Devolatilization of coal in northeastern India in argon, air and oxygen-enriched air under fluidized beds. It is certified that the work contained in the thesis entitled "Devolatilization of Coal of Northeastern India in Argon, Air, and Oxygen-Enriched Air under Fluidized Bed Conditions", by Ramesh Chandra Ramesh Chandra Ramesh Chandra Ramesh Chandra Borah.
Characterization of coal
Proximate and ultimate analyses of the coal samples
Appendix 3.4 Calculation of volatile matter of the coal samples on air- dried basis
Calculation of the ash of the coal sample on air-dried basis
DEVOLATILIZATION OF COALS IN ARGON ATMOSPHERE UNDER
UNDER FLUIDIZED BED CONDITIONS
OXYGEN-ENRICHED AIR UNDER FLUIDIZED BED CONDITIONS
CONCLUSIONS AND SCOPE FOR FUTURE RESEARCH
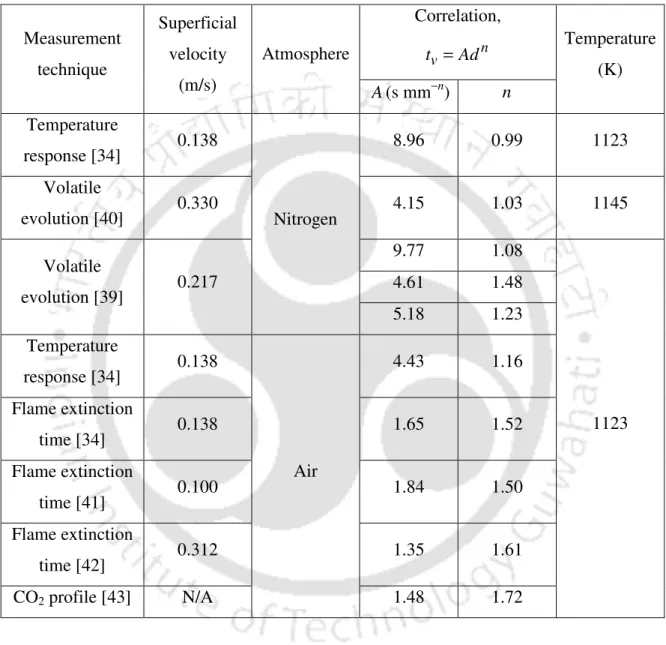
LIST OF TABLES
INTRODUCTION TO COAL CONVERSION PROCESSES
- Introduction
- Coal reserves in India
- Background of the present research
- Importance of the present work
- Objectives of the present work
About 10% of Assam coal is used in the coal mix charged to coke ovens. In most of the works reported in the literature, only the time of expropriation has been studied.
LITERATURE REVIEW
Introduction
The fundamentals of coal devolatilization .1 The chemical structure of low-rank coals
The decrease in the yield of the heavier products indicates that secondary degradation has taken place. The amount of deposition increases with the increase in the size of the particle, and therefore the yield of volatile matter decreases.
![Figure 2.1 Model of bituminous coal structure [2] (reproduced by permission from Elsevier Ltd., 1984)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/41.892.138.778.155.826/figure-model-bituminous-coal-structure-reproduced-permission-elsevier.webp)
Particle diameter (mm)
It can be seen from Figure 2.14 and Table 2.4 that the specific heat increases with the increase in the content of volatile substances. Specific heat can be an important factor in the volatility of coal, as it plays an important role in the rate at which coal is heated. There may be differences in the recovery of volatiles during de-evaporation depending on the content of volatiles in the coal.
A fluidized bed consists of a collection of particles suspended in a gas stream flowing upward at such a velocity that the particles do not escape from the vessel, but continue to circulate vigorously within the vessel. Because the bed provides resistance to flow, the drag forces, given by the pressure drop across the bed, are sufficient to support the weight of the bed. For solid fuels such as coal, the carbon content of the bed is no more than a few percent.
Temperature (K)Specific heat (kJ kg-1 K-1)
Models of coal devolatilization
These models address the structure of the parent coal and require knowledge of the functional groups present in the coal and their nature. Structural models are generally complex because they depend on the chemical structure of the parent coal. The CPD model describes coal conversion behavior based on the chemical structure of the parent coal [67].
The model requires three sets of input parameters: (i) coal-independent kinetic parameters, which are rate parameters, (ii) coal-dependent parameters, which are used to determine the chemical structure of the parent coal, and (iii) a elemental analysis of parent coal. The FG-DVC model starts with the parent coal structure as in the CPD model, but tar formation and the behavior of the carbon produced during devolatilization are treated more extensively. The second advantage is that extensive data on coal structure are not required in these models.
Modeling of devolatilization of large coal particles
They reported that with increase in fluidization rate, there is increase in the loss of carbon during devolatilization. This has been attributed to the higher rate of transfer of oxygen to the surface of the particle. Relatively few studies of mass transfer in the devolatilization of large particles have been reported.
Peters and Bertling [24] investigated the pyrolysis of coking coal in the size range 1 to 15 mm. Essenhigh [63] investigated the devolatilization of particles with a diameter ranging from 0.295 to 4.76 mm in a non-fluidized combustion system. The approach of LaNauze [49] is similar to that of Essenhigh [63], except in the treatment of the diffusion process.
Conclusions
Niksa, Modeling the devolatilization behavior of high-volatile bituminous coals, Proceedings of the 22nd International Simposium on Combustion, The Combustion Institute, Pittsburgh, 1988, pp. Saville, On the role of heating rate in rapid coal devolatilization, Proceedings of the 20th International Simposium on Combustion, The Combustion Institute, Pittsburgh, 1985, pp. Meissner, Rapid devolatilization of pulverized coal, Proceedings of the 15th International Simposium on Combustion, The combustion Institute, Pittsburgh, 1975, pp.
Stubington, The role of volatile coals in fluidized bed combustion, Journal of the Institute of Energy. Pillai, Influence of coal type on devolatilization and combustion in fluidized beds, Journal of Institute of Energy. Sarofim, Coal devolatilization at high temperatures, Proceedings of the 16th International Symposium on Combustion, The Combustion Institute, Pittsburgh, 1976, pp.
Introduction
It has been reported in the literature that the volatile material can burn in the freeboard [20], reducing the degree of heat transfer from the bed. There is inconsistency in the literature regarding the effect of gas velocity on the rate of devolatilization. In the gas monitoring method, the dilution ratio of gas is large at the higher velocity, and the inaccuracy due to dilution is large.
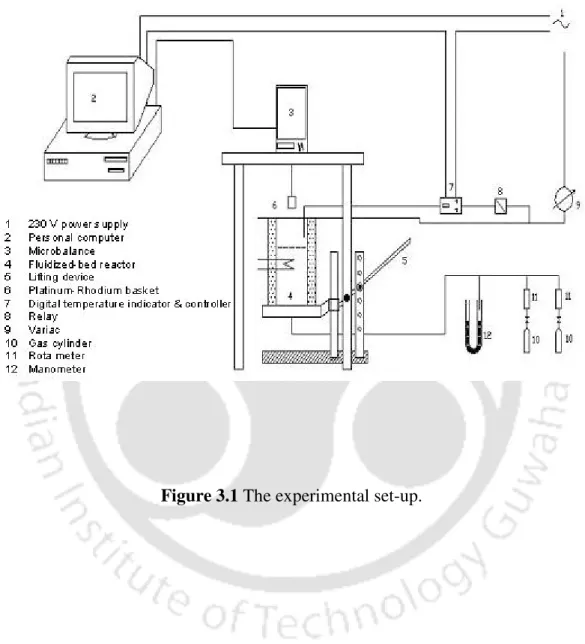
In this method, the mass loss measurement will be performed continuously by a sensitive and accurate microbalance during the time the sample is exposed to turbulent conditions in the freeboard of a hot fluidized bed. The use of this setup for collecting data on devolatilization in inert atmosphere, air and enriched air will be described. Next, the procedure applied for measuring the size of the particles will be explained.
Experimental studies on coal devolatilization .1 Experimental set-up
A cromel-alumel thermocouple was installed in the upstream part of the bed to measure and control the temperature. In order to determine the minimum fluidization velocity, a graph of the pressure drop across the layer versus the superficial gas velocity was made (Figure 3.2). The selection of particle mass (eg g) was chosen for each of the coal samples to calculate the mass equivalent of volume diameter ( )dv.
By subtracting the mass of the hanging wire from its weight in water, the volume of the coal was calculated based on the effect of displacement by water of known density (i.e. the Archimedean principle). When calculating the volume of displaced water, the density adjustments corresponding to the room temperature were taken into account. The mass of the nearly spherical particle was divided by the density of the coal to obtain the apparent volume of the coal particle.
Velocity of air (m/s)
Calculation of density of coal particle
Calculation of error
Calculation of the moisture content of coal sample on air-dried basis
Calculation of the volatile matter of the coal sample on air-dried basis
Determination of the percentages of carbon and hydrogen
Determination of nitrogen in coal
Determination of sulfur in coal
Calculation of gross calorific value [33]
As the mass of the barium sulphate found in the determination of sulfur in coal, kg B volume of standard sulfuric acid solution used in the blank determination of. Bs mass of the barium sulphate found in the determination of sulfur in the blank Eschka mixture, kg. M1 mass scale used for determining moisture content with lid, kg M2 mass scale used for determining moisture content with lid and coal.
M3 mass of container for determining moisture content with lid and coal sample after removal of moisture, kg. M1′ mass of the crucible for determining volatile substances with a lid, kg M2′ mass of the crucible for determining volatile substances with a lid and coal. M3′ mass of the crucible used for the determination of volatile substances with the lid and the residue after the removal of volatile substances from the coal sample, kg.
DEVOLATILIZATION OF COAL SAMPLES IN ARGON ATMOSPHERE UNDER FLUIDIZED BED CONDITIONS
Introduction
The volatile yield can be determined by pyrolysis, which provides the proportion of coal volatilized during the devolatilization step. The rate at which coal is vaporized in a fluidized bed combustor depends on several variables, such as particle size, atmosphere, fluidization rate, bed temperature, coal type and the amount of volatiles. However, the large particles can release volatiles above the bed because they are heated more slowly than the smaller particles [3].
Therefore, it is important to determine the amount of volatile material released by a single coal particle over time after its injection into the bed. In this chapter, volatilization studies of coal samples collected from five coals of northeastern India in argon atmosphere are reported. The performance of these correlations for single coal particles, and for a bundle of coal particles is investigated.
Analysis of the data on devolatilization in argon atmosphere
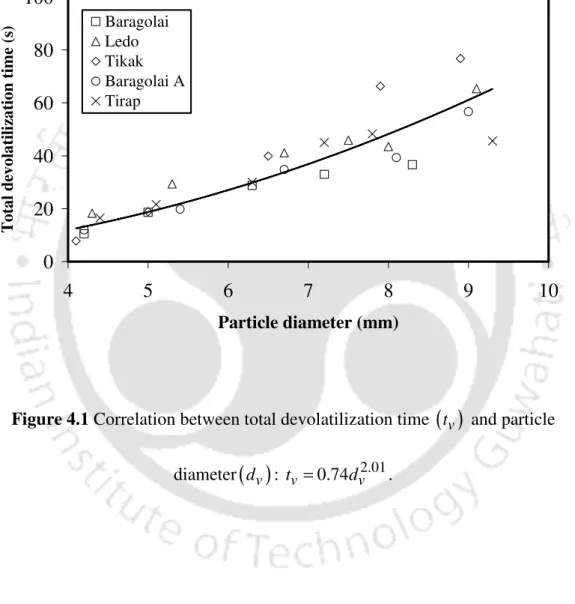
- Correlation between devolatilization time and particle diameter
This may be due to the fact that the heterogeneity in the composition of the coals of different coals caused the variations in the escape times, and this effect was significant for the large particles. There is no report in the literature on the escape time of a coal particle in a fluidized bed in an inert atmosphere at a flow velocity of 1 m/s. The likely reason for this variation lies in the type of coal studied and the superficial velocity used.
The values of A1 and n1 obtained in this work agree well with the values reported in the literature [3,11,12]. The coal used in other studies is different in composition from the coal used in the present study. However, based on the available data, they describe certain trends in the variation of the correlation parameters A1 and n1.
Particle diameter (mm)95% devolatilization time (s)Bulli
Correlation for mass-loss with time
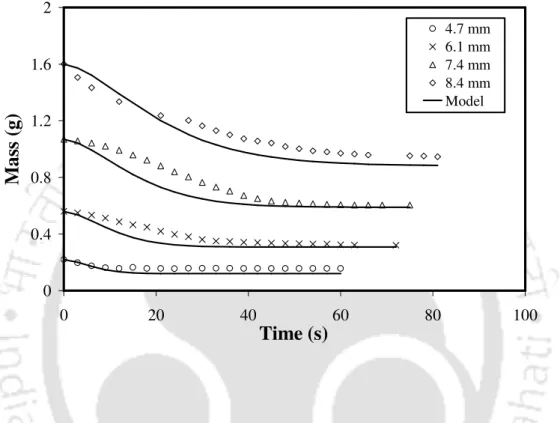
The change in particle mass over time for five different sized coals during devolatilization is shown in Figures 4.5–4.9. The initial rate of mass loss was lower for the larger particles than that for the smaller particles because the heating rate of the larger particles was slower than that of the smaller particles [3]. The devolatilization rate during this period depends on the particle size and volatile content of the particle.
The fractional mass loss (i.e. the fractional emission of volatile matter at time t), v(t), was represented by a simple profile. The ratio of volatile yield to air-dried near volatile content ranged from 0.98 to 1.24. The total volatile yield (V0− ∞V) can be taken to be 1.107 times the estimated volatile content of the coal.
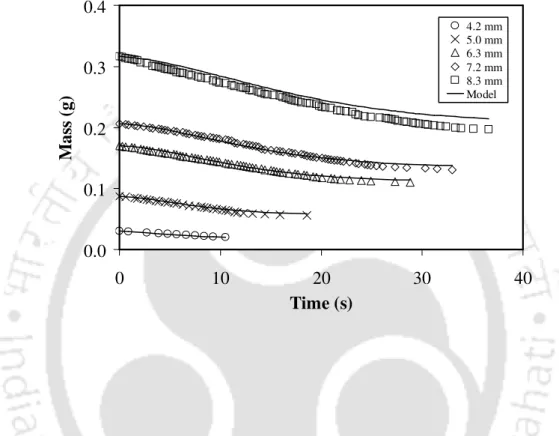
Variation of the yield of volatile matter with the volatile content of coals The ratio of the yield of volatile matter to the proximate volatile content
4.3) can be used to calculate the mass of a single coal particle until volatilization is complete. This also supports the fact that the low volatile coals are heated faster than the high volatile coals. Therefore, the residence time of volatiles is shorter for the low-volatile coal than that for the high-volatile coal.
Therefore, the ratio of the volatile yield to the nearest volatile content decreased with the increasing volatile content of the coals.
Devolatilization in multi-particle system
Visual observation of the coal particles at different times of pyrolysis
The central regions of the partially pyrolyzed coal particles were clearly unreacted and resembled the original coal.
Mechanism of devolatilization
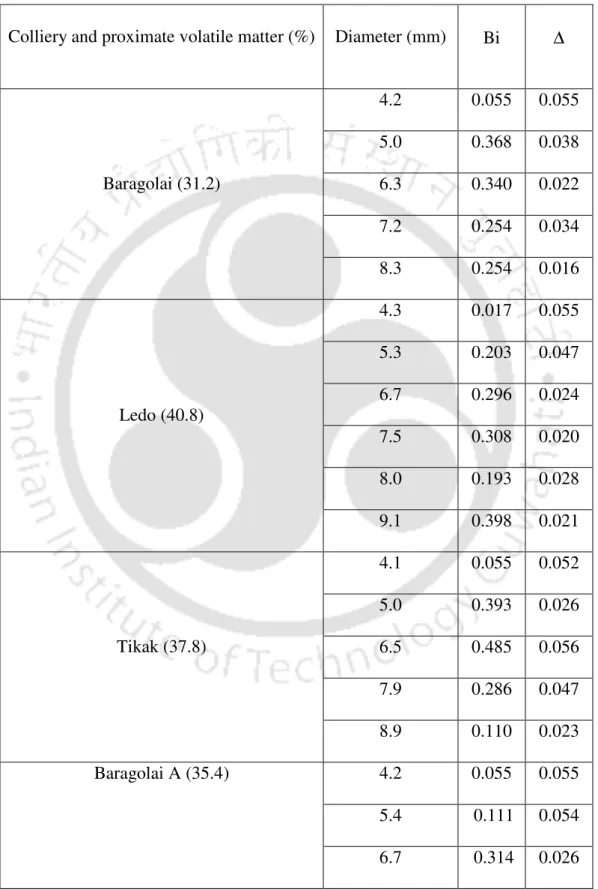
The values of the Biot number Bi=ha ( )3k for the coal particles are shown in table 4.4. Biottal less than 0.1 indicates that the heat conduction resistance inside the particle is much lower than the heat transfer resistance at the surface. The lump capacitance model of transient heat transfer is applicable for Biot numbers less than 0.1 [24].
For Biot numbers greater than unity, the internal heat transfer resistance is higher than external heat transfer resistance. The Biot numbers of the particles (≥5 mm) used in our study fall in the same range as reported by Chern and Hayhurst [23]. The apparent decrease in Biot number is a consequence of the reduction in the heat transfer coefficient with the increase in particle size.
Calculation of root mean square error (RMSE)
Sample calculation for the determination of Biot number
Thring, Measurement of the Burning Times of Individual Coal Particles, Proceedings of the Conference on Scientific use of Coal, Sheffield, 1958, pp. Essenhigh, Influence of coal rank on the burning times of individual trapped particles, Journal of Engineering for Power. Keairns, Coal Devolatilization Investigation in Support of the Westinghouse Fluidized Bed Coal Gasification Process, Fuel.
Hofbauer, Temperature in a fuel particle burning in a fluidized bed: the effect of drying, devolatilization and char burn-off, Combust and Flame. Code, Experimental study on devolatilization of large coal particles in a fluidized bed, Proceedings of 9th International Conference on Fluidized Bed Combustion, vol. Code, Devolatilization and Combustion of Large Coal Particles in a Fluidized Bed, Canadian Journal of Chemical Engineering.
DEVOLATILIZATION OF COAL SAMPLES IN AIR UNDER FLUIDIZED BED CONDITIONS
Introduction
![Figure 2.2 The main reactions which occur during coal pyrolysis as per the mechanism of devolatilization proposed by van Heek and Hodek [5] (adapted by](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/43.892.165.730.230.704/figure-reactions-pyrolysis-mechanism-devolatilization-proposed-hodek-adapted.webp)
![Figure 2.3 Hypothetical chemical structure of a sub-bituminous coal: (a) parent coal, and (b) during devolatilization [3] (reproduced by permission from Elsevier Ltd., ](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/45.892.179.732.131.935/hypothetical-chemical-structure-bituminous-devolatilization-reproduced-permission-elsevier.webp)
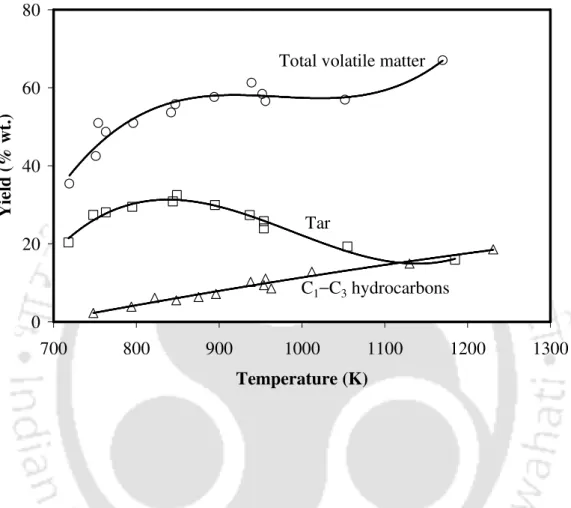
![Figure 2.5 Effect of temperature on the yields of C 3 H 6 and C 3 H 8 [16] (adapted by permission from Elsevier Ltd., 1979)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/51.892.157.737.250.864/figure-effect-temperature-yields-adapted-permission-elsevier-1979.webp)
![Figure 2.6 Effect of temperature on the yield of CH 4 [16] (adapted by permission from Elsevier Ltd., 1979)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/52.892.175.733.284.794/figure-effect-temperature-yield-adapted-permission-elsevier-1979.webp)
![Figure 2.7 Effect of temperature on the yields of C 2 H 4 and C 2 H 6 [16] (adapted by permission from Elsevier Ltd., 1979)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/53.892.158.736.299.877/figure-effect-temperature-yields-adapted-permission-elsevier-1979.webp)
![Table 2.1 Effect of temperature on the yield of products from a bituminous coal [12]](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/54.892.128.796.270.954/table-effect-temperature-yield-products-bituminous-coal-12.webp)
![Fig. 2.8 Variation of average heating rate with particle diameter [34] (adapted by permission from Elsevier Ltd., 2000)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10473859.0/60.892.154.738.283.883/variation-average-heating-particle-diameter-adapted-permission-elsevier.webp)