-affected sediment on the continental margin near Los Angeles, CA. Trans. Am. Geophys. Union 76, 37.
Mackay, D., Shiu, W.Y., 1977. Aqueous solubility of polynuclear aromatic hydrocarbons. J. Chem. Eng. Data 22, 399–402.
Madsen, S.D., Forbes, T.L., Forbes, V.E., 1997. Particle mixing by the polychaete Capitella species 1: coupling fate and effect of a particle-bound organic contaminant (fluoranthene) in a marine sediment. Mar. Ecol. Prog. Ser. 147, 129–142.
´
Marsh, A.G., Gremare, A., Tenore, K.R., 1989. Effect of food type and ration on growth of juvenile Capitella
capitata sp. I (Annelida: Polychaeta): macro- and micronutrients. Mar. Biol. 102, 519–527.
Mayer, L.M., Schick, L.L., Sawyer, T., Plante, C.J., Jumars, P.A., Self, R.L., 1995. Bioavailable amino acids in sediments: a biomimetic, kinetics-based approach. Limnol. Oceanogr. 40, 511–520.
McElroy, A.E., 1985. Physiological and biochemical effects of the polycyclic aromatic hydrocarbon benz[a]anthracene on the deposit feeding polychaete Nereis virens. In: Gray, J.S., Christiansen, M.E. (Eds.), Marine Biology of Polar Regions and Effects of Stress On Marine Organisms, Wiley, Chichester, pp. 527–543.
McElroy, A.E., Farrington, J.W., Teal, J.M., 1990. Influence of mode of exposure and the presence of a tubiculous polychaete on the fate of benz[a]anthracene in the benthos. Environ. Sci. Technol. 24, 1648–1654.
Petch, D.A., 1986. Selective deposit-feeding by Lumbrineris cf. latreilli (Polychaeta: Lumbrineridae), with a new method for assessing selectivity by deposit-feeding organisms. Mar. Biol. 93, 443–448.
´
Pierard, C., Budzinski, H., Garrigues, P., 1996. Grain-size distribution of polychlorobiphenyls in coastal sediments. Environ. Sci. Technol. 30, 2776–2783.
Plante, C.J., Jumars, P.A., Baross, J.A., 1990. Digestive associations between marine detritivores and gut bacteria. Annu. Rev. Ecol. Syst. 21, 93–127.
Prahl, F.G., Carpenter, R., 1983. Polycyclic aromatic hydrocarbon (PAH)-phase associations in Washington coastal sediment. Geochim. Cosmochim. Acta 47, 1013–1023.
Saulnier-Michel, C., 1992. Polychaeta: digestive system. In: Harrison, F.W., Gardiner, S.L. (Eds.), Microscopic Anatomy of Invertebrates: Annelida, Wiley, New York, pp. 53–69.
Self, R.F.L., Jumars, P.A., 1978. New resource axes for deposit feeders? J. Mar. Res. 36, 627–641. Self, R.F.L., Jumars, P.A., 1988. Cross-phyletic patterns of particle selection by deposit feeders. J. Mar. Res.
46, 119–143.
Shimeta, J., 1996. Particle-size selection by Pseudopolydora paucibranchiata (Polychaeta: Spionidae) in suspension feeding and deposit feeding: influences of ontogeny and flow speed. Mar. Biol. 126, 479–488. Stull, J.K., Haydock, C.I., Montagne, D.E., 1986a. Effects of Listriolobus pelodes (Echiura) on coastal shelf benthic communities and sediments modified by a major California wastewater discharge. Estuar. Coast. Shelf Sci. 22, 1–17.
Stull, J.K., Haydock, C.I., Smith, R.W., Montagne, D.E., 1986b. Long-term changes in the benthic community on the coastal shelf of Palos Verdes, southern California. Mar. Biol. 91, 539–551.
Taghon, G.L., 1982. Optimal foraging by deposit-feeding invertebrates: roles of particle size and organic coating. Oecologia 52, 295–304.
Taghon, G.L., 1989. Modeling deposit feeding. In: Lopez, G., Taghon, G., Levinton, J. (Eds.), Ecology of Marine Deposit Feeders, Springer, New York, pp. 223–246.
Taghon, G.L., Nowell, A.R.M., Jumars, P.A., 1984. Transport and breakdown of fecal pellets: biological and sedimentological consequences. Limnol. Oceanogr. 29, 64–72.
Tenore, K.R., 1977. Growth of Capitella capitata cultured on various levels of detritus derived from different sources. Limnol. Oceanogr. 22, 936–941.
Tsutsumi, H., Fukunaga, S., Fujita, N., Sumida, M., 1990. Relationship between growth of Capitella sp. and organic enrichment of the sediment. Mar. Ecol. Prog. Ser. 63, 157–162.
Weis, J.S., Weis, P., Heber, M., Vaidya, S., 1981. Methylmercury tolerance of killifish (Fundulus heteroclitus) embryos from a polluted vs. nonpolluted environment. Mar. Biol. 65, 283–287.
Whitlatch, R.B., Weinberg, J.R., 1982. Factors influencing particle selection and feeding rate in the polychaete