Short Communication
Changes in soil enzyme activities following additions of cyanobacterial
biomass and exopolysaccharide
G.Z. de Caire
a,*, M.S. de Cano
a, R.M. Palma
b, C.Z. de Mule´
aaFacultad de Ciencias Exactas y Naturales, Intendente Gu¨iraldes 2620, C1428EHA, Buenos Aires, Argentina bFacultad de Agronomı´a, Av. San Martı´n 4453, C1417ASK, Buenos Aires, Argentina
Accepted 10 June 2000
Abstract
The aim of this research was to establish changes in the overall activity of extracellular enzymes:b-glucosidase, phosphomonoesterase, arylsulphatase, protease and urease and the intracellular enzyme dehydrogenase produced by the addition ofTolypothrix tenuisand Micro-chaete tenera (Cyanobacteria) exopolysaccharide (EPS) and biomass to a silty clay loam soil. Both biomass and EPS of M. tenera
significantly p,0:05 increasedb-glucosidase activity by 0.1 and 29.3%, respectively. Both cyanobacteria species significantly p,
0:05increased urease activity by 15^1%:Cyanobacterial biomass and EPS fromT. tenuisandM. teneraproduced significant increases in
protease (76–90%, 101–136%), phosphomonoesterase (19–27%, 13–22%), arylsulphatase (148–167%, 406–174%) and dehydrogenase activity (16–32%, 43–30%).q2000 Elsevier Science Ltd. All rights reserved.
Keywords: Extracellular enzymes; Intracellular enzymes; Cyanobacteria; Exopolysaccharides
Decomposition of residues in soil releases nutrients such as nitrogen, phosphorus and sulphur required for plant and microbial growth. Various processes are involved in this nutrient cycling including reactions caused by inorganic catalysts (Conti, 1998) and those catalysed by intracellular enzymes (e.g. dehydrogenase) and extracellular enzymes such asb-glucosidase, protease, phosphomonoesterase and arylsulphatase. The extracellular enzymes are derived from microorganisms, plant roots and soil animals (Martens et al., 1992).
Incorporation of organic materials into soil promotes microbial and soil enzyme activity. Soil enzymes are thought to be largely of microbial origin and some are obviously associated with viable cells; however many enzymes can remain catalytic in cell debris, in soil solution or complexed with clay or organic colloids. Although the ecological role of cell debris and extracellular complexed enzymes is yet to be completely explored, Burns (1982) hypothesized that humic–enzyme complexes may benefit some organisms by hydrolyzing substrates that are too large or insoluble for microbial uptake. Soils that have been managed to promote soil quality (e.g. minimum tillage, organic amendments, crop rotations) should have higher
biological activity than intensively used soils, and this should be reflected in greater enzyme production and possi-bly greater potential to stabilize and protect extracellular enzymes by forming soil enzyme complexes (Dick et al., 1996).
Cyanobacteria, such as Nostoc muscorum and Tolypo-thrix tenuis, produce extracellular enzymes that decompose organic residues (Hussein et al., 1989a,b). They are also used as inoculants to increase the polysaccharide content and microbial activity of soil (Storni de Cano et al., 1997; Zulpa de Caire et al., 1997; Zaccaro de Mule´ et al., 1999). The aim of this research was to identify changes in the activity of soil extracellular enzymes b-glucosidase, phos-phomonoesterase, arylsulphatase, protease and urease and the intracellular dehydrogenase following the addition of cyanobacterial exopolysaccharide and biomass to soil.
Strains ofT. tenuis(N840d) andMicrochaete tenera(N8
13a) were used. These were maintained in Allen and Stanier (1968) medium and are from the culture collection of the Biology of Cyanobacteria Laboratory, University of Buenos Aires. They were chosen from a number of species by comparing their growth on soil by dry weight assessment at day 30. To obtain the exopolysaccharide (EPS) and suffi-cient biomass, the strains were cultured in 2-l Erlenmeyer flasks with Allen and Stainer modified medium (without sodium nitrate) and incubated at 28^18C under fluorescent
Soil Biology & Biochemistry 32 (2000) 1985–1987
0038-0717/00/$ - see front matterq2000 Elsevier Science Ltd. All rights reserved. PII: S 0 0 3 8 - 0 7 1 7 ( 0 0 ) 0 0 1 7 4 - 7
www.elsevier.com/locate/soilbio
* Corresponding author. Fax:154-11-4524-8057.
light at 45mE m22s21. At the end of the exponential growth phase (25 days) the cyanobacterial biomass was separated by centrifugation (8000×g). EPS was isolated from the growth medium (i.e. supernatant after centrifugation) according to Nakagawa et al. (1987), but without a dialysis step. The wet weight harvested was 4.6 g l21for T. tenuis and 2.65 g l21forM. tenera. The pH of the final solutions were 8.6 and 8.4 forT. tenuisandM. tenera, respectively.
A poorly drained soil (Typic Argialboll USDA, 1994) with silty clay loam texture from Vieytes, Province of Buenos Aires, Argentine, was collected at 0–10 cm depth.
In order to establish the effect of biomass and exopoly-saccharide additions on the enzyme activities of the soil, two treatments were performed:
(i) Inoculation with biomass. Non-sterile soil samples dried, sieved (2 mm pore diam.) and 120 g dispensed in each of 30 plastic boxes 13×8×5 cm3:The soil was
saturated with distilled water (ù160 g final weight). The
boxes were kept at 25^18C and with constant moisture content throughout the experiment and placed under 45mmol photon m22s21 irradiance, 12 h photoperiod.
Ten boxes containing the soil were inoculated on the surface with 6 g wet weight T. tenuis and 10 with 6 g wet weight M. tenera (corresponding to 0.044 and 0.13 g dry weight, respectively). The remaining 10 boxes were kept as controls (received distilled water but no inoculum).
(ii) Addition of exopolysaccharide. Twenty boxes were prepared, as above. Ten boxes were amended with 40 ml T. tenuis EPS solution and 10 with 40 ml ofM. tenera EPS solution. Corresponding controls were set up.
After 90 days, the activities of b-glucosidase, urease, protease, phosphomonoesterase, arylsuphatase and dehy-drogenase were determined using the methods described by Alef and Nannipieri (1995).
An analysis of variance using a randomized complete block design was performed. The homogeneity of experi-mental error mean squares for treatment was examined using Bartlett’s test for homogeneity of variances. The Duncan Test was used to compare means (Steel and Torrie, 1985).
The biomass in the inoculated soil treatments grew during 90 days producing a film that covered 60–90% of the surface with mean thickness of 2–3 mm.
Theb-glucosidase, urease, protease, phosphomoesterase, arylsulphatase and dehydrogenase activities increased in most cases following cyanobacterial inoculation and EPS amendment (Tables 1 and 2). A small p,0:05 4%
increase in b-glucosidase activity was measured whenM. tenera biomass was added but there was no effect withT. tenuis. As the inoculants do not contain cellulose, there was no obvious addition of inducer molecules and cyanobacter-ial addition did not result in an increase in the biosynthesis of b-glucosidase by the indigenous soil microflora. The addition of M. tenera EPS did result in an increase in b -glucosidase probably because it provided organic nutrients forb-glucosidase-producing soil microflora thereby stimu-lating the synthesis of this enzyme.
The increase of urease activity produced following inocu-lation by both strains was significant p,0:05;but the low
values obtained were probably due to the low substrate concentration in the original soils without fertilization and after treatment.
The addition M. tenera, T. tenuis and their EPS led to large increases (100–140%) in soil protease activity, which may reflect the excretion of enzyme by the actively growing cyanobacteria although not all cyanobacteria excrete proteolytic enzymes. However, cyanobacterial EPS is known to exert a stimulating effect on microbial enzyme production (Dmitrovskii and Sadchikov, 1994).
Both the cyanobacterial inoculants and EPS increased acid phosphatase activity. T. tenuis probably produced extracellular cell-bound phosphatase as enzyme activity increased by 27%, while EPS addition produced an increase of 13%.M. tenerabiomass and exopolysaccharide produced similar effects: 19 and 22% increase in enzyme activity,
G.Z. de Caire et al. / Soil Biology & Biochemistry 32 (2000) 1985–1987
1986
Table 1
Soil enzyme activities after addition ofT. tenuis(40d) andM. tenera(13a) biomass. (Different letters between columns indicate significant differences p,0:05:^indicates standard deviation)
Enzyme activities T. tenuis40d M. tenera13a Control b-Glucosidase 188.98 b* 197.36 a 190.16 b mgp-nitrophenol g21h21
^19.5 ^20.5 ^18.5
Urease 191.43 a 180.53 b 165.61 c
mg N-urea g21h21
^20.1 ^17.6 ^18.8
Protease 119.96 a 129.62 a 68.06 b
mg tyrosine g21h21 ^12.6 ^14.2 ^7.3 Phosphatase 1360.66 a 1274.83 b 1068.13 c mgp-nitrophenol g21h21 ^139.8 ^135.5 ^100.5
Arylsulphatase 75.17 a 80.82 a 30.28 b
mgp-nitrophenol g21h21 ^8.1 ^9.2 ^3.2
Dehydrogenase 6.55 a 5.77 b 4.98 c
mg formazan g2120 h21 ^0.8 ^0.7 ^0.5
Table 2
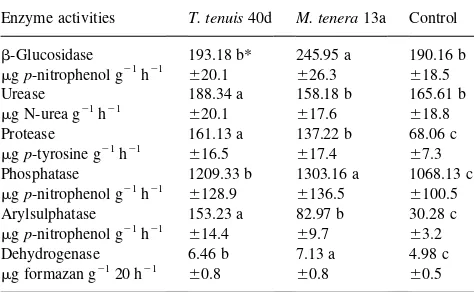
Soil enzyme activities after addition ofT. tenuis(40d) andM. tenera(13a) exopolysaccharide saccharides. (Different letters between columns indicate significant differences p,0:05:^indicates standard deviation) Enzyme activities T. tenuis40d M. tenera13a Control b-Glucosidase 193.18 b* 245.95 a 190.16 b mgp-nitrophenol g21h21
^20.1 ^26.3 ^18.5
Urease 188.34 a 158.18 b 165.61 b
mg N-urea g21h21
^20.1 ^17.6 ^18.8
Protease 161.13 a 137.22 b 68.06 c
mgp-tyrosine g21h21
^16.5 ^17.4 ^7.3 Phosphatase 1209.33 b 1303.16 a 1068.13 c mgp-nitrophenol g21h21
^128.9 ^136.5 ^100.5 Arylsulphatase 153.23 a 82.97 b 30.28 c mgp-nitrophenol g21h21
^14.4 ^9.7 ^3.2
Dehydrogenase 6.46 b 7.13 a 4.98 c
mg formazan g2120 h21
respectively. These results imply that M. tenera excreted, which is generally induced by P deficiency, is widespread among cyanobacteria; in several species it is optimal between pH 8.0 and 10.0 coinciding with the pH of Typic Argialboll (Healy, 1982).
Both strains produced significantly high increases in aryl-sulphatase activity. The values were highest with EPS addi-tion, especially for T. tenuis when a 400% increase was recorded. This increase may have been due to a combination of enzyme secretion and the stimulation of biosynthesis by other microorganisms.
An increase of dehydrogenase activity was stimulated by addition of both strains either as biomass or as EPS (16– 43%). Although dehydrogenase has been shown to be sensi-tive to soil management effects (Martens et al., 1992), it is less useful as a measure of long-term changes in soil quality because it cannot accumulate in a complexed form in soils and is best used as an indicator of the viable microbial populations (Dick et al., 1996).
In general, EPS addition (especiallyT. tenuis) produced a higher increase of the enzymatic activity than biomass addi-tion. The difference between the increments produced by biomass or EPS addition was caused in part by the necessity for the biomass to undergo cellular lysis prior to the libera-tion of intracellular enzymes as the results indicate very low excretion of extracellular enzymes. EPS solution contained proteins some of which could have exoenzymatic activity and other substances that could behave as inducer molecules that stimulate exoenzyme synthesis and secretion by indi-genous soil microorganisms.
According to our previous experience and the present results, addition of cyanobacteria could be a promising cultural practice for the amelioration of degraded soils.
References
Alef, K., Nannipieri, P., 1995. Methods in Applied Soil Microbiology and Biochemistry. Academic Press, London (576pp.).
Allen, M.M., Stanier, R.Y., 1968. Growth and division of some unicellular blue-green algae. Journal of General Microbiology 51, 199–202. Burns, R.G., 1982. Enzyme activity in soil: location and possible role in
microbial ecology. Soil Biology & Biochemistry 14, 423–427. Conti, M.E., 1998. Introduccio´n a la EdafologI´a con e´nfasis en suelos
argentinos. Distribucio´n Orientacio´n Gra´fica, Buenos Aires, Argentina (350pp.).
Dick, R.P., Breakwell, D.P., Turco, R.F., 1996. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In: Doran, J.W., Jones, A.J. (Eds.). Methods for Assessing Soil Quality, Soil Science Society of America, Madison, WI (410 pp.).
Dmitrovskii, L.G., Sadchikov, A.P., 1994. Stimulation of proteolytic bacteria activity by certain algae. Gidrobiologicheskii Zhurnal 30, 53–59.
Healy, F.P., 1982. Phosphatase. In: Carr, N.G., Whitton, B.A. (Eds.). The Biology of Cyanobacteria, Blackwell, Oxford (688 pp.).
Hussein, Y.A., Shalan, S.N., Abb-El-Wahab, S.M., Hassan, M.E., 1989a. The degradation of lignin by blue-green algae. International Journal of Experimental Botany (Phyton) 49, 1–4.
Hussein, Y.A., Abb-El-Wahab, S.M., Hassan, M.E., Shalan, S.N., 1989b. The specific degradation of lignin by blue-green alga. International Journal of Experimental Botany (Phyton) 49, 13–15.
Martens, D.A., Johanson, J.B., Frankerberger Jr, W.T., 1992. Production and persistence of soil enzymes with repeated addition of organic resi-dues. Soil Science 153, 53–61.
Nakagawa, M., Takamura, J., Yagi, O., 1987. Isolation and characterization of the slime from a cyanobacterium Mycrocystis aeruginosaK-3A. Agricultural Biology and Chemistry 51, 329–332.
Steel, R.G.D., Torrie, J.H., 1985. Principles and Procedures of Statistics. 2nd ed. McGraw-Hill, New York.
Storni de Cano, M., Zaccaro de Mule´, M.C., Zulpa de Caire, G., Palma, R.M., Colombo, K., 1997. Aggregation of soil particles by Nostoc muscorumAg. (cyanobacteria). International Journal of Experimental Botany (Phyton), 35–40.
United States Department of Agriculture (USDA), 1994. Key to Soil Taxonomy. 6th ed. (306 pp.).
Zaccaro de Mule´, M.C., Zulpa de Caire, G., Storni de Cano, M., Palma, R.M., Colombo, K., 1999. Effect of cyanobacterial inoculation and fertilizers on Rice seedlings and post-harvest soil structure. Commu-nication of Soil Science and Plant Analysis 30 (1–2), 204–210. Zulpa de Caire, G., Storni de Cano, M., Zaccaro de Mule´, M.C., Palma,
R.M., Colombo, K., 1997. Exopolysaccharide of Nostoc muscorum Ag. (Cyanobacteria) in the aggregation of soil particles. Journal of Applied Phycology 9, 249–253.