Dolly has become a synonym for one of the greatest
breakthroughs in animal reproductive biology: the regeneration of a whole mammal from a somatic cell nucleus. The equivalent experiments in plants — the regeneration of whole plants from single differentiated cells — are comparatively easy. Does this apparent difference in the developmental potential of animal and plant somatic cells reflect mechanistic differences in the
regulation and maintenance of their respective cell differentiation?
Addresses
ZMBP, Entwicklungsgenetik, Universität Tübingen, Auf der Morgenstelle 1, D-72076 Tübingen, Germany
*e-mail: [email protected]
†e-mail: [email protected] ‡e-mail: [email protected] Current Opinion in Plant Biology1999, 2:508–512
1369-5266/99/$ — see front matter © 1999 Elsevier Science Ltd. All rights reserved.
Abbreviation
GFP green fluorescent protein
Introduction
During normal development, most cells enter specific dif-ferentiation programs that change their physiological and morphological properties. As cell differentiation progresses it is believed that the potential of cells to change their developmental specialization is increasingly restricted (Figure 1). To understand differences in the developmen-tal potential of animal and plant cells it is therefore important to explore the role of the mechanisms that initi-ate and maintain cell differentiation.
Most of our knowledge about the regulation of cell differ-entiation comes from animal studies. In animal cells, differentiation is controlled by the interplay of intrinsic systems that operate in a cell-autonomous manner and extrinsic signals that are received from the surrounding tis-sue [1] (Figure 1). In some cases, such as the stem cells, continuous extrinsic signaling is necessary to maintain dif-ferentiation throughout development [2]. Usually, cell differentiation is maintained by intrinsic systems, such as the cell-cycle machinery or chromatin-dependent gene silencing. During recent years evidence has accumulated to suggest that the mechanisms underlying the regulation of cell differentiation in plants are similar to those already known in animals. Our review discusses examples of the respective roles of intrinsic and extrinsic signals in the reg-ulation of cell differentiation in plants.
Extrinsic control of cell differentiation states
A well-studied example of the extrinsic control of cell dif-ferentiation in plants is the specification of stem cells. Inplants, stem cells are found in small populations in the shoot and root meristems where they remain in an undif-ferentiated state before undergoing differentiation to give rise to most tissues of the mature plant [3,4].
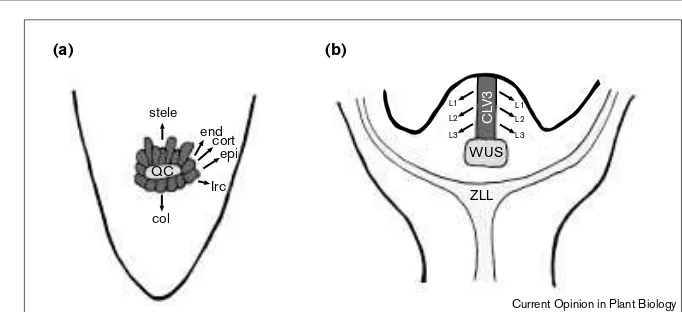
In the root meristem, stem cells are located in immediate contact with a group of four nondividing cells, which are collectively known as the quiescent center, and that are the initials of different cell types [5] (Figure 2a). In a series of ablation experiments it was demonstrated that the removal of a single quiescent center cell resulted in the differentiation of those stem cells that had been in imme-diate contact with it [6]. This finding indicates that differentiation in root stem cells is inhibited by short-range signaling from the quiescent center cells.
As in the root, stem cells in the shoot meristem are also specified nonautonomously. Maintaining shoot stem cells requires the activity of WUSCHEL (WUS), a gene that encodes a homeodomain protein [7••]. WUSis expressed in a small group of cells that are found immediately below the stem cells, the WUS domain (Figure 2b). A second gene involved in stem cell specification is ZWILLE(ZLL) [8•], which is allelic to PINHEAD [9]. ZLL encodes a protein that is homologous to the translation initiation factor eIF2C. During the initial stages of shoot meristem formation in embryogenesis, and also during post-embryonic development, ZLL is expressed in the vascular precursor cells, which are found below the WUS domain (Figure 2b). In zllmutants all apical cells differ-entiate, suggesting that ZLL-dependent long-range signaling is required for the maintenance of stem cells. One possibility is that ZLLpositions the WUSexpression domain correctly (T Laux, personal communication). Interestingly, the ZLL homolog in Drosophila, the PIWI gene, is required for the nonautonomous maintenance of stem cells in the germ line [10]. A ZLL homolog in Caenorhabditis elegans has also been implicated in germ cell maintenance [10].
Cell proliferation occurs only along the central-peripheral axis of the meristem where stem cells form a core of slowly divid-ing undifferentiated cells. On leavdivid-ing the core, cells proliferate more rapidly and begin to differentiate. The dynamic of this system poses the question of how the balance between stem cell self-renewal and differentiation might be maintained. Two recent findings suggest that a group of three genes, the CLAVATA(CLV) genes, are involved in this process. The CLVgenes encode components of a receptor–ligand sys-tem that controls the size of the merissys-tem [11,12,13••]. The increased meristem size of clvmutants is mainly due to their enlarged stem cell population. The central domain of slowly dividing cells, one of the main characteristics of stem cells, has
Plant cells — young at heart?
been shown to be larger in clv3mutants than in wildtypes [14•]. In addition, it was shown that CLV3 is specifically expressed in the presumptive stem cells, and hence it can be considered as a molecular stem cell marker [13••] (Figure 2b). In clv1 and clv3 mutants the expression domain of CLV3 -expressing cells is expanded, supporting the idea that the number of stem cells is greatly increased by these mutations [13••]. Thus, the CLVgroup seems to play a vital role in pro-moting the transition from stem to non-stem cells. The links between the CLVand WUS-dependent control of stem cell maintenance are not understood. It will be interesting to know whether CLV-dependent stem cell control acts
independently of WUS, or whether the CLV genes are involved in the regulation of WUS.
Intrinsic control of cell differentiation states
In animals, the onset of cell differentiation is closely asso-ciated with changes to intrinsic systems, such as the cell cycle or chromatin-dependent gene silencing [1].The role of the cell cycle and endoreduplication in cell differentiation
A correlation between cell differentiation and cell cycle regulation is also observed during leaf hair (trichome)
Figure 1
Stem cell
Trichome cell
Tissue-layer signals
Protodermal cell
e.g. GL1 expression, endoreduplication,
branching elongation
WUS, ZLL, CLV
GL1 Tissue-layer signals
Undifferentiated cell
Differentiated cell
? e.g. Gene
expression, morphological
changes, physiological
changes Ex
trinsic signals
Ex
trinsic signals
Intrinsic signals
Intrinsic signals
Current Opinion in Plant Biology
(a) (b)
e.g. AtML1 expression [43]
The regulation of cell differentiation. (a)The progression from an undifferentiated cell to a differentiated cell involves changes in properties such as gene expression, morphology and physiology. These changes are associated with a loss of potential for cells to change their developmental specialization. Both of these aspects are controlled by the combination of extrinsic and intrinsic signals.
Generally, intrinsic signals become more important in later stages of development. (b)The progression from stem cells to trichome cells as an example of the progression of cell differentiation. Note, that during trichome differentiation intrinsic factors, as exemplified by GL1
expression, become more important than extrinsic signals.
Figure 2
Models for stem-cell maintenance in the root and shoot meristem. (a)In the root meristem, the quiescent center (QC) functions as an organizing center. Those cells in immediate contact with the QC are stem cells (dark shading), which are initials for all cell files: endodermis (end), cortex (cort), epidermis (epi), lateral root cap (lrc) and columella (col).
(b)In the shoot meristem, WUSis functionally equivalent to the QC in the root meristem in maintaining the presence of stem cells (dark shading). Long-range signaling for stem cell maintenance is also postulated for ZLL, which is expressed in the vascular cells. CLV3is expressed in the presumptive stem cells that are the initials for the L1 (epidermis), L2 (subepidermal tissues, i.e. mesophyll cells) and L3 (subepidermal tissues, i.e. vascular cells) tissue layers.
WUS
ZLL
L3 L2 L3
(a) (b)
QC
col Irc
epi cort end
stele CLV
3
L1 L2 L1
development. Trichomes are initiated at the base of young leaves in a field of dividing cells [15,16]. In experiments in which trichome formation was induced by the overexpres-sion of known positive regulators of trichome initiation, it was shown that trichome initiation is only possible in tis-sues that are proliferating. This was demonstrated in Arabidopsis by plants overexpressing the maize R gene [17] and in tobacco plants overexpressing the Anthirrinum MIXTA gene [18].
Trichome initiation is closely coupled with the onset of endoreduplication [19]. Trichome precursor cells stop divid-ing but continue DNA replication. In addition, it has been shown that two genes that are involved in trichome initiation are also important in regulating the number of endoredupli-cation cycles. Overexpression of GLABRA1(GL1), a positive regulator of trichome initiation, promotes further endoredu-plication cycles in trichome cells [20••]. Mutations in the TRIPTYCHON(TRY) gene, a negative regulator of trichome initiation, also result in an increased DNA content [20••]. Although GL1and TRYmay have independent functions in trichome patterning and endoreduplication, an attractive hypothesis is that the initiation of trichome development by these two genes is directly linked to the cell cycle machinery.
An expanding role of cell cycle regulators in plants? During recent years evidence has accumulated to show that, in animals, various cell cycle genes are involved not only in the regulation of the cell cycle, but also directly in cell differentiation [21]. For example, proteins encoded by p21 CKI, an inhibitor of mitosis, and that encoded by Retinoblastoma(Rb), an inhibitor of cell cycle progression, also affect the cell differentiation of keratinocytes and myoblasts, respectively [22,23]. There is scant evidence of a direct role of cell cycle genes in plant cell differentiation. Some observations, however, now suggest a connection between cell cycle regulation and cell differentiation in plants. In Arabidopsis, leaf aging and differentiation are associated with increased expression of CYCLIN DEPEN-DENT KINASE INHIBITOR1 (ICK1) [24], which shows homology to p21 CKI. Similarly, in maize, leaf tissues con-taining older, differentiated cells show higher concentrations of Rb than do younger leaf tissues [25]. It is tempting to speculate that these plant homologs are also directly involved in cell differentiation. More direct evi-dence of an additional role for a cell cycle gene in differentiation was found for CycD3 in Arabidopsis [26••]. Expression of CycD3 activates cell divisions at the G1–S cell cycle phase transition. Plants overexpressing CycD3 have abnormal meristems and leaves. Calli that have been transformed so that they express CycD3 differentiate, become green and cannot be regenerated to form shoots. These findings suggest that CycD3has an additional role in the regulation of cell differentiation.
Chromatin-dependent gene silencing
In animals, chromatin-dependent gene silencing is a com-mon mechanism for maintaining cell differentiation. In
Drosophila, a large number of genes, known collectively as the Polycomb (Pc) group, regulate the accessibility of chro-mosomal regions, and as a consequence transcriptionally repress gene expression in particular chromosomal regions [27].
The first Pc-like gene described in plants is the CURLY LEAF (CLF) gene of Arabidopsis. clfmutants display a wide range of morphological defects in their leaves and flowers [28]. Most of these defects are caused by the ectopic expres-sion of the floral homeotic gene AGAMOUS(AG), indicating that CLFrepresses the expression of AG. Pc genes also play an important role in suppressing the development of unfer-tilized seeds. In medea (mea) and fertilization-independent endosperm (fie) mutants endosperm and sometimes also embryo development is initiated without fertilization [29•,30,31]. The two proteins which are defective in these mutants contain motifs found in the Pc-group proteins of Drosophila: MEAcontains a SET domain similar to enhancer of zeste [29•,32•,33•], and FIE shares a sequence identity with the WD domain of extra sex combs[34•].
On the basis of homology with their animal counterparts, Pc-like proteins are thought to function as chromatin regu-lators in plants. The current data, however, are consistent with the involvement of Pc-like proteins in three different mechanisms of gene regulation.
First, evidence of the conservation of a function for Pc-like proteins in the regulation of chromatin comes from recent experiments in which a green fluorescent protein (GFP) that had been fused with the Drosophila Pc -chro-modomain was expressed in tobacco [35•]. In Drosophila, the chromodomain mediates the targeting of Pcto specif-ic chromosomal regions through protein–protein interactions [36]. The Drosophilachromodomain has been shown to bind to distinct chromosomal regions in tobacco, and its overexpression caused a variety of morphological defects during the development of tobacco, including curly leaves or abnormal flower development. The data suggest that the heterologous chromodomain interferes with an endogenous chromatin-regulating mechanism. Because the Drosophilachromodomain is thought to func-tion only as part of a large protein complex, these findings suggest that the underlying molecular mechanisms involved in regulating chromatin are conserved in both animals and plants.
expressed [37,38]. The recent identification of CMT1, a DNA methyltransferase containing a chromodomain in Arabidopsis, also suggests that chromodomain-targeted repression of downstream genes can result from DNA methylation [39•].
Third, the suppression of seed development by MEA and FIEcould involve transcriptional regulation via DNA bind-ing, rather than chromatin regulation. fertilization independent seed(fis2) mutants show the same phenotype as meaand fie mutants and FIS2encodes a putative zinc-finger transcrip-tion factor [34•]. Although it is possible that FIS2 transcriptionally activates MEA and FIE, an alternative explanation is that FIS2 forms a complex with MEA and FIEand targets them to particular promotor regions, which regulate the transcriptional activation of downstream genes.
Intrinsic programs versus extrinsic cues
It is difficult to determine the extent to which differentiat-ed cells are kept in their differentiatdifferentiat-ed state by intrinsic programs, or by continuous tissue-specific signaling. Some information that relates to tissue-layer-specific cell differ-entiation is available. Three distinct tissue layers are established during embryogenesis and remain separate throughout development [40]. Each tissue layer gives rise to specific cell types. The analysis of chimeras has shown that cells that are displaced from the epidermal cell layer to a subepidermal layer change their fate according to their new position [41]. This implies that tissue-layer-specific positional information continuously determines the fate of cells according to their position. It was therefore surprising to find that tissue-layer-specific restrictions can be overri-den by manipulating the expression of genes required for the initiation of one epidermis-specific cell type. Overexpression of one key positive regulator of trichome initiation, GL1, a myb-homolog transcription factor, in the absence of the important negative regulator, TRY, is suffi-cient to trigger trichome formation in the subepidermal tissue layers [20••,42••]. As GL1and TRYare both cell-type-specific regulators, they can be considered as intrinsic factors that can overcome extrinsic cues (Figure 1b). This result is remarkable because it lacks a counterpart in animal systems suggesting that, in animals, tissue-layer-specific signals impose tighter restrictions on the fate of cells.
Conclusions
Plant development is evidently more flexible than animal development in many respects. It is conceivable that this dif-ference in flexibility is due to the fact that cell differentiation in plants is based more on extrinsic regulation than that in animals. However, plants and animals clearly share various mechanisms that control their differentiation state. This, in turn, implies that the difference between animal and plant cells is in the extent to which commitment is irreversible.
Acknowledgements
We thank members of the Zentrum für Molekularbiologie der Pflanzen (Center of Plant Molecular Biology) for reading this manuscript critically.
Work in the authors’ laboratory is supported by the Volkswagen Stiftung and by grants from the Deutsche Forschungsgemeinschaft.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Edlund T, Jessell TM:Progression from extrinsic to intrinsic
signaling in cell fate specification: a view from the nervous
system.Cell1999, 96:211-224.
2. Morrison SJ, Shah NM, Anderson DJ:Regulatory mechanisms in
stem cell biology.Cell1997, 88:287-298.
3. Doerner P:Shoot meristems: Intercellular signals keep the
balance.Curr Biol 1999, 20:377-380.
4. Lenhard M, Laux T:Shoot meristem formation and maintenance.
Curr Opin Plant Biol 1999, 2:44-50.
5. Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C,
Weisbeek P:Embryonic origin of the Arabidopsisprimary root and
root meristem initials.Development 1994, 120:2475-2487.
6. van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B:
Short-range control of cell differentiation in the Arabidopsisroot
meristem.Nature 1997, 390:287-289.
7. Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T:Role
•• of WUSCHELin regulating stem cell fate in the Arabidopsisshoot
meristem.Cell 1998, 95:805-815.
WUSCHEL (WUS), a gene required for stem-cell specification in
Arabidopsis, encodes a novel homeodomain protein. WUSis expressed in a small group of cells immediately below the presumptive stem cells and is therefore postulated to specify stem cells nonautonomously.
8. Moussian B, Schoof H, Haecker A, Juergens G, Laux T:Role of the
• ZWILLEgene in the regulation of central shoot meristem cell fate
during Arabidopsisembryogenesis.EMBO J1998, 17:1799-1809.
ZWILLE(ZLL), a gene specifically required for maintaining meristematic cell
status, encodes a protein of a novel family. ZLLis expressed in vascular
pre-cursor cells underlying the shoot meristem from early embryo stages onwards.
9. McConnell JR, Barton MK:Effects of mutations in the PINHEAD
gene of Arabidopsison the formation of shoot apical meristems.
Dev Genet1995, 16:358-366.
10. Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H:A novel class of
evolutionarily conserved genes defined by piwiare essential for
stem cell self-renewal.Genes Dev 1998, 12:3715-3727.
11. Clark SE, Running MP, Meyerowitz EM:CLAVATA3is a specific
regulator of shoot and floral meristem development affecting the
same processes as CLAVATA1.Development 1995, 121:2057-2067.
12. Clark SE, Williams RW, Meyerowitz EM:The CLAVATA1gene
encodes a putative receptor-kinase that controls shoot and floral
merstem size in Arabidopsis.Cell 1997, 89:575-585.
13. Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM:
•• Signaling of cell fate decisions by CLAVATA3in Arabidopsisshoot
meristems.Nature 1999, 283:1911-1914.
The CLV3gene, required for the control of the shoot meristem, encodes a small,
predicted extracellular protein and clonal analysis showed that it acts
non-autonomously. CLV3mRNA expression is found in a small group of cells at the
summit of the meristem in each tissue layer — the presumptive stem cells. CLV3
is postulated to be the ligand of CLV1, which encodes a leucine-rich repeat
transmembrane receptor serine-threonine kinase. Together, CLV1 and CLV3 are thought to coordinate proliferation and differentiation in the meristem.
14. Laufs P, Grandjean O, Jonak C, Kieu K, Traas J:Cellular parameters
• of the shoot apical meristem in Arabidopsis.Plant Cell 1998,
10:1375-1390.
A study of the spatial distribution of mitotic activity in the shoot apical meris-tem. The central domain of cells with low levels of mitotic activity is larger in
clv3mutants than in the wildtype.
15. Larkin JC, Marks MD, Nadeau J, Sack F:Epidermal cell fate and
patterning in leaves.Plant Cell 1997, 9:1109-1120.
16. Hülskamp M, Schnittger A:Spatial regulation of trichome formation
in Arabidopsis thaliana.Sem Cell Dev Biol1998, 9:213-220.
17. Lloyd AM, Schena M, Walbot V, Davis RW:Epidermal cell fate
determination in Arabidopsis: patterns defined by
18. Glover BJ, Perez-Rodriguez M, Martin C:Development of several epidermal cell types can be specified by the same MYB-related
plant transcription factor.Development 1998, 125:3497-3508.
19. Hülskamp M, Misera S, Jürgens G:Genetic dissection of trichome
cell development in Arabidopsis.Cell 1994, 76:555-566.
20. Schnittger A, Jürgens G, Hülskamp M:Tissue layer and organ
•• specificity of trichome formation are regulated by GLABRA1and
TRIPTYCHONin Arabidopsis.Development 1998, 125:2283-2289.
Overexpression of GLABRA1(GL1) in the absence of TRIPTYCHON(TRY)
was shown to result in ectopic trichome initiation in the subepidermal tissue
layers of Arabidopsis. This is the only report to date of the expression of
cell-type-specific genes overriding tissue-layer-specific restrictions.
21. Jacks T, Weinberg RA:The expanding role of cell cycle regulators.
Science 1998, 280:1035-1036.
22. Cunto FD, Topley G, Calautti E, Hsiao J, Ong L, Seth PK, Dotto GP: Inhibitory function of p21Cip/WAF1 in differentiation of primary
mouse keratinocytes independent of cell cycle control.Science
1998, 280:1069-1072.
23. Novitch BG, Mulligan GJ, Jacks T, Lassar AB:Skeletal muscle cells
lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle.
J Cell Biol 1996, 135:441-456.
24. Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC:ICK1, a
cyclin-dependent protein kinase inhibitor from Arabidopsis
thalianainteracts with both Cdc2a and CycD3, and its expression
is induced by abscisic acid.Plant J1998, 15:501-510.
25. Huntley R, Healy S, Freeman D, Lavender P, de Jager S, Greenwood J,
Makker J, Walker E, Jackman M, Xie Q et al.:The maize
retinoblastoma protein homologue ZmRb-1 is regulated during leaf development and displays conserved interactions with G1/S
regulators and plant cyclin D (CycD) proteins.Plant Mol Biol1998,
37:166-169.
26. Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH:Cytokinin
•• activation of Arabidopsiscell division through a D-type cyclin.
Science 1999, 283:1541-1544.
The activation of cell divisions by cytokinin was shown to be closely
corre-lated with the up-regulation of CycD3in Arabidopsis, and the formation of
calli was independent of cytokinin when CycD3was overexpressed. Plants
overexpressing CycD3showed abnormal meristems and leaves, pointing to
an additional role for CycD3in the control of cell differentiation.
27. Pirrotta V:Chromatin-silencing.Trends Genet1997, 13:314-318.
28. Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM,
Coupland G:A polycomb-group gene regulates homeotic gene
expression in Arabidopsis.Nature 1997, 386:44-51.
29. Grossniklaus U, Vielle-Calzada J, Hoeppner MA, Gagliano WB:
• Maternal control of embryogenesis by MEDEAa polycomb group
gene in Arabidopsis.Science 1998, 280:446-450.
Medea(mea) is a gametophytic maternal effect mutant displaying excessive
embryo growth. MEAencodes a polycomb group gene containing a SET
domain that is similar to the Enhancer of zestein Drosophila.
30. Ohad N, Margossian L, Hsu Y, Williams C, Repetti P, Fischer RL: A mutation that allows endosperm development without
fertilization.Proc Natl Acad Sci USA 1996, 93:5319-5324.
31. Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ:
Fertilization-independent seed development in Arabidopsis
thaliana.Proc Natl Acad Sci USA 1997, 94:4223-4228.
32. Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM:
• Genes controlling fertilization-independent seed development in
Arabidopsis thaliana.Proc Natl Acad Sci USA1999, 96:296-301.
This paper reports the cloning of FIS1and FIS2. FIS1encodes a
Polycomb-like protein, whereas FIS2encodes a C2H2zinc-finger-containing protein.
FIS2is one of three genes identified that are required to prevent the
devel-opment of unfertilized seeds. It is interesting that FIS2is the only gene of
this class that does not encode a putative Polycomb-like protein.
33. Kiyosue T, Ohad N, Yadegari R, Hannon M, Dinneny J, Wells D, Katz A,
• Margossian L, Harada JJ, Goldberg RB et al.:Control of
fertilzation-independent endosperm development by the MEDEApolycomb
gene in Arabidopsis.Proc Natl Acad Sci USA 1999, 96:4186-4191.
This paper reports the cloning of f644, a gene involved in the regulation of embryo and endosperm development. f644 encodes a Polycomb-like protein
that is identical to the MEDEAgene. The analysis of new alleles displaying
a different phenotype to those of previously characterized alleles revealed
new insights into the function of MEDEA.
34. Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Goldberg JJ,
• Fischer RL:Mutations in FIE, a WD polycomb group gene, allow
endosperm development without fertilzation.Plant Cell 1999,
11:407-416.
The cloning of FIE, a gene required to inhibit endosperm development without
fertilization, is reported. FIEencodes a WD-motif-containing Polycomb protein.
35. Ingram R, Charrier B, Scollan C, Meyer P:Transgenic tobacco plants
• expressing the Drosophila Polycomb (Pc) chromodomain show
developmental alterations: possible role of Pc chromodomain proteins in chromatin-mediated gene regulation in plants.
Plant Cell 1999, 11:1047-1060.
The chromodomain of the DrosophilaPolycomb protein, which mediates
tar-geting to chromosomal regions, was fused to GFP and introduced into tobacco. The analysis of the GFP–chromodomain fusion in the nuclei of transgenic plants showed that it localized to distinct chromosomal regions. The transgenic plants revealed various developmental abnormalities includ-ing deformed flowers and leaves. In addition, a homeodomain gene that is normally down-regulated in leaves showed enhanced expression.
36. Messmer S, Franke A, Paro R:Analysis of the functional role of the
Polycomb chromodomain in Drosophila melanogaster.Genes Dev
1992, 5:1241-1254.
37. Finnegan EJ, Peacock WJ, Dennis ES:Reduced DNA methylation in
Arabidopsis thalianaresults in abnormal plant development.Proc Natl Acad Sci USA 1996, 93:8449-8454.
38. Ronemus MJ, Galbiati M, Ticknor C, Chen J, Dellaporta SL:
Demethylation-induced developmental pleiotropy in Arabidopsis.
Science 1996, 273:654-657.
39. Henikoff S, Comai L:A DNA methyltransferase homolog with a
• chromodomain exists in multiple polymorphic forms in
Arabidopsis.Genetics 1998, 149:307-318.
CMT1, a DNA methyltransferase that contains a chromodomain that is
embedded within the catalytic region, was identified in Arabidopsis. A
sequence comparison between different ecotypes indicates that this gene is
not essential in Arabidopsis. It has, however, been conserved during
evolu-tion and is also found in Cardaminopsis arenosa, an outcrossing relative.
40. Satina S, Blakeslee AF, Avery A:Demonstration of three germ
layers in the shoot apex of Datura by means of induced
polyploidy in periclinal chimeras.Amer J Bot1940, 27:895-905.
41. Stewart RN, Burk LG:Independence of tissues derived from apical
layers in ontogeny of the tobacco leaf and ovary.Amer J Bot 1970,
57:1010-1016.
42. Szymanski DB, Marks MD:GLABROUS1overexpression and
•• TRIPTYCHONalter the cell cycle and trichome cell fate in
Arabidopsis.Plant Cell 1998, 10:2047-2062.
Plants overexpressing GL1in a trymutant background are shown to
pro-duce subepidermal trichomes. In 35S:GL1, tryand the double mutant cell
cycle control was altered in trichomes, the epidermis and the mesophyll.
43. Lu P, Porat R, Nadeau JA, O’Neill SD: Identification of a meristem
L1 layer-specific gene in Arabidopsisthat is expressed during
embryonic pattern formation and defines a new class of