Brain Research 883 (2000) 119–124
www.elsevier.com / locate / bres
Short communication
Possible regulatory role of dendritic spikes in induction of long-term
potentiation at hippocampal Schaffer collateral-CA1 synapses
1
*
Yoshikazu Isomura , Nobuo Kato
Department of Integrative Brain Science, Graduate School of Medicine, Kyoto University, 606-8501 Kyoto, Japan
Accepted 15 August 2000
Abstract
The amplitude of backpropagating action potentials (BAPs) is attenuated, either activity- or neurotransmitter-dependently in the apical dendrite of hippocampal pyramidal neurons. To test the possibility that this BAP attenuation may contribute to regulating the inducibility of long-term potentiation (LTP), BAPs evoked by theta-burst stimulation (TBS), a standard protocol for LTP induction, to apical dendrite synapses were subjected to perturbation by conditioning stimuli to basal dendrite synapses. During this conditioned TBS (cTBS), the amplitude of BAPs was noticeably attenuated, but that of somatic action potentials was not. In the distal dendrite area, cTBS-induced LTP was much smaller than that induced by TBS. By contrast, no difference was observed between TBS- and cTBS-induced LTP in the proximal dendrite area. These findings suggest that the activity-dependent attenuation of BAPs, propagating along the apical dendrite, may
serve to regulate hippocampal synaptic plasticity. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Long-term potentiation: physiology
Keywords: Action potential; Backpropagation; Calcium; CA1 pyramidal cell; Dendrite; Schaffer collateral
In hippocampal and neocortical pyramidal cells, synapti- [21] and serotonin [15]. An enhancement of single BAPs cally evoked action potentials, which initially occur in the by acetylcholine, noradrenalin and dopamine has also been axon, are backpropagated actively into the dendritic tree reported [6]. The functional significance of such regulation [18]. Backpropagating action potentials (BAPs) become of BAPs has been unknown to date.
smaller in amplitude as they are propagated more distally It has become increasingly evident that not just somatic along the apical dendrite of hippocampal CA1 pyramidal action potentials but BAPs are required for induction of cells [17]. Even at the same distance from the soma, the long-term potentiation (LTP) in the hippocampus [11,16] amplitude of BAPs shows a gradual reduction in a (for review see [10]). The recent finding that bursting repetitive spike train [4,17]. This activity-dependent at- action potentials are more effective for hippocampal LTP tenuation of BAPs, attributable to prolonged inactivation induction than single action potentials [13,19] also points
1
of tetrodotoxin-sensitive Na channels [5,8], is regulated to importance of the difference in spatial and temporal by neurotransmitters such as GABA [20], acetylcholine patterns of action potentials within single pyramidal neu-rons during LTP induction. In the present study, we attempted to investigate the functional roles of dendritic *Corresponding author. Tel.: 181-75-753-4661; fax: 181-75-753- and somatic action potentials during LTP induction in
4486. hippocampal CA1 pyramidal cells, with a special focus on
E-mail address: [email protected] (N. Kato). assessing what roles might be assigned to the activity-1
Present address: Department of Neurobiology, Tokyo Metropolitan
dependent regulation of BAPs. The results suggest that the Institute for Neuroscience, 183-8526 Tokyo, and also affiliated to
susceptibility to LTP at distal dendritic synapses may be CREST, Japan Science and Technology Corporation, 332-0012 Saitama,
Japan. switched off by activity-dependent attenuation of BAPs.
120 Y. Isomura, N. Kato / Brain Research 883 (2000) 119 –124
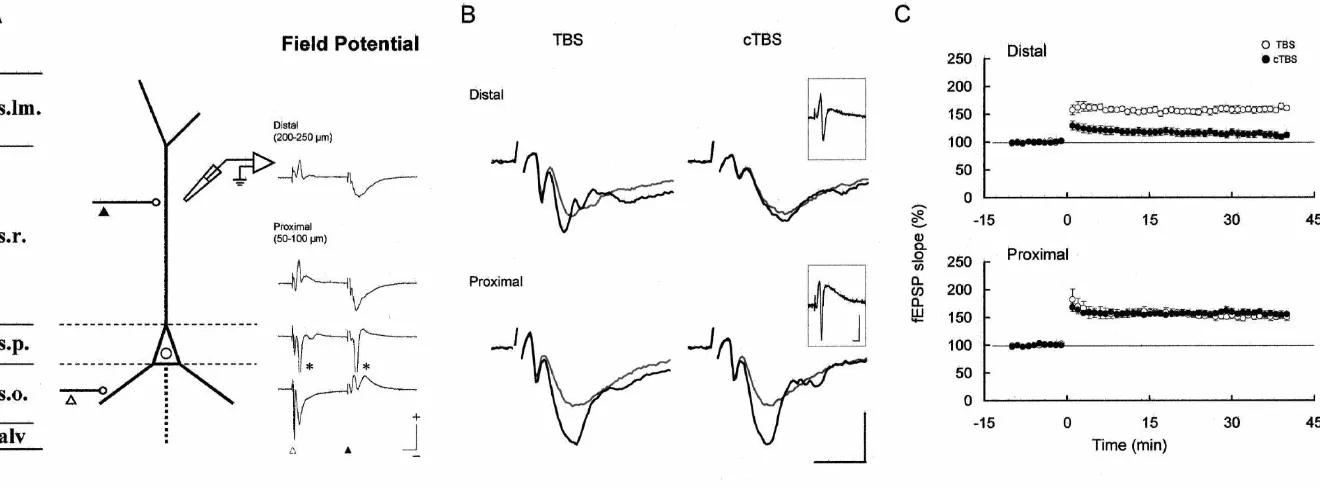
Hippocampal slices (300mm thick) were prepared from ‘distal’ sites of the stratum radiatum of CA1 region ether anesthetized Wistar rats (P18–24) with a microslicer (proximal, 50–100 mm, and distal, 200–250 mm distant (DTK-1000; Dosaka EM, Kyoto, Japan), and allowed to from the stratum pyramidale) [7]. In some experiments, a recover in artificial cerebrospinal fluid (aCSF) at room single recording electrode was penetrated sequentially into temperature for at least 60 min. The aCSF consisted of 124 the distal and proximal sites of stratum radiatum, the mM NaCl, 3.4 mM KCl, 1.3 mM KH PO , 26 mM2 4 stratum pyramidale, and the stratum oriens to obtain the NaHCO , 2.0 mM MgSO , 2.5 mM CaCl3 4 2 and 25 mM depth profile of the current sink. To monitor field EPSPs,
D-glucose and saturated with 95% O –5% CO . Each slice2 2 test pulses were delivered every 12 s with the intensity
was cut between CA3 and CA1 regions and transferred adjusted to evoke field EPSPs ranging from 0.5 to 0.75 mV into a submerged-type recording chamber continuously in amplitude (2.5–5 V, 200 ms). Five consecutive field circulated with aCSF at 308C [7,9]. EPSP slopes were averaged for each data point. LTP was To evoke action potentials antidromically, the axon of induced by two trains of TBS or cTBS at 20-s intervals. the recorded neuron was stimulated repetitively at 12.5 Hz Data are expressed as means6S.D. and Student’s t-test with a tungsten bipolar electrode placed in the alveus was applied for statistical comparison. All experiments (5–12 V, 200 ms), under the blockade of excitatory were carried out in accordance with the Principles of postsynaptic potentials (EPSPs) with 10 mM 6-cyano-7- Laboratory Animal Care (NIH publication No. 86-23, dinitroquinoxaline-2,3-dione (CNQX; RBI, Massachusetts, 1985) and the Guiding Principles for the Care and Use of USA). To evoke action potentials synaptically, we stimu- Animals of the Physiological Society of Japan, 1988. lated the Schaffer collateral fibers, which travel in parallel For somatic recording, microelectrodes were inserted with the stratum pyramidale and form synapses on both the into the stratum pyramidale, which was clearly identified apical and basal dendrites [1]. Bipolar electrodes were by inspection under a dissection microscope. For dendritic placed in the stratum radiatum for stimulation of synapses recordings, neuronal structures in the stratum radiatum on the apical dendrite (s.r. stimulation), and in the stratum were penetrated and it was checked whether action po-oriens for synapses on the basal dendrite (s.o. stimulation), tentials were evoked. The neuronal structures that we respectively (2.5–5 V, 200ms). For induction of LTP at the recorded from were considered to be neuronal somata or Schaffer collateral-CA1 pyramidal cell synapses onto the dendrites rather than axons, since we could record not only apical dendrite, theta-burst stimulation (TBS; 10 bursts at 5 action potentials but also EPSPs. To clarify whether the Hz, with each burst consisting of four pulses at 100 Hz) action potential that we recorded is dendritic or somatic in was given by stimulating the stratum radiatum. In con- origin, the amplitude of action potentials occurring in ditioned theta-burst stimulation (cTBS) protocol, additional response to repetitive antidromic stimulation was examined synaptic stimulation to the basal dendrite in the stratum in the presence of 10 mM CNQX, a potent non-NMDA oriens was delivered twice, at 40 and 80 ms, before each receptor antagonist. The amplitude of action potentials burst of the TBS. recorded in the stratum radiatum was greatly reduced to Intracellular recordings were obtained from soma or 38.2614.0% in an activity-dependent manner (Fig. 1A; apical dendrite of hippocampal CA1 pyramidal cells n55; the first action potentials 59.662.5 mV, the last [22,23]. Sharp glass microelectrodes, made with a mi- 22.868.5 mV; P,0.001). Much the same activity-depen-cropipette puller (P-2000; Sutter Instrument, California, dent spike attenuation was described in the previous USA), were filled with 2.5 M K acetate (100–150 MV). studies [4,17], in which the apical dendrites were visually Somatic membrane potential was recorded from the soma identified before being patch-clamped. On the other hand, in the stratum pyramidale, while dendritic membrane somatic action potentials failed to show this activity-potential was recorded from the apical dendrite in the dependent attenuation of the peak amplitude (Fig. 1A; stratum radiatum (150–250 mm distant from the stratum 94.361.8%, n58; the first 74.865.6 mV, the last 70.665.5 pyramidale), which was identified visually under a dissec- mV). We therefore regarded these recordings from the tion microscope (SMZ-1B; Nikon, Tokyo, Japan). They stratum radiatum as dendritic in origin and, in the present were amplified with an amplifier (IR-183; NeuroData experiments, such activity-dependent spike attenuation was Instruments, New York, USA), lowpass-filtered at 10 kHz, adopted as the criterion for dendritic action potentials and digitized at 5–20 kHz with an A / D interface (Digidata backpropagating into the apical dendrite of CA1 pyramidal 1200; Axon Instruments, California, USA). Identification cells.
of dendritic recordings was based on activity-dependent As the first step in examining the relationship between attenuation of action potentials (see results). In this study, dendritic action potential firing and LTP induction, action resting membrane potentials were 262.461.8 and potentials during TBS, a typical procedure for LTP
Y
.
Isomura
,
N
.
Kato
/
Brain
Research
883
(2000
)
119
–
124
121
122
Y
.
Isomura
,
N
.
Kato
/
Brain
Research
883
(2000
)
119
–
124
Y. Isomura, N. Kato / Brain Research 883 (2000) 119 –124 123
(n55). In somatic recordings, on the other hand, neither neurons [14], which suggested that the s.o and s.r stimula-the burst-by-burst reduction nor stimula-the within-burst reduction tions elicit such differentially localized synaptic events as of the action potential amplitude was observed (n57). In we observed. Also, population spikes were generated in the the present experiments (Fig. 1B, insets), the duration of stratum pyramidale (s.p.) in the present experiments (aster-the second and third action potentials during each burst of isks in Fig. 2A right, s.p.), suggesting that the intensities of the TBS with burst firing was much longer than the first both stimulations were sufficient to evoke action poten-action potential, or than solitary poten-action potentials during tials.
the TBS with non-burst firing. Such a longer duration of LTP induction at Schaffer collateral synapses was large depolarization may help postsynaptic neurons to attempted by TBS or cTBS protocol, and assessed by surpass the voltage threshold for LTP induction during recording field EPSPs at two distinct locations in the burst firing [2]. Indeed, previous reports showed that stratum radiatum that contained distal and proximal apical induction of LTP by high-frequency synaptic stimulation dendrites of pyramidal neurons, respectively (Fig. 2B and like TBS requires postsynaptic bursting of action potentials C). The intensities of the conditioning stimuli for cTBS instead of a solitary occurrence of single action potentials were the same in both recording sites (usually 3–4 V), and [13,19]. population spikes were clearly evoked by the conditioning We then examined the difference in membrane potential stimuli during the cTBS in all the recordings (Fig. 2B, profiles during TBS and cTBS (Fig. 1C). This cTBS insets). Both TBS and cTBS elicited LTP in the proximal consisted of TBS (indicated by filled triangles) and a site (Fig. 2B and C, proximal): no significant difference preceding conditioning stimulation to the stratum oriens was observed between TBS- and cTBS-induced LTP (indicated by open triangles). As seen from the two most (TBS, n55, 150.3613.6%; cTBS, n55, 155.069.3%; P.
left-hand illustrations in Fig. 1C, the peak amplitude of 0.5). However, in the distal site (Fig. 2B and C, distal) dendritic action potentials was significantly smaller during only TBS but not cTBS elicited LTP: the change in field each burst of cTBS than during TBS, with this trend EPSP slope induced by cTBS was much smaller than that particularly evident for the second and later bursts (n54; induced by TBS (TBS, n57, 160.5612.0%; cTBS, n57, 85.565.7% for the first burst, P,0.02; 75.167.3% for the 112.1612.1%; P,0.001). The slopes of field EPSP before second burst, P,0.01). By contrast, somatic action po- LTP induction were not different between TBS and cTBS tentials were not significantly changed in peak amplitude groups in either recording sites.
(Fig. 1C right; n54; 100.161.9% for the first burst; BAPs in the apical dendrite of hippocampal pyramidal 98.660.9% for the second burst). Also, the reduction in cells are subject to activity-dependent regulation. From the the size of dendritic action potentials was not observed present experiments, we propose that one functional role of when, by chance, the conditioning stimulation to the s.o. this BAP regulation may be to control the induction of failed to induce dendritic action potentials. These findings hippocampal LTP at distal dendritic synapses. In the distal indicate that dendritic action potentials elicited by the s.r. area of the stratum radiatum and, more distally in the stimulation, which are known to be intimately correlated stratum lacunosum-moleculare, the apical dendrite of CA1 with induction of LTP at Schaffer collateral synapses [11], pyramidal cells receives massive synaptic inputs from the are reduced in amplitude by the conditioning s.o. stimula- CA3 region and from the entorhinal cortex [1]. The tion delivered preceding the s.r. stimuli. This effect of the plasticity of those distally located synapses may depend on conditioning stimulation presumably represents an activity- BAPs more crucially than thus far recognized.
neu-124 Y. Isomura, N. Kato / Brain Research 883 (2000) 119 –124
sodium action potentials in dendrites of hippocampal CA1 pyrami-rons [6,15,21], could have also been activated during the
dal neurons, J. Neurophysiol. 74 (1995) 1395–1403. cTBS but not the TBS. This possibility remains open and
[5] C.M. Colbert, J.C. Magee, D.A. Hoffman, D. Johnston, Slow cannot actually be considered until quantitative details are 1
recovery from inactivation of Na channels underlies the activity-known of terminal distribution of such neurons along the dependent attenuation of dendritic action potentials in hippocampal proximal–distal dendritic axis of hippocampal pyramidal CA1 pyramidal neurons, J. Neurosci. 17 (1997) 6512–6521.
[6] D.A. Hoffman, D. Johnston, Neuromodulation of dendritic action cells.
potentials, J. Neurophysiol. 81 (1999) 408–411. Induction of LTP is thought to depend on postsynaptic
[7] Y. Isomura, N. Kato, Action potential-induced calcium dynamics calcium increase mediated by NMDA receptors and / or
correlated with synaptic plasticity in developing hippocampal voltage-dependent calcium channels (VDCCs) [2,11]. We pyramidal cells, J. Neurophysiol. 82 (1999) 1993–1999.
have proposed that the significance of action potentials [8] H.Y. Jung, T. Mickus, N. Spruston, Prolonged sodium channel during LTP induction resides in their activation of VDCCs inactivation contributes to dendritic action potential attenuation in hippocampal pyramidal neurons, J. Neurosci. 17 (1997) 6639–6646. rather than NMDA receptors [7]. Given that a much higher
[9] N. Kato, Dependence of long-term depression on postsynaptic membrane potential is required for activation of VDCCs
metabotropic glutamate receptors in visual cortex, Proc. Natl. Acad. ($220–30 mV) than for that of NMDA receptors ($250
Sci. USA 90 (1993) 3650–3654.
mV), action potentials may be a prerequisite for VDCC [10] D.J. Linden, The return of the spike: postsynaptic action potentials activation but may not necessarily be required for NMDA and the induction of LTP and LTD, Neuron 22 (1999) 661–666. receptor activation. Our previous study indeed showed that [11] J.C. Magee, D. Johnston, A synaptically controlled, associative
signal for Hebbian plasticity in hippocampal neurons, Science 275 action potential-induced dendritic calcium increase is
(1997) 209–213. drastically reduced by blocking high-threshold VDCCs [7].
[12] C.J. McBain, T.J. DiChiara, J.A. Kauer, Activation of metabotropic In the present experiments, the peak of dendritic action
glutamate receptors differentially affects two classes of hippocampal potentials during each burst of TBS was above the interneurons and potentiates excitatory synaptic transmission, J. threshold of VDCCs. On the other hand, the peak of those Neurosci. 14 (1994) 4433–4445.
dendritic action potentials during each burst of cTBS, [13] F.G. Pike, R.M. Meredith, A.W.A. Olding, O. Paulsen, Postsynaptic bursting is essential for Hebbian induction of associative long-term which failed to induce LTP at distally located synapses,
potentiation at excitatory synapses in rat hippocampus, J. Physiol. remained below the threshold for VDCCs, albeit above the
(Lond.) 518 (1999) 571–576. threshold of NMDA receptors (Fig. 1C), indicating that
[14] W.G. Regehr, D.W. Tank, Postsynaptic NMDA receptor-mediated activation of NMDA receptors alone is not sufficient to calcium accumulation in hippocampal CA1 pyramidal cell dendrites, induce LTP. This finding is consistent with our proposal Nature 345 (1990) 807–810.
that LTP induction may be regulated by changing calcium [15] V.M. Sandler, W.N. Ross, Serotonin modulates spike backpropaga-21
tion and associated [Ca ] changes in the apical dendrites of influx through VDCCs activated by action potentials. i
hippocampal CA1 pyramidal neurons, J. Neurophysiol. 81 (1999) 216–224.
[16] H.E. Scharfman, J.M. Sarvey, Postsynaptic firing during repetitive
Acknowledgements stimulation is required for long-term potentiation in hippocampus,
Brain Res. 331 (1985) 267–274.
[17] N. Spruston, Y. Schiller, G. Stuart, B. Sakmann, Activity-dependent The authors are grateful to Drs. K. Hashimoto and K.
action potential invasion and calcium influx into hippocampal CA1 Yamamoto for help during the experiments, and to Ms. T.
dendrites, Science 268 (1995) 297–300. Ishimaru for secretarial assistance. We also thank Drs. M.
¨
[18] G. Stuart, N. Spruston, B. Sakmann, M. Hausser, Action potential Takada, T. Fukai and S. Kawaguchi for helpful discussion initiation and backpropagation in neurons of the mammalian CNS, and encouragement. This work was supported by grants Trends Neurosci. 20 (1997) 125–131.
from the Ministry of Education, Science, Sports and [19] M.J. Thomas, A.M. Watabe, T.D.T.D. Moody, M. Makhinson, T.J. O’Dell, Postsynaptic complex spike bursting enables the induction Culture of Japan.
of LTP by theta frequency synaptic stimulation, J. Neurosci. 18 (1998) 7118–7126.
[20] H. Tsubokawa, W.N. Ross, IPSPs modulate spike backpropagation 21
References and associated [Ca ] changes in the dendrites of hippocampal CA1
i
pyramidal neurons, J. Neurophysiol. 76 (1996) 2896–2906. [21] H. Tsubokawa, W.N. Ross, Muscarinic modulation of spike back-[1] D.G. Amaral, M.P. Witter, Hippocampal formation, in: G. Paxinos
propagation in the apical dendrites of hippocampal CA1 pyramidal (Ed.), The Rat Nervous System, 2nd Edition, Academic Press, San
neurons, J. Neurosci. 17 (1997) 5782–5791. Diego, 1995, pp. 443–493.
[22] R.W. Turner, D.E.R. Meyers, T.L. Richardson, J.L. Barker, The site [2] T.V.P. Bliss, G.L. Collingridge, A synaptic model of memory:
for initiation of action potential discharge over the somatodendritic long-term potentiation in the hippocampus, Nature 361 (1993)
axis of rat hippocampal CA1 pyramidal neurons, J. Neurosci. 11 31–39.
(1991) 2270–2280. [3] E.H. Buhl, K.K. Halasy, P. Somogyi, Diverse sources of