INTRODUCTION
- Background of Study
- Problem Statement
- Project Objective
- Scope of Study
- Relevancy of Project
- Feasibility of Project
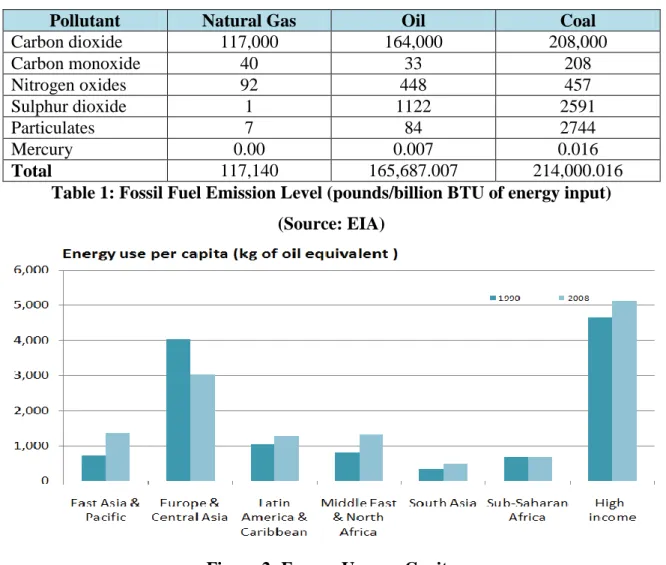
Apart from the CO2 impact on the environment, the removal of CO2 from the feed gas is essential for the gas and petrochemical industry to prevent dry ice formation in downstream low-temperature equipment, which can lead to equipment failure and production loss. For oxyfuel combustion, the high concentration of CO2 in flue gases is due to the fact that fuel is burned in almost pure oxygen instead of air. Chemical absorption of CO2 from gas streams in e.g. flue gases depends on acid-base neutralization reaction using basic solvents.
The solubility of CO2 in the solvent (absorbent) is at its best at higher CO2 partial pressure and lower temperature. This technology involves the selective removal of CO2 from a gas stream in absorbents (zeolite or charcoal), followed by regeneration (desorption), which can be achieved either by reducing the pressure (Pressure Adsorption or PSA), or by increasing the temperature ( Temperature-Swing Adsorption, or TSA) or by passing an electric current through the adsorbent (Electrical Swing Adsorption, or ESA) or process hybrids (PTSA) or washing according to Olajire, 2011. The corrosion problem in certain sections of the CO2 plant can be a major disadvantage as it leads to a decrease in efficiency and an increase in the operating cost of the process.
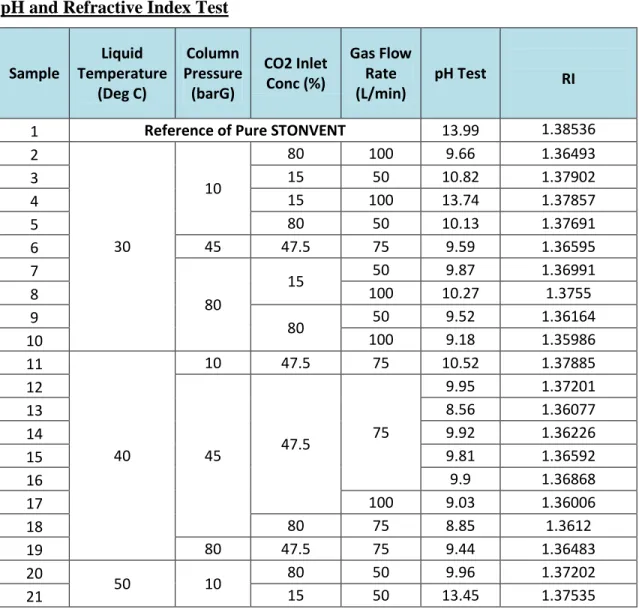
Differential level of CO2 inlet concentration and gas flow rate is introduced to the sample. In this article, the characteristic of STONVENT by-product will be identified, which will confirm whether STONVENT by-product properties undergo the same changes as amine by-product, which becomes the oxidizing agent with its protonated amine ion (Veiwab, 2009), so that a new solution can be proposed in to overcome major problems such as corrosion and degradation of CO2 scavenger.
LITERATURE REVIEW
- Environmental Issue
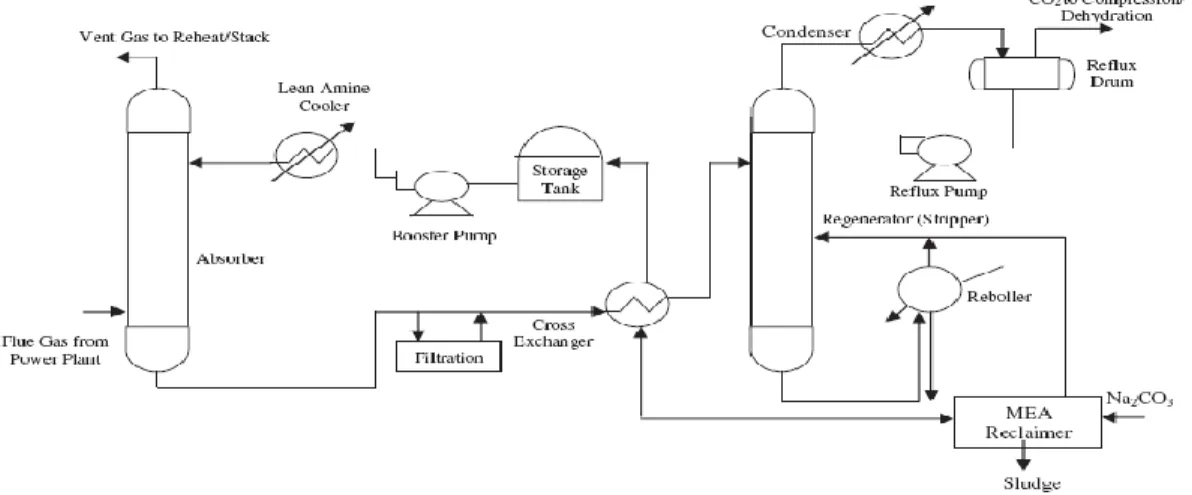
- CO 2 -Hydrocarbon Absorption System
- Alkanolamines Absorption
- Adverse Effect of Amine Based
- Solvent Development
From the data presented it is clear that it is very important to reduce the amount of CO2 in the atmosphere and many technologies are available nowadays. The choice of CO2 separation technique depends on a number of factors, for example the partial pressure of CO2 in the gas stream, the extent of CO2 recovery required, solvent regeneration, sensitivity to impurities such as acid gases and particulates, the purity of the desired product, capital and operating process costs, additive costs , needed to overcome fouling and corrosion, and, where appropriate, environmental impact [Yagi et.al., 2004]. The cryogenic method of CO2 purification takes place at low temperatures, which involves the separation of gas mixtures by fractional condensation and distillation.
Absorption of CO2 at low temperatures (35- . 50°C) and relatively low CO2 partial pressure (proportional to concentration) can occur if the solvent is strongly attracted to the solute, but this high attraction between the solvent and CO2 causes regeneration energy to be high. The absorption technology using amine solution or aqueous alkanolamines is considered the most mature technology, while mixed amine has been reported to maximize the desired qualities of the individual amines. Amines fall into different classes depending on how many of the hydrogen atoms are replaced.
Primary amines have only one of the hydrogen atoms in the ammonia molecule replaced. In secondary amine, alkyl groups have replaced two of the hydrogens in an ammonia molecule. Tertiary alkanolamines do not have a hydrogen atom attached to the nitrogen atom, as in the case of primary and secondary alkanolamines.
The low reactivity is because no carbamate reaction takes place because there is no hydrogen atom bonded to the nitrogen atom. By using sterically hindered amine, the molecular configuration attaching a bulky substitute to the nitrogen atom of the amine molecule affects the absorptivity and desorption temperature. The rotation of the bulky alkyl group around the aminocarbamate group will result in significantly low stability of the carbamate compound.
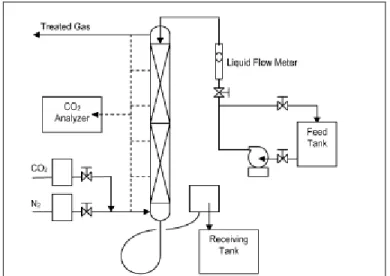
For the experiments, an aqueous solution of alkanolamine (eg MEA, DEA, etc.) was prepared in the feed tank by diluting the concentrated alkanolamine with deionized water to a given concentration. Since sulfur dioxide is one of the gases produced in the process, it can cause the degradation of amine to become worse. There are different ways to react by using solvent, for example, solvent can be a medium to combine reactants together, can act as a carrier to deliver chemical compounds in solutions in the required amount.
METHODOLOGY
- Research Methodology
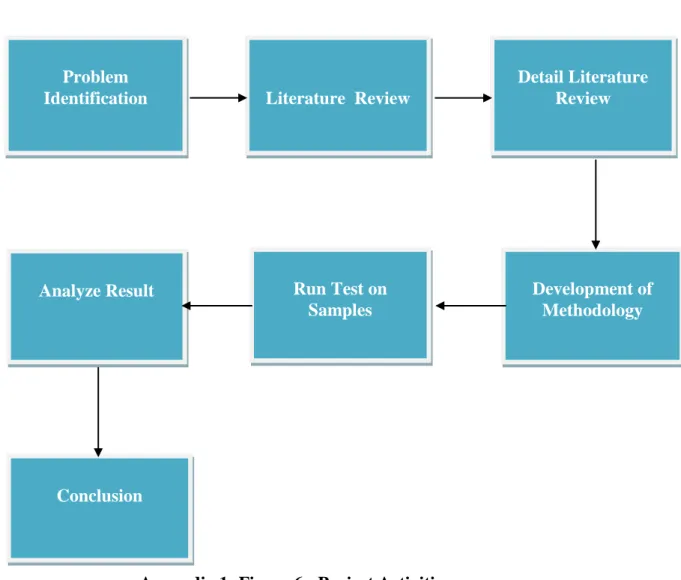
- Project Activities
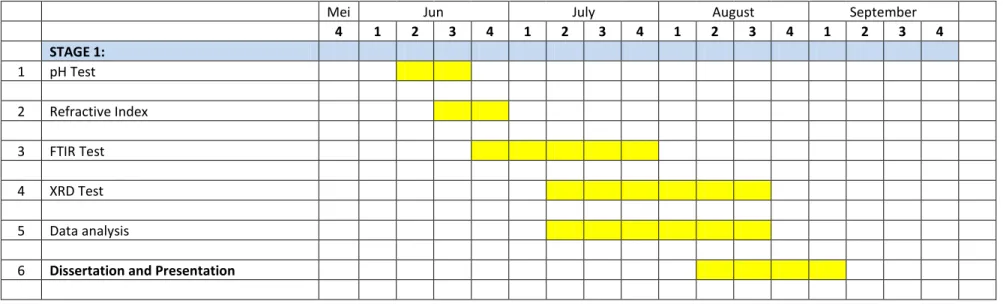
- Key Milestone / Gant Chart
- Tools Required
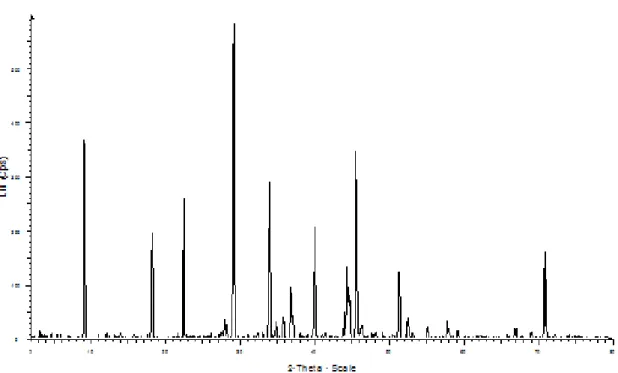
Function To characterize crystallographic structure, crystallite size (grain size) and preferred orientation in polycrystalline or powdered solid samples. Powder diffraction is commonly used to identify unknown substances by comparing diffraction data to a database maintained by the International Center for Diffraction Data. It can also be used to characterize heterogeneous solid mixtures to determine relative abundance of crystalline compounds.
To record the interaction of infrared radiation with a sample, measuring the frequencies at which the sample absorbs the radiation and the intensity of the absorption.
RESULT AND DISCUSSION
Result
Discussion
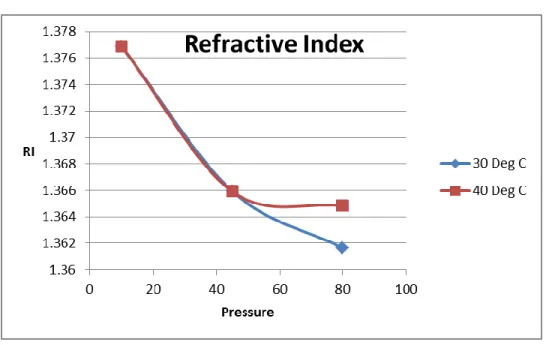
When the temperature rise and pressure remain constant, 10 bar G, the pH value and the refractive index are decreasing. As temperature and pressure increase, the RI value decreases, proving that the active ingredient in the solvent is reduced after reaction with CO2. Before reaction, from 3200 cm-1 to 3600 cm-1, the line was largely stretched, and alcohol functional group with O-H bond or alkane group with CH bond could be present.
A recurring small peak found from 1200cm-1 to 1000cm-1 which probably originates from the carboxylic acid functional group, C-O bond and amine functional group with C-N bond. From 1000cm-1 to 650cm-1, a slightly sharp peak may represent the amine functional group with C-H alkene bond. Before reaction, from 3200cm-1 to 3600cm-1, the line was largely stretched and alcohol functional group with OH bond or alkane group with CH bond may be present.
Found a repeating small peak from 1200 cm-1 to 1000 cm-1 that probably originates from the carboxylic acid functional group, the C-O bond and the amine functional group with C-N bond. From 1000 cm -1 to 650 cm -1 , a slightly sharp peak could represent the amine functional group with C-H alkene bond. It is observed that the peak of the C≡C binding which in the range from 2000 cm-1 to 2400 cm-1 bends more, this shows that the solid STONVENT by-product could associate with the C≡C moiety at point B.
At point D, the alkylamine C-H bond is present within the range from 650cm-1 to 1200cm-1 indicating the presence of the amine functional group in the solvent. From the XRD test, it is observed that both results indicate the presence of Trona, Na3H(CO3)2.2H2O.
CONCLUSION AND RECOMMENDATION
Bandyopadhyaya; (2001) "Penghilangan karbon dioksida melalui penyerapan dalam campuran amina: pemodelan penyerapan dalam air. Eidi-Haugmo, I., Brakstad, O. Ng Meng Wai, Alejandro Camerlengo & Ahmad Khairi Abdul Wahab (2005), "Studi tentang Pemanasan Global di Malaysia”, Jurnal Teknologi, 42(P) Juni. Salsabila Ahmad, Mohd Zainal Abidin Ab Khadir & Suhaidi Shafie (2011), “Perspektif Saat Ini tentang Energi Terbarukan di Malaysia”, Review Energi Terbarukan dan Berkelanjutan.