Genotypic difference in salinity and water stress tolerance of fresh
market tomato cultivars
Akram Alian
a, Arie Altman
a, Bruria Heuer
b,*
aDepartment of Horticulture,The Otto Warburg Center for Agricultural Biotechnology,Faculty of Agriculture,
The Hebrew Uni6ersity of Jerusalem,Reho6ot,Israel
bInstitute of Soil,Water and En6ironmental Sciences,ARO,The Volcani Center,Bet Dagan,Israel
Received 23 June 1999; received in revised form 22 October 1999; accepted 22 October 1999
Abstract
The physiological response and tolerance of four fresh-market, cultivated tomatoes (Lycopersicon esculentumcvs. ‘F121’, ‘F144’, Fireball and Patio) to water stress and salinity was determined. Under salinity, ion accumulation, osmotic potential and dry matter production were highly correlated. No such correlation was found under water stress. No correlation between plant tolerance and accumulation of proline could be determined. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Ion content;Lycopersicon esculentum; Osmotic potential; Proline
www.elsevier.com/locate/plantsci
1. Introduction
Tomatoes, one of the world’s most important and widespread crops, are classified as moderately salt tolerant [1]. In general, most of the research on tomatoes was devoted to the study of the differential responses to salinity and water stress of wild versus domesticated species, aiming at donor selection for tomato breeding programs [2 – 4]. Very few reports on the genotypic variation in cultivated Lycopersicon esculentum are available [5,6]. Although tomato plants usually require a high water potential for optimal growth [7], the information on tomato response to water or salin-ity stresses is rather scarce [8,9].
Osmotic adjustment, at the physiological level, is an adaptive mechanism involved in drought or salinity tolerance which permits the maintenance of turgor under conditions of water deficit [10 –
12]. Under salt stress conditions, osmotic adjust-ment is usually achieved by the uptake of inorganic ions from the growth media [13,14]. This accumulation of ions is often accompanied by mineral toxicity and nutritional imbalance. Con-trariwise, under water stress, osmotic adjustment is obtained by production and accumulation com-patible organic solutes in the cytoplasm, in addi-tion to several other cellular and molecular mechanisms. The major compatible solutes include free amino acids [15], glycine betaine [16], sugars [17], proline [18,19] and ectoine [20]. The few available reports on the osmotic adjustment of wild or cultivated tomatoes pertain to short peri-ods of stress, up to 15 days, and the results are not consistent. In some studies partial osmotic adjust-ment was reported [5,9], while in others no os-motic adjustment could be determined [8].
This study is aimed at providing additional in-formation on the ability of domesticated tomato cultivars to osmotically adjust to long-term expo-sure to water stress and salinity, and to determine possible relationship between osmotic adjustment and plant tolerance to environmental stress.
* Corresponding author. Tel.:+972-3-968-3704; fax:+ 972-3-960-4017.
E-mail address:[email protected] (B. Heuer)
Table 1
Fresh matter accumulation (g plant−1) in four tomato cultivars exposed to water stress and salinity
Young leaves
Treatment Old leaves Stems Roots
‘F144’
25.9092.70 51.8493.62
43.8495.10 Control 43.0695.14
135 mM NaCl 22.5992.35 45.4395.69 31.5493.69 21.7593.13 10% PEG 34.5093.00 24.4691.76 34.8292.30 20.1291.78
5.66
LSD0.05 8.05 13.95 7.11
‘F121’
Control 46.0195.85 38.6292.63 44.6594.08 28.9792.12 135 mM NaCl 24.2692.82 45.9494.40 21.8992.09 31.8893.90 13.2290.92 21.9290.11
15.5891.38 10% PEG 29.5691.76
5.70
LSD0.05 8.46 6.67 5.76
Fireball
34.4096.02 Control 36.7193.62 118.70924.03 29.7095.52
135 mM NaCl 43.3197.64 86.28917.29 26.9193.10 25.5392.54 10% PEG 32.5695.40 85.28916.45 30.6393.50 31.9494.51
8.12 38.03
LSD0.05 11.26 8.92
Patio
Control 73.9198.24 71.9096.34 41.5292.56 35.6193.83 20.7793.21 24.4191.92
51.1496.25 135 mM NaCl 57.9596.45
18.0194.93 10% PEG 42.0196.92 34.7296.39 27.3494.43
7.84
LSD0.05 14.08 12.31 6.14
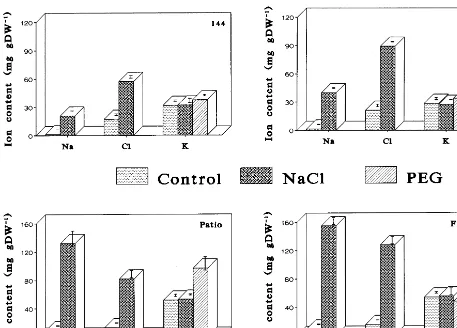
Fig. 2. Accumulation of Na+, Cl−and K+in young leaves of four tomato cultivars as affected by water stress and salinity.
2. Materials and methods
2.1. Plant material and growth conditions
Seeds of tomato (Lycopersicon esculentum Mill. cvs. ‘F121’ and ‘F144’ from Hazera, Israel), and Lycopersicon esculentum L. cvs. Fireball (Stokes Seeds, USA) and Patio (Tomato Growers supply company, USA) were germinated in vermiculite and grown in a growth chamber at 2591°C, 50910% relative humidity and a 13-h photope-riod at a quantum flux density of 420mE m−2s−1
at plant height, provided by fluorescent lamps (F58 Polylux 940, General Electric). Fourteen days after emergence, seedlings were transferred to half-strength Hoagland nutrient solution [21] which was continuously aerated and deionized water was added regularly to replace the water lost by tran-spiration. After three additional weeks, the plants were divided into three groups and subjected for 4 weeks to the following treatments: (1) gradual salinization of the medium by daily addition of 45
mM, to a final concentration of 135 mM NaCl (equivalent to an osmotic potential of −0.6 MPa); (2) lowering the osmotic potential of the nutrient solution with two consecutive additions of 5% polyethyleneglycol (PEG) 6000 to a final con-centration of 10% (w/v), equivalent to −0.112 MPa [22]; (3) standard nutrient solution (as non-salinized plants). The experiments were not carried out using solutions of iso-osmotic potential, since a concentration of 135 mM NaCl affects tomato plants without killing them, while PEG concentra-tions at the same osmotic potential are toxic. The experiments were repeated twice with three repli-cates of each treatment.
2.2. Growth and chemical analysis
top) and old (sixth leaf from top) leaves, stems and roots.
2.3. Ion content
Sodium and K+ content was determined in dry plant material following wet ashing with concen-trated H2SO4with a Corning 400 flame photome-ter. For chloride determination, dry plant material was extracted with 0.1 N HNO3 in 10% (v/v) acetic acid and Cl−was determined in the extracts using a Buckler-Cotlove chloridometer.
2.4. Osmotic potential
The osmotic potential (spontaneous osmotic po-tential) of leaf and root samples removed from each treatment was determined following their freezing and thawing with a Precision 5004 os-mometer.
2.5. Proline content
Proline content was measured according to the method of Bates [23]. Plant material was extracted
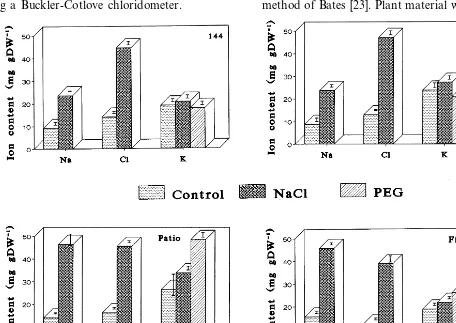
Fig. 3. Accumulation of Na+, Cl−and K+in roots of four tomato cultivars following plant exposure to water stress and salinity for 4 weeks.
Table 2
Sodium:potassium ratio in leaves and roots of four tomato cultivars exposed to 135 mM NaCl
Non saline control
Cultivar 135 mM NaCl
Young leaves Old leaves
Young leaves Old leaves Roots Roots
2.82 2.94 2.14 Fireball 0.17 0.10 0.84
0.61 2.49 2.61
Patio 0.18 0.10 1.72
1.96 0.87 ‘F121’ 0.05 0.12 0.40 1.45
0.48 0.65 0.40
Table 3
Proline content (mmole g FW−1) in tomato plants as affected by water stress and salinity
Old leaves Stems
Treatment Young leaves Roots
‘F144’
LSD0.05 0.18 0.89 0.51
‘F121’
LSD0.05 0.69 0.60 0.77
Fireball
1.290.07 0.890.03 0.890.05 Control 4.290.12
4.490.10 4.890.09
at room temperature with distilled water for sugar determination and with 3% aqueous 5-sulfosali-cylic acid for proline determination.
2.6. Statistical analysis
The data are presented with the respective stan-dard errors of the means, and the least significant difference (LSD0.05) between treatments, derived from an analysis of variance.
3. Results
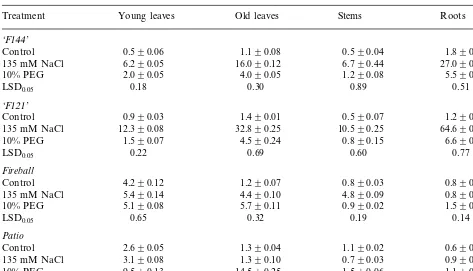
Plant growth of cv. Fireball, determined as the accumulation of fresh weight, was not significantly affected by the salinization of the nutrient solution (Table 1). The same was true for roots and old leaves of cvs. ‘F144’ and ‘F121’, while the fresh weight of young leaves of these cultivars were severely reduced. It is noteworthy that the young leaves developed after the stress treatments were imposed. Growth of all organs of cultivar Patio was significantly reduced by salinity. Similarly to salt stress, water stress also did not affect growth of cv. Fireball (Table 1). Between the four
culti-vars, the most pronounced influence of water stress could be determined in cvs. ‘F121’ and Patio. A similar response was found when the dry weight was measured (data not shown). Conse-quently, it can be concluded that cv. Fireball showed full tolerance to water or salt stress on the basis of fresh or dry weight increase of different plant organs. To the best of our knowledge, this is the first time that such a clear response to environ-mental stress could be observed among fresh-mar-ket, cultivated tomatoes. These results suggest that the different cultivars are able to fully or partially adjust to the reduction of the osmotic potential in the growth media.
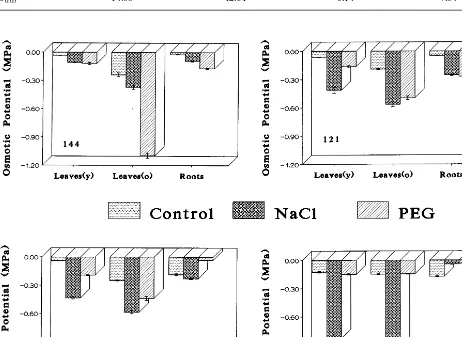
and the roots of both cultivars fully adjusted. In general, the osmotic potential of old leaves is more negative than that of the young ones.
Plant exposure to salinity induced the uptake and accumulation of considerable amounts of sodium and chloride which was cultivar dependent and organ specific. Cultivar Fireball accumulated the highest amount of both ions (Fig. 2). In Fire-ball young leaves, the content of Na+ was by 1.17, 3.86 and 7.42 higher than in cvs. Patio, ‘F121’ and ‘F144’, respectively, (Fig. 2). Similar accumulation of Na+ was found in the old leaves (data not shown). The content of Cl− in young Fireball leaves was by 1.56, 1.43 and 2.23 higher than in cvs. Patio, ‘F121’ and ‘F144’, respectively, while its old leaves accumulated by 1.34 and 2.36 more Cl− than cvs. Patio and ‘F144’. However, the roots of Fireball contained 17, 18 and 13% less Cl− than the roots of Patio, ‘F121’ and ‘F144’, respectively (Fig. 3). Surprisingly, they also con-tained 28% less Na+ than in Patio roots, but almost a double amount as compared with roots of ‘F144’ and ‘F121’. Older leaves of all cultivars had higher Cl− concentrations than younger leaves. As a result, their osmotic potential was more negative (Fig. 1). The fluctuations in the content of K+ were relatively small in all the plants. The calculated Na:K ratio was significantly increased in Fireball, Patio and ‘F121’ but much less in ‘F144’ (Table 2).
Compatible organic solutes, accumulate follow-ing exposure to water and salinity stress. These, in addition to ions, play a role in osmoregulation. Consequently, we measured proline accumulation in different plant organs of the four cultivars. Both, salinity and water stress, had only little effect (although significant) on young leaf and root proline content in cv. Fireball (Table 3). It was increased in old leaves and stems by both treatments, and especially by salinity. In cv. Patio plants salinity had only a slight effect on proline content, while water stress increased it signifi-cantly, especially in the leaves. On the contrary, salinity and less so water stress, remarkably in-creased proline accumulation in the leaves of both, ‘F121’ and ‘F144’ (Table 3). The highest accumu-lation was found in roots of cv. ‘F121’, by 53 times more than in the non-saline controls. This amount contributes about 0.101 MPa to their osmotic potential and is equivalent to 49.5% of the net root osmotic potential.
4. Discussion
Genetic variability within a species offers a valu-able tool for studying mechanisms of salt toler-ance. One of these mechanisms depends on the capacity for osmotic adjustment which allows growth to continue under saline conditions. This is basically true for water stress, although osmotic adjustment is not achieved in the same way under both stresses. Under salt stress, this process is accomplished by uptake and accumulation of inor-ganic ions, mainly Na+ and Cl−. Under water stress, it is achieved by synthesis and accumulation of organic compatible solutes. When the four tested cultivars were compared, very significant differences in Na+ and Cl− concentrations were found, with the highest accumulation in cv. Fire-ball (Figs. 2 and 3). Consequently, the Na:K ratio was also significantly different (Table 2). It was reported that the Na:K ratio might serve as an indicator of crop tolerance to stress as the increase of Na+ in salt-tolerant species is generally associ-ated with a decrease in K+ [24]. However, salt tolerance is actually often associated with peticular ‘capacities’ to maintain K+ content high. Older leaves of all cultivars had higher Cl− concentra-tions than younger leaves, probably because of a rapid area increase in expanding leaves.
-tions (Table 3). These results contradict short term response of tomatoes reported elsewhere [9].
Based on growth parameters, ion accumulation and young leaves osmotic potentials, the salt toleance of the different fresh market cultivars might be classified in the following decreasing order: Fireball\Patio\‘F121’\‘F144’. As al-ready mentioned, to the best of our knowledge, it is the first time that a completely different toler-ance of cultivated (and not in relation to wild type) tomatoes to drought and salinity could be demonstrated. Salt tolerance of plants has been usually expressed as the yield decrease for a given level of soluble salts in the root zone as compared with the yield of non saline controls. Although cv. Fireball tolerated the highest levels of Na+ in the shoots and roots, its fresh and dry weights were the lowest, suggesting that this cultivar may serve as a potential source for salt-tolerance breeding programs of cultivated tomatoes, and especially for those characterized by high vegetative growth and severe stress effects.
Acknowledgements
Contribution from the Agricultural Research Organization, Institute of Soils, Water and Envi-ronmental Sciences, Bet Dagan, Israel, No. 633-1999.
References
[1] E.V. Maas, Salt tolerance of plants, Appl. Agric. Res. 1 (1986) 12 – 26.
[2] M.C. Bolarin, F.G. Fernandez, V. Cruz, J. Cuartero, Salinity tolerance in four wild tomato species using vege-tative yield-salinity response curves, J. Am. Soc. Hort. Sci. 116 (1991) 286 – 290.
[3] J. Cuartero, A.P. Yeo, T.J. Flowers, Selection of donors for salt-tolerance in tomato using physiological traits, New Phytol. 12 (1992) 63 – 69.
[4] G. Guerrier, Fluxes of Na+, K+and Cl−and osmotic adjustment in Lycopersicon pimpinellii folium and L.
esculentum during short- and long-term exposure to NaCl, Physiol. Plant. 97 (1996) 583 – 591.
[5] J.J. Alarcon, M.J. Sanchez-Blanco, M.C. Bolarin, A. Torrecillas, Growth and osmotic adjustment of two tomato cultivars during and after saline stress, Plant Soil 166 (1994) 75 – 82.
[6] M. Caro, V. Cruz, J. Cuartero, M.T. Estan, M.C. Bo-larin, Salinity tolerance of normal-fruited and cherry tomato cultivars, Plant Soil 136 (1994) 249 – 255. [7] P.D. Waister, J.P. Hudson, Effect of soil moisture
regimes on leaf deficit transpiration and yield of toma-toes, J. Hort. Sci. 45 (1970) 359 – 370.
[8] F. Perez-Alfoncea, M.T. Estan, M. Caro, G. Guerrier, Osmotic adjustment inLycopersicon esculentum andL.
penellii under NaCl and polyethyleneglycol 6000 isoos-motic stresses, Physiol. Plant. 87 (1993) 493 – 498. [9] A. Torrecillas, C. Guillaume, J.J. Alarcon, M.G.
Ruiz-Sanchez, Water relations of two tomato species under water stress and recovery, Plant Sci. 105 (1995) 169 – 176. [10] J.M. Cutler, D.W. Rains, Effect of water stress and hardening on the internal water relations and osmotic constituents of cotton leaves, Physiol. Plant. 2 (1978) 261 – 268.
[11] T.C.E. Hsiao, E. Acevedo, E. Fereres, D.W. Henderson, Water stress, growth and osmotic adjustment, Philos. Trans. R. Soc. Lond. Series B. 278 (1973) 479 – 500. [12] J.M. Morgan, Osmoregulation and water stress in higher
plants, Annu. Rev. Plant Physiol. 35 (1984) 299 – 319. [13] T.J. Flowers, P.F. Troke, A.R. Yeo, The mechanism of
salt tolerance in halophytes, Annu. Rev. Plant Physiol. 28 (1977) 89 – 121.
[14] D. Prat, R.A. Fathi-Ettai, Variation in organic and mineral components in youngEucalyptusseedlings under saline stress, Physiol. Plant. 79 (1990) 479 – 486. [15] R.F. Meyer, J.S. Boyer, Osmorelation solute distribution
and growth in soybean seedlings having low water poten-tials, Planta 151 (1981) 482 – 489.
[16] D. Rhodes, A.D. Hanson, Quartenary ammonium and tertiary sulfonium compounds in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 44 (1993) 357 – 384. [17] R. Munns, R. Weir, Contribution of sugars to osmotic adjustment in elongating and expanded zones of wheat leaves during moderate water deficits at low light levels, Aust. J. Plant Physiol. 8 (1981) 93 – 105.
[18] A.J. Delauney, D.P.S. Verma, Proline biosynthesis and osmoregulation in plants, Plant J. 4 (1993) 215 – 223. [19] B. Heuer, Osmoregulatory role of proline in water and
salt-stressed plants, in: M. Pessarakli (Ed.), Handbook of Plant and Crop Stress, Marcel Dekker, NewYork, 1994, pp. 363 – 381.
[20] R.A. Ciulla, M.R. Diaz, B.F. Taylor, M.F. Roberts, Organic osmolytes in aerobic bacteria from mono lake, an alkaline, moderately hypersaline environment, Appl. Environ. Microbiol. 63 (1997) 220 – 226.
[21] D.J. Arnon, Microelements in culture experiments with higher plants, Ann. J. Bot. 25 (1938) 322 – 340.
[22] N.P. Money, Osmotic pressure of aqueous Polyethylene glycols, Plant Physiol. 91 (1989) 766 – 769.
[23] L.S. Bates, R.P. Waldren, I.D. Teare, Rapid determina-tion of free proline for water stress studies, Plant Soil 39 (1973) 205 – 207.
[24] H. Greenway, R. Munns, Mechanisms of salt tolerance in nonhalophytes, Ann. Rev. Plant Physiol. 31 (1980) 149 – 190.