www.elsevier.com / locate / bres
Research report
Immunohistochemical study on the distribution of Bcl-2 and Bax in
the central nervous system of the transgenic mice expressing a human
Cu / Zn SOD mutation
a a a d,e
Chung-Min Shin , Yoon Hee Chung , Myeung Ju Kim , Dong Hoon Shin
,
b f c a ,
*
Yong Sik Kim , Mark E. Gurney , Kwang Woo Lee , Choong Ik Cha
a
Department of Anatomy, Seoul National University College of Medicine, 28 Yongon-Dong, Chongno-Gu, Seoul 110-799, South Korea
b
Department of Pharmacology, Seoul National University College of Medicine, 28 Yongon-Dong, Chongno-Gu, Seoul 110-799, South Korea
c
Department of Neurology, Seoul National University College of Medicine, 28 Yongon-Dong, Chongno-Gu, Seoul 110-799, South Korea
d
Neuroscience Research Institute, Medical Research Center, Seoul National University, 28 Yongon-Dong, Chongno-Gu, Seoul 110-799, South Korea
e
Department of Anatomy, Dankook University College of Medicine, Chonan, South Korea
f
CNS Diseases Research Unit Pharmacia Upjohn, Inc., Kalamazoo, MI 49001, USA
Accepted 19 September 2000
Abstract
In the present study, we performed immunohistochemical studies to investigate the changes of Bcl-2 and Bax in the central nervous system of the transgenic mice expressing a human Cu / Zn SOD mutation. In contrast to the controls, a high density of Bcl-2-IR astrocytes were detected all around the gray matter of the spinal cord of the mutant transgenic mice. Bcl-2-IR astrocytes were also detected in the cerebellum and brainstem of transgenic mice. Specific immunoreactivity for Bax was seen in the spinal cord and brainstem of transgenic mice. Immunostaining for Bax was identified only in neurons and not in glial cells. Our present study demonstrated the distribution of Bcl-2 and Bax in detail using immunohistochemical methods through the central nervous system of the transgenic mice, for the first time.
2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Neuromuscular diseases
Keywords: Amyotrophic lateral sclerosis; Transgenic mice; Astrocyte; Bcl-2; Bax
1. Introduction possibly related to a toxic gain in function of mutant SOD1
(mSOD1) [10].
Amyotrophic lateral sclerosis (ALS) is a neurodegenera- Several lines of evidence suggest that neuronal death in tive disease characterized by progressive muscle weakness, ALS is apoptosis [9,18,21,22]. Apoptosis or programmed atrophy and spasticity, which ultimately leads to paralysis cell death is likely to be an important mechanism of cell and death within 3–5 years [12]. Ninety percent of ALS loss in neurodegenerative diseases. Explosive research cases are sporadic with no identifiable genetic or en- during the last several years has led to the identification of vironmental risk factors. The remaining 10% shows famili- apoptosis-associated molecules, including Bcl-2, Bax, p53 al inheritance and one fourth of these are associated with and interleukin 1b-converting enzyme. In vitro expression missense mutations in the antioxidant enzyme copper / zinc of mSOD1 can induce neuronal apoptosis and abnor-superoxide dismutase (SOD1). This neurodegeneration is malities in the production of free radicals [9,22]. Because motor neuron survival depends on trophic factors, abnor-malities in neurotrophic support may result in apoptotic death of motor neurons in ALS by inducing a programmed cell death mechanism involving the generation of reactive *Corresponding author. Tel.:182-2-740-8205; fax:182-2-745-9528.
E-mail address: [email protected] (C.I. Cha). oxygen species. This hypothesis is supported indirectly by
310 C.-M. Shin et al. / Brain Research 887 (2000) 309 –315
experiments showing oxidative damage in CNS tissues of tal conditions. We selected five slides in each area of the ALS subjects and abnormalities in mRNA levels coding transgenic (n55) and control mice (n55), and counted all for Bcl-2 and Bax in spinal motor neurons in ALS [21]. immunoreactive cells in each corresponding area of the Recently, several studies have shown that, aside from spinal cord and brain. Visual assessment and densitometric the dramatic loss of motor neurons, gliosis is, as in human measurement using a NIH image program (Scion Image) ALS, a striking neuropathological feature of the spinal determined the density of immunoreactive cells.
cord of an animal model of familial ALS (FALS) [2– 4,11,12,24]. In the previous studies, we reported increased
expression of NOS, nitrotyrosine and p53 in the astrocytes 3. Results
in the brain of the mutant transgenic mice that are used as
an animal model of FALS [2–4]. Troost et al. [26] In the spinal cord of the transgenic mice, immuno-reported Bcl-2 immunoreactivity in the astrocytes in the histochemistry showed intensely stained Bcl-2-immuno-brains of ALS patients. In the present study, we performed reactive (IR) glial cells with the appearance of astrocytes immunohistochemical studies to investigate the changes of (Table 1), but no Bcl-2-IR glial cells were observed in the Bcl-2 and Bax in the central nervous system of the spinal cord of the control mice (Fig. 1). The Bcl-2-IR glial transgenic mice expressing a human SOD1 mutation. cells showing the morphology of astrocytes were con-firmed as astrocytes by immunohistochemistry using an-tiserum directed against GFAP, which is the specific
2. Materials and methods marker for astrocytes (Fig. 2). Some GFAP-IR astrocytes
were observed in the spinal cord and brainstem of the Five mutant transgenic and five control mice developed control mice, but these astrocytes did not show any by Gurney et al. [10]were used for these experiments. immunoreactivity with the Bcl-2 antiserum. In contrast to They were bred by The Jackson Laboratory (Bar Harbor, the controls, a high density of Bcl-2-IR astrocytes were ME) under the Strain designations B6SJL-TgN (SOD1- detected all around the gray matter of the spinal cord of the G93A) 1 Gur and B6SJL-TgN (SOD1) 2 Gur for mutant mutant transgenic mice. In the cervical, thoracic and transgenic and control mice, respectively. All animals were lumbar segment, Bcl-2-IR astrocytes were distributed sacrificed at the age of 9 months, when clinical symptoms diffusely in the gray matter except in superficial layers. were manifested in the mutant transgenic mice. To avoid Bcl-2-IR astrocytes were also detected in the cerebellum suffering during the experimental procedures, the authors
conformed to the Ethical Committee Guidelines for
Lab-Table 1
oratory Animals. Distribution of Bcl-2 and Bax protein in the central nervous system of the
a
The mice were perfused transcardially with cold phos- transgenic mice phate buffered saline (PBS, 0.02 M, pH 7.4), and then with
Area Subdivision Bcl-2 Bax
ice-cold 4% paraformaldehyde for 10 min at a flow rate of
Spinal cord Anterior horn 111 111
50–60 ml / min. Brains were immediately removed and
Posterior horn 11 1
sliced into blocks 4–6 mm thick. Spinal cords were also
Cerebellum Molecular layer 1 2
removed and sliced into cervical, thoracic and lumbar
Purkinje cell layer 2 111
segments of 3–10 mm in length. These blocks were
Granular layer 11 2
immersed in a cold fixative for 12 h and then cryoprotected Deep cerebellar nuclei 111 11 in a series of cold sucrose solutions of increasing
con-Medulla Reticular formation 11 11
centrations. Frozen sections were cut at 40 mm in the Inferior olivary nucleus 11 2 coronal plane. Immunohistochemistry was performed in Hypoglossal nucleus 111 111 accordance with the free floating method described earlier Medial vestibular nucleus 111 2
Spinal vestibular nucleus 11 2 [2–4]. Polyclonal rabbit anti-rat / mouse Bcl-2 antibody
Dorsal motor nucleus of vagus 111 111 (PharmingenE, USA, Cat. No. 13456E, at a dilution of
1:1000), polyclonal rabbit anti-rat / mouse Bax antibody Pons Reticular formation 11 11
Trigeminal nucleus 11 11
(PharmingenE, USA, Cat. No. 13686E at a dilution of 1:
Facial nucleus 11 11
2000) and GFAP (SerotecE Cat. No. MCA 363A at a
Midbrain Periaqueductal gray matter 111 2 dilution of 1:500) were used as primary antibodies.
Reticular formation 11 2
A sample of sections was reacted without primary
antiserum, and a different sample was exposed to primary Motor cortex 2 11
antiserum that had been preincubated for 24 h with Bcl-2, Hippocampus CA1 2 1
Bax or GFAP. Sections from these samples did not exhibit CA2 2 1
CA3 2 111
any of immunoreactivity described in this report. Sections
a
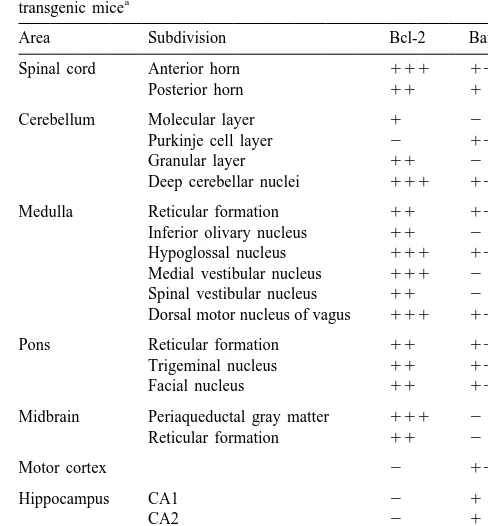
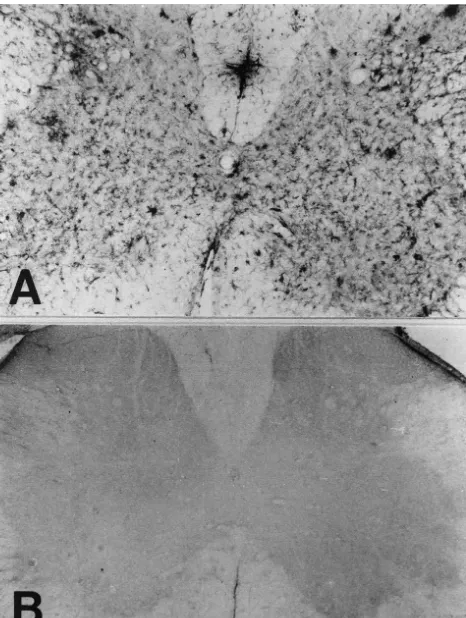
Fig. 2. Bcl-2-IR glial cells (A) showing the morphology of astrocytes Fig. 1. Changes in Bcl-2-IR astrocytes in the spinal cord of SOD mutant were confirmed as astrocytes by immunohistochemistry in the adjacent transgenic mice (A) compared with those of the control mice (B). In the sections using antiserum directed against GFAP (B), which is the specific spinal cord of the mutant transgenic mice, strong immunoreactivity for marker for astrocytes. Scale bar550mm (A, B).
Bcl-2 was observed, but no specific immunostaining was observed in the spinal cord of the control mice. Scale bar5100mm (A, B).
nucleus, facial nucleus and pontine reticular formation. In the cerebellum, Bax-IR neurons were observed in the and brainstem of transgenic mice (Table 1). In the Purkinje cell layer and cerebellar nuclei (Fig. 6). In the cerebellum, intensely stained Bcl-2-IR astrocytes were motor cortex, Bax-IR neurons in the pyramidal cell layer detected in the fastigial, interposed and dentate nucleus showed intense staining (Fig. 7A). In the hippocampus, (Fig. 3). In the medulla, Bcl-2-IR astrocytes were observed Bax-IR neurons in the CA3 region showed intense stain-in the medullary reticular formation, stain-inferior olivary ing, and the immunoreactivity was localized mainly in the nucleus, hypoglossal nucleus, medial vestibular nucleus, pyramidal cell layer (Fig. 7B). In contrast to the transgenic spinal vestibular nucleus and dorsal motor nucleus of the mice, no Bax-IR neurons were detected in the brainstem vagus. In the pons, Bcl-2-IR astrocytes were noted in the and spinal cord of the control mice.
pontine reticular formation and trigeminal and facial nuclei. In the midbrain, Bcl-2-IR astrocytes were detected
in the periaqueductal gray matter (Fig. 4) and mesence- 4. Discussion
phalic reticular formation. In contrast to transgenic mice,
312 C.-M. Shin et al. / Brain Research 887 (2000) 309 –315
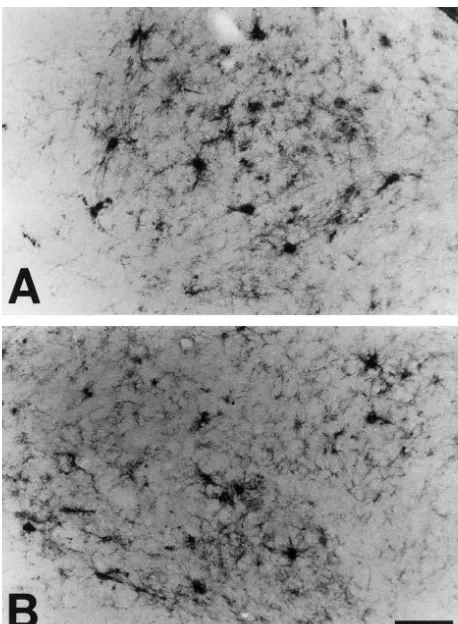
Fig. 3. Bcl-2-IR astrocytes were detected in the intracerebellar nuclei of Fig. 4. Bcl-2-IR astrocytes were detected in the periaqueductal gray the mutant transgenic mice. Intensely stained Bcl-2-IR astrocytes were matter in the midbrain of the mutant transgenic mice (A, B). In the higher observed in the dentate nucleus (A) and interposed nucleus (B) of the magnification (B), immunohistochemistry clearly showed the morphology mutant transgenic mice. Scale bar550mm (A, B). of intensely stained Bcl-2-IR astrocytes. Scale bar5100mm (A); 50mm
(B).
molecules, enhances the capacity of neutralizing Bax
through the formation of Bax:Bcl-2 heterodimers. Al- The Bcl-2 upregulation by astrocytes might have numer-though the cascade of molecular events underlying apop- ous functional consequences for the ALS brain. Astrocytes tosis is quite complex and involves a large number of remove excitotoxins, produce extracellular matrix and proteins, both the above cited in vivo and in vitro adhesion molecules, restore the ionic milieu, and are an observations strongly suggest that Bcl-2 plays a key role in essential component of the blood–brain barrier [6]. In the mSOD1-mediated neurodegeneration. previous studies using the mutant transgenic mice, we The precise mechanism by which Bcl-2 prevents cell reported that expression of NOS, nitrotyrosine and p53, death is uncertain, but several hypotheses involve its which are known to be associated with apoptosis, in-intracellular location at mitochondrial, endoplasmic re- creased in the astrocytes [2–4]. Troost et al. [26] reported ticulum, and nuclear membranes, which are also implicated Bcl-2 immunoreactivity in the astrocytes in the brains of as sites of oxygen free radical generation [13]. Brain cells ALS patients. Therefore, an increase in astrocyte size, and neurons, in particular, are very vulnerable to oxidative activity, or survival via an upregulation of Bcl-2 might be stress because of their high phospholipid content, high beneficial by increasing or helping maintain production of levels of respiration, relatively low levels of antioxidants, neurotrophic factors including nerve growth factor (NGF), and high content of catecholamines and oxidases. Ghadge basic fibroblast growth factor (bFGF), platelet-derived et al. [9] reported that the Bcl-2, which is known to affect growth factor (PDGF), ciliary neurotrophic factor and free radical generation and cell viability [14], protected insulin-like growth factor [23], and protecting or helping cells from apoptosis induced by mSOD. In their studies maintain the integrity of the blood–brain barrier [6]. It can [9], reactive oxygen species (ROS) appeared to be in- be hypothesized that Bcl-2 expression in the astrocytes volved in the mSOD-induced cell death. Therefore, the may partially underlie astrocytic resistance and protection Bcl-2 expression in the astrocytes might represent a of motor neurons in ALS.
mechanism for counter balancing ROS-mediated damage In the present study we demonstrated an increased
Fig. 5. In the transgenic mice (A), specific immunoreactivity for Bax was seen in the gray matter of the spinal cord and especially in the anterior horn, compared with no specific immunostaining of the control mice (B). Immunostaining for Bax was identified only in neurons and not in glial cells. Scale bar5100mm (A, B).
transgenic mSOD1 mice (Table 1). Our findings agreed with the report of Ekegren et al. [7] and Vukosavic et al. [27]. Ekegren et al. [7] reported increased Bax protein in the spinal cord of ALS patients. Vukosavic et al. [27] reported increased Bax immunoreactivity in mice with a human SOD1 mutation, but they performed immuno-histochemical staining only in the spinal cord. So our present study demonstrated the distribution of Bax in detail
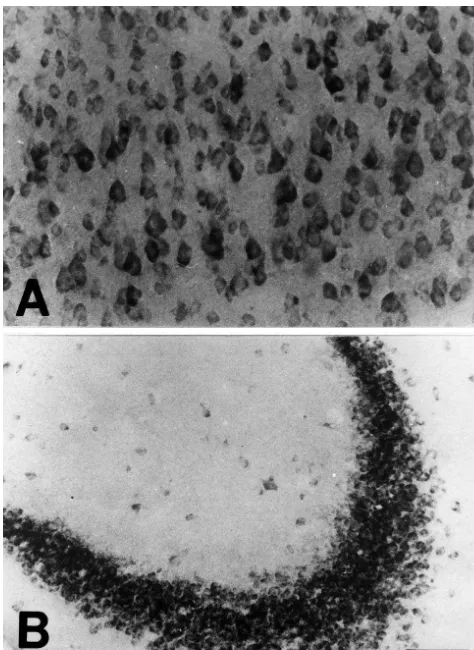
Fig. 6. In the cerebellum of the mutant transgenic mice, a high density of for the first time using immunohistochemical methods in Bax-IR neurons was observed in the Purkinje cell layer (A) and cerebellar the whole brains of the transgenic mice. nuclei (C). In contrast to the transgenic mice, no Bax-IR neurons were One of the most striking findings in the present study is detected in the Purkinje cell layer of the control mice (B). P, Purkinje cell
layer. Scale bar550mm (A, B, C). the increased Bax immunoreactivity in the CA3 region of
hippocampus. Waldemar et al. [28] reported results of single photon emission computed tomography study of 14
314 C.-M. Shin et al. / Brain Research 887 (2000) 309 –315
References
[1] S.E. Browne, A.C. Bowling, M.J. Baik, M. Gurney, R.H. Brown Jr, M.F. Beal, Metabolic dysfunction in familial, but not sporadic, amyotrophic lateral sclerosis, J. Neurochem. 71 (1998) 281–287. [2] C.I. Cha, J.M. Kim, D.H. Shin, Y.S. Kim, J. Kim, M.E. Gurney,
K.W. Lee, Reactive astrocytes express nitric oxide synthase in the spinal cord of transgenic mice expressing a human Cu / Zn SOD mutation, Neuroreport 9 (1998) 1503–1506.
[3] C.I. Cha, Y.H. Chung, C.M. Shin, D.H. Shin, Y.S. Kim, M.E. Gurney, K.W. Lee, Immunocytochemical study on the distribution of nitrotyrosine in the brain of the transgenic mice expressing a human Cu / Zn SOD mutation, Brain Res. 853 (2000) 156–161.
[4] K.J. Cho, Y.H. Chung, C. Shin, D.H. Shin, Y.S. Kim, M.E. Gurney, K.W. Lee, C.I. Cha, Reactive astrocytes express p53 in the spinal cord of transgenic mice expressing a human Cu / Zn SOD mutation, Neuroreport 10 (1999) 3939–3943.
[5] A.S. David, R.A. Gillham, Neuropsychological study of motor neuron disease, Psychosomatics 27 (1986) 441–445.
[6] B. Dehouck, M.P. Dehouck, J.C. Fruchart, R. Cecchelli, Upregula-tion of the low density lipoprotein receptor at the blood–brain barrier: intercommunications between brain capillary endothelial cells and astrocytes, J. Cell. Biol. 126 (1994) 465–473.
[7] T. Ekegren, E. Grundstrom, D. Lindholm, S.M. Aquilonius, Upregu-lation of Bax protein and increased DNA degradation in ALS spinal cord motor neurons, Acta Neurol. Scand. 100 (1999) 317–321. [8] R. Gallassi, P. Montagna, A. Morreale, S. Lorusso, P. Tinuper, R.
Daidone, E. Lugaresi, Neuropsychological, electroencephalogram and brain computed tomography findings in motor neuron disease, Eur. Neurol. 29 (1989) 115–120.
[9] G.D. Ghadge, J.P. Lee, V.P. Bindokas, J. Jordan, L. Ma, R.J. Miller, R.P. Roos, Mutant superoxide dismutase-1-linked familial amyot-rophic lateral sclerosis: molecular mechanisms of neuronal death Fig. 7. In the motor cortex, Bax-IR neurons in the pyramidal cell layer and protection, J. Neurosci. 17 (1997) 8756–8766.
showed intense staining (A). In the hippocampus, Bax-IR neurons in the [10] M.E. Gurney, H. Pu, A.Y. Chiu, M.C. Dal Canto, C.Y. Polchow, CA3 region showed intense staining, and the immunoreactivity was D.D. Alexander, J. Caliendo, A. Hentati, Y.W. Kwon, H.X. Deng et localized mainly in the pyramidal cell layer (B). Scale bar550mm (A, al., Motor neuron degeneration in mice that express a human Cu, Zn
B). superoxide dismutase mutation, Science 264 (1994) 1772–1775.
[11] E.D. Hall, J.A. Oostveen, M.E. Gurney, Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS, Glia 23 (1998) 249–256. [12] A. Hirano, Neuropathology of ALS: an overview, Neurology 47 the Bax protein [20], as increased Bax expression has been
(1996) S63–S66.
shown to impair mitochondrial membrane potential [29]. It [13] D.M. Hockenbery, G. Nunez, C. Milliman, R.D. Schreiber, S.J. is important to point out that Bcl-2 can prevent the Korsmeyer, Bcl-2 is an inner mitochondrial membrane protein that deleterious effects of Bax on mitochondria [25]. blocks programmed cell death, Nature 348 (1990) 334–336.
[14] D.M. Hockenbery, Z.N. Oltvai, X.M. Yin, C.L. Milliman, S.J. Our present study demonstrated the distribution of Bcl-2
Korsmeyer, Bcl-2 functions in an antioxidant pathway to prevent and Bax in detail for the first time using
immunohistoch-apoptosis, Cell 75 (1993) 241–251. emical methods through the central nervous system of the
[15] Y. Iwasaki, M. Kinoshita, K. Ikeda, K. Takamiya, T. Shiojima, transgenic mice. The results of the present study combined Cognitive impairment in amyotrophic lateral sclerosis and its with the previous studies [2–4] suggest that reactive relation to motor disabilities, Acta Neurol. Scand. 81 (1990) 141–
143. astrocytes may play an important role in the pathogenesis
[16] A. Kakita, K. Oyanagi, H. Nagai, H. Takahashi, Eosinophilic and progress of ALS.
intranuclear inclusions in the hippocampal pyramidal neurons of a patient with amyotrophic lateral sclerosis, Acta Neuropathol. (Berl) 93 (1997) 532–536.
[17] V. Kostic, V. Jackson-Lewis, F. de Bilbao, M. Dubois-Dauphin, S.
Acknowledgements Przedborski, Bcl-2: prolonging life in a transgenic mouse model of
familial amyotrophic lateral sclerosis, Science 277 (1997) 559–562. [18] L.J. Martin, Neuronal death in amyotrophic lateral sclerosis is This work was supported by Korea Research Foundation
apoptosis: possible contribution of a programmed cell death mecha-Grant (98-021-F00024). This study was supported in part nism, J. Neuropathol. Exp. Neurol. 58 (1999) 459–471.
superox-ide dismutase on neuronal survival andL-DOPA-induced toxicity in [25] S. Shimizu, Y. Eguchi, W. Kamiike, Y. Funahashi, A. Mignon, V. postnatal midbrain culture, J. Neurochem. 69 (1997) 21–33. Lacronique, H. Matsuda, Y. Tsujimoto, Bcl-2 prevents apoptotic [20] D.E. Merry, S.J. Korsmeyer, Bcl-2 gene family in the nervous mitochondrial dysfunction by regulating proton flux, Proc. Natl.
system, Annu. Rev. Neurosci. 20 (1997) 245–267. Acad. Sci. USA 95 (1998) 1455–1459.
[21] X. Mu, J. He, D.W. Anderson, J.Q. Trojanowski, J.E. Springer, [26] D. Troost, J. Aten, F. Morsink, J.M. de Jong, Apoptosis in Altered expression of Bcl-2 and Bax mRNA in amyotrophic lateral amyotrophic lateral sclerosis is not restricted to motor neurons. sclerosis spinal cord motor neurons, Ann. Neurol. 40 (1996) 379– Bcl-2 expression is increased in unaffected post-central gyrus,
386. Neuropathol. Appl. Neurobiol. 21 (1995) 498–504.
[22] S. Rabizadeh, E.B. Gralla, D.R. Borchelt, R. Gwinn, J.S. Valentine, [27] S. Vukosavic, M. Dubois-Dauphin, N. Romero, S. Przedborski, Bax S. Sisodia, P. Wong, M. Lee, H. Hahn, D.E. Bredesen, Mutations and Bcl-2 interaction in a transgenic mouse model of familial associated with amyotrophic lateral sclerosis convert superoxide amyotrophic lateral sclerosis, J. Neurochem. 73 (1999) 2460–2468. dismutase from an antiapoptotic gene to a proapoptotic gene: studies [28] G. Waldemar, S. Vorstrup, T.S. Jensen, A. Johnsen, G. Boysen, Focal in yeast and neural cells, Proc. Natl. Acad. Sci. USA 92 (1995) reductions of cerebral blood flow in amyotrophic lateral sclerosis: a
3024–3028. [99mTc]-d,l-HMPAO SPECT study, J. Neurol. Sci. 107 (1992)
[23] J.S. Rudge, Astrocyte-derived neurotrophic factors, in: S. Murphy 19–28.
(Ed.), Astrocytes. Pharmacology and Function, Academic Press, San [29] J. Xiang, D.T. Chao, S.J. Korsmeyer, BAX-induced cell death may Diego, CA, 1993, pp. 267–305. not require interleukin 1 beta-converting enzyme-like proteases, [24] D. Schiffer, S. Cordera, P. Cavalla, A. Migheli, Reactive astrogliosis Proc. Natl. Acad. Sci. USA 93 (1996) 14559–14563.