DOI 10.1007/s10751-008-9840-4
Mössbauer study on microwave synthesized (Cu,Fe)
sulfide composites and correlation with natural
mineral—cubanite
Sarita Pareek·Anwar Rais· Amita Tripathi·Usha Chandra
Published online: 10 October 2008
© Springer Science + Business Media B.V. 2008
Abstract In nature, the mineral ‘Cubanite’ with composition CuFe2S3 occurs in
orthorhombic structure in the matrix of chalcopyrite and pyrrhotite. Synthesis of this mineral in the laboratory conditions has not been reported yet. An attempt to synthesize the orthorhombic Cu–Fe–Sulfide (cubanite) by resistive as well as microwave heating technique is reported. MW-heated sample shows the presence of orthorhombic component along with isocubanite and pyrrhotite. The synthesized samples were studied in details through XRD and Mössbauer spectroscopy. The synthesis process, responsible for different proportions of the minerals, may indicate the conditions of their genesis in nature. Formation of isocubanite (cubic cubanite) seems unavoidable under the conditions of synthesis. The striking indistinguishable character of cubanite and chalcopyrite could be challenged through Mössbauer hy-perfine parameter—the hyhy-perfine field of chalcopyrite (∼37 T) being quite different from that of cubanite (∼33 T).
Keywords Orthorhombic cubanite·Isocubanite·Mössbauer spectroscopy·
Cu–Fe–S·Chalcopyrite–pyrrhotite
1 Introduction
In mineral deposits, cubanite often occurs as a lath in a chalcopyrite matrix. Inter-growth of cubanite and chalcopyrite has been described from a number of localities
S. Pareek·U. Chandra (
B
)High-Pressure Physics Laboratory, Department of Physics, University of Rajasthan, Jaipur 302055, India
e-mail: ushac_jp1@sancharnet.in A. Rais
Geological Survey of India, Jaipur 302017, India A. Tripathi
by Schwartz [1]. The origin of the certain of these intergrowths is clearly due to the breaking down of a solid solution—via exsolution of chalcopyrite into orthorhombic cubanite with its characteristics striation. The crystal structure of natural cubanite (orthorhombic Pcmn,a= 6.467 Å,b= 11.11 Å andc= 6.23 Å) have been described
widely by several investigators [2]. Both Cu and Fe are tetrahedrally coordinated by four S atoms; One third of the S has 2 Cu and 2 Fe neighbours and the other 2/3 of S have 3 Fe and one Cu. The Fe atoms are bonded together in pairs to give rise to ferromagnetism.
Various techniques like57
Fe Mössbauer spectroscopy, X-ray diffraction, electrical resistivity etc., used under high pressure on the natural specimen show an insulator– metal phase transition in the range 3.4–5.8 GPa coinciding with structural transition from an orthorhombic to a hexagonal structure [3–5]. The room temperature data shows that the metallization process concurs with a gradual transition from a mag-netically ordered phase at low pressure to a non-magnetic (or paramagnetic phase)at high-pressure. When orthorhombic cubanite is heated above 200◦C in vacuum, an
irreversible order–disorder transition to a cubic phase (known as isocubanite— an isomorphous phase of cubanite) is observed. In nature, a sub-solidus process occurs with transformation of cubic (iso) cubanite to orthorhombic structure. Such transformation during synthesis may be decided by the physico-chemical conditions existing in the laboratory. No successful attempt to synthesize cubanite in the orthorhombic structure under laboratory conditions has been reported.
In this report, an attempt to synthesize and correlate the conditions of the natural genesis of the mineral in orthorhombic structure through resistive and microwave heating methods is elaborated under compositional variations as well as under var-ious conditions of heating and cooling cycles. The composite samples thus obtained are analyzed through XRD and57
Fe Mössbauer Spectroscopy.
2 Experimental procedure
Stoichiometric proportion of Cu,Fe and S were heated up to 800◦C in an evacuated
quartz tube using a resistive furnace under controlled heating rate. The samples thus heated were:
(a) Cu–Fe–S (1:2:3) cooled slowly up to RT—sample ‘a’ (b) Cu–Fe–S (1:2:3) quenched in ice water—sample ‘b’
(c) Cu–Fe–S (1:2:3) cooled down to 400◦C and then quenched—sample ‘c’.
(d) sample ‘d’—Cu–Fe–S (1:2:1.5), in an evacuated quartz tube was heated using domestic microwave oven at full power of 600 W for few seconds. Sample was slowly cooled down to room temperature.
All the samples were characterized by recording X-ray diffraction patterns using Philips diffractometers with Fe Kαand Cu Kαradiations respectively. The composite pattern was analyzed through specific program for the respective mineral composi-tions (for chalcopyrite (CuFeS2), pyrrhotite (Fe1−xS) and cubanite–orthorhombic as
well as cubic) using standard tables. The Mössbauer measurements were done using a 10 mCi Co57
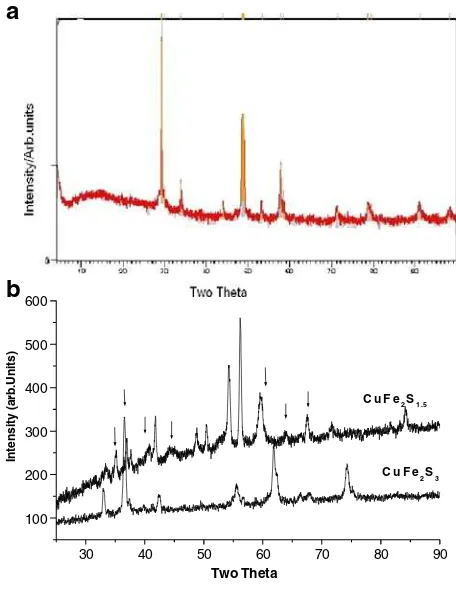
Fig. 1 aX-ray diffraction pattern of Cu–Fe–S (1:2:3) resistive heated up to 800◦
C and slowly cooled to room temperature using Cu Kα radiation.bX-ray diffraction patterns of microwave heated samples Cu–Fe–S (1:2:1.5) and Cu–Fe–S (1:2:3) respectively using Fe Kαradiation. The
arrowsdisplay the peaks due
to orthorhombic cubanite
with two sextets and a doublet with a reasonable goodness of fit parameter and line width using the analysis program developed by Jernberg and Sundquist [6].
3 Results and discussions
The dependency of the synthesis process is well depicted in the Fig.1. The X-ray pat-tern of sample ‘a’ has been analyzed as a mixture of isocubanite, pyrrhotite (Fe1−xS)
and chalcopyrite (CuFeS2)while the microwave heated sample CuFe2S1.5showed the
presence of orthorhombic cubanite (having distinct peak withd= 3.22 Å) marked
by the arrows along with good amount of pyrrhotite. On the contrary, microwave heated CuFe2S3 (with excess sulphur) displays very few peaks representing the
presence of isocubanite/chalcopyrite and little amount of pyrrhotite. XRD pattern of the sample ‘b’ indicates only chalcopyrite and pyrrhotite while sample ‘c’ shows good amount of chalcopyrite/isocubanite with less quantity of pyrrhotite. Cabri [2] by studying in details the orthorhombic cubanite heated at 200◦
C in vacuum, established similarity in ‘d’ values of the high temperature phase(isocubanite) and chalcopyrite. Isocubanite, a cubic polymorph of cubanite witha= 5.303 Å shows strongest lines in
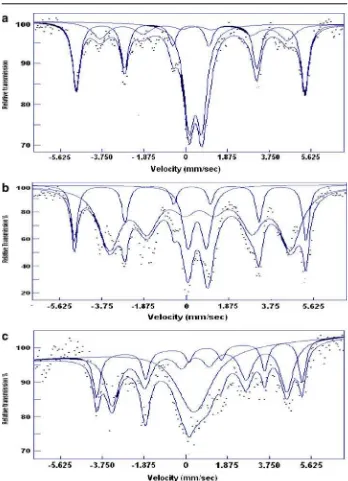
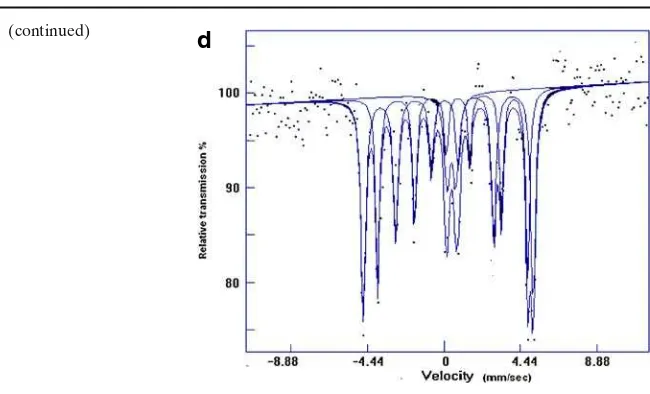
Fig. 2 Room temperature Mössbauer spectra of resistively heated Cu–Fe–S (1:2:3)acooled slowly to RTbquenched in ice water andccooled down to 400◦
C then quenched to RT.dMicrowave heated Cu–Fe–S (1:2:1.5). The velocity scale is relative to metallic Fe
Fig. 2 (continued)
d
hyperfine field of chalcopyrite is larger (∼36 T) as compared to cubanite (∼33 T). Figure 2 and Table 1 show the Mössbauer spectra and the analyzed parameters of the resistive as well as microwave heated samples. Isomer shifts (IS) 0.60 and 0.20 mm/s are typical values for high spin Fe2+ and Fe3+ respectively in metal–
iron sulfide compounds when iron is tetrahedrally coordinated by sulfur [9]. Using XRD analysis, sample ‘a’ could be resolved into two magnetic sextets with IS = 0.23 and 0.46 mm/s respectively and a nonmagnetic doublet with IS of 0.46 mm/s and QS of 0.61 mm/s assigned to chalcopyrite (Cu+1
Fe3+S
2), pyrrhotite(Fe1−xS)
and cubanite (CuFe2S3) respectively. If one considers the value of hyperfine field
alone, the magnetic sextet with field∼33 T should be assigned to cubanite instead to chalcopyrite, however the IS of 0.23 mm/s seems to be typical value of Fe3+ at
the tetrahedral symmetry. The nonmagnetic component can also be fitted with two singlets having IS = 0.15 and 0.772 mm/s respectively with relaxation line broadening implying the electrons moving rapidly between Fe2+and Fe3+ions in the cubanite
structure. The fitting of the Mössbauer spectra of sample ‘b’ and ‘c’ was not easy since the phases were not very well formed. Nevertheless one can see the prominent chalcopyrite field of 33.9 T with IS = 0.26 mm/s as well as a broad non-magnetic isocubanite with IS = 0.50 mm/s and pyrrhotite for sample ‘b’. In sample ‘c’, the chalcopyrite phase was missing. Sextet related to pyrrhotite phase with IS of 0.782 and 0.551 mm/s belonging to Fe2+ were seen along with very broad non-magnetic
doublet. Both these quenched samples have shown large proportion of magnetic components while the spectrum of the slowly cooled sample resembled that of orthorhombic cubanite at high pressure (4.5 GPa) [4]. The microwave heated sample ‘d’, analyzed as composed of orthorhombic cubanite showed a sextet with 34.3 T along with pyrrhotite and non-magnetic doublet (Fig.2d). Orthorhombic cubanite transforms into cubic cubanite above 200◦C. The transition is accompanied by the
S.
Pareek
et
al.
Table 1 Room temperature Mössbauer parameters of resistively heated samples Cu–Fe–S (1:2:3) at various conditions of synthesis as well as microwave heated Cu–Fe–S (1:2:1.5)
Sample description Components Isomer shift Quadrupole Width (mm/s) Intensity % Magnetic hyperfine
metallic Fe (mm/s) splitting (mm/s) field (Tesla)
Sample ‘a’—resistive Magnetic sextet P1 0.235 −0.011 0.440 40.70 33.95
heated-slowly cooled
Magnetic sextet P2 0.466 −0.166 0.910 19.94 27.10
Nonmagnetic doublet 0.460 0.611 0.600 39.36 –
Sample ‘b’—resistive Magnetic sextet P1 0.218 −0.093 0.354 17.96 33.98
heated- quenched
Magnetic sextet P2 0.623 0.027 1.299 66.67 26.50
Nonmagnetic doublet 0.502 0.869 0.507 15.37 –
Sample ‘c’—resistive Magnetic sextet P1 0.782 −0.277 0.398 19.98 30.50
heated- quenched at 400◦
C
Magnetic sextet P2 0.551 0.194 0.794 44.53 25.94
Nonmagnetic doublet 0.395 0.417 1.803 35.70 –
Sample ‘d’—microwave Magnetic sextet P1 0.152 0.227 0.363 50.40 34.33
heated CuFe2S1.5
Magnetic sextet P2 0.728 0.352 0.303 36.96 30.36
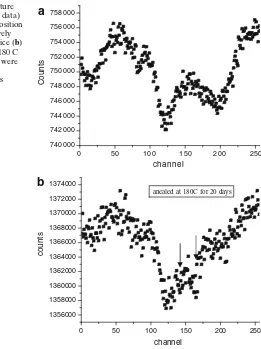
Fig. 3 aRoom temperature Mössbauer spectra (raw data) of sample ‘b’ with composition Cu– Fe–S (1:2:3) resistively heated but quenched in ice (b) the sample annealed at 180 C for 20 days. The spectra were taken at velocity range related to inner two lines of metallic Fe
anealed at 180C for 20 days
a
b
Figure3shows the raw Mössbauer patterns (unanalyzed) of the sample ‘b’ after annealing at 180◦C for 20 days. The new inner peaks emerge at the expenses of
outer peaks consisting of chalcopyrite and pyrrhotite [2], thus demonstrating nicely exsolution process occurring in nature. The optical reflection technique also confirms the exsolution of chalcopyrite into cubanite and the presence of cubanite in the sample.
4 Conclusion
minerals in the laboratory. Mössbauer spectroscopy seems to be a sensitive and better technique to analyze the phases.
Acknowledgements We are grateful to DST, New Delhi and PLANEX (PRL), Ahmedabad for the financial support; Magnetism and Mössbauer lab., U.O.R., Jaipur for X-ray diffraction; GTZ, Germany for gifting Mössbauer set up. We thank referees for the valuable comments and suggestions.
References
1. Schwartz, G.M.: Intergrowth of chalcopyrite and cubanite; experimental proof of the origin of intergrowth and their bearing on the geologic thermometer. Econ. Geol.22, 44–61 (1927) 2. Cabri, L.J., Hall, S.R., Szymanski, J.T., Stewart, J.M.: On the transformation of cubanite. Can.
Min.12, 33–38 (1973), and references therein
3. Rosenberg, G.Kh., Pasternak, M.P., Hearne, G.R., McCammon, C.A.: High-pressure metalliza-tion and electronic–magnetic properties of hexagonal cubanite (CuFe2S3). Phys. Chem. Miner.
24, 569–573 (1997)
4. McCammon, C.A.: Equation of state, bonding character and phase transition of cubanite, CuFe2S3studied from 0 to 5 GPa. Amer. Miner.80, 1–8 (1995)
5. McCammon, C.A., Zhang, J., Hazen, R.M., Finger, L.W.: High-pressure crystal chemistry of cubanite CuFe2S3. Amer. Miner.77, 937–944 (1992)
6. Jernberg, P., Sundquist, T.: Report UUIP-1090, Institute of Physics, University of Uppsala, Uppsala, Sweden (1983)
7. Caye, R., Cerevelle, B., Cesborn, F., Oudin, E., Picot, P., Pillard, F.: Isocubanite, a new definition of the cubic polymorph of cubanite CuFe2S3. Miner. Mag.52, 509–514 (1988)
8. Greenwood, N.N., Whitfield, H.J.: Mössbauer effect studies on cubanite and related iron sulfides. J. Chem. Soc. (A) 1697–1699 (1968)