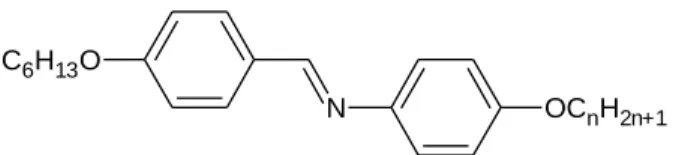
In partial fulfillment of the requirements for the Bachelor of Science (Hons.) Chemistry degree. A series of liquid crystal p-n-(Dimethylamino)benzylidene-p-alkyloxyanilines, DBA-One with different numbers of carbon atoms on the terminal alkyl chain has been successfully synthesized. The structure of the compounds is confirmed by infrared (IR) spectroscopy, nuclear magnetic resonance (1 H and 13 C NMR) spectroscopy and electron ionization mass spectrometry (EI-MS).
Sebatian DBA-O6 hingga DBA-O12 telah tehkan pasti sebagai nematik fasa basaan permerhatian textur hablur liquid di bawah POM. I certify that, this project report entitled "SYNTHESIS AND CHARACTERIZATION OF MESOGENIC SHIFT ETHER, P-N-(DIMETHYLAMINO)BENZYLIDEN-P-ALKYLOXIANILINE". It is certified that KHOR KE XIN (ID No: 07ANB07423) has completed this report entitled “SYNTHESIS AND CHARACTERIZATION OF SCHIFF BASE MESOGENIC ETHER, P-N-(DIMETHYLAMINO) BENZYLIDINALKYALOXE” under the supervision of P-N.
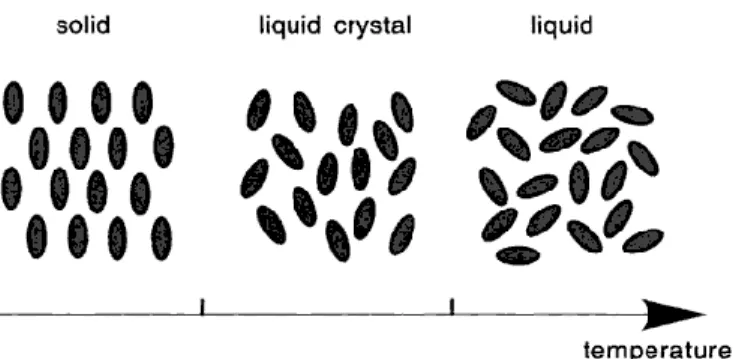
Liquid Crystal
History of Liquid Crystal
The study of liquid crystals began in 1888, when Austrian botanist Friedrich Reinitzer reported color phenomena occurring in melts of compounds known as cholesteryl benzoate and cholesteryl acetate, which are natural products occurring in plants and animals. In the case of cholesteryl benzoate, the crystal transformed into a cloudy liquid at 145.5 °C and a clear liquid at 178.5 °C. Otto Lehmann, a German physicist who studied the crystallization properties of various substances, studied samples from Reinitzer through his polarizing microscope.
3 He discovered that materials flow like liquids and behave optically like a crystal. Lehmann then named them liquid crystal after ensuring that the opaque phase was a single phase of matter that shared the properties of liquids and solids.
Orientational and Positional Order
- Thermotropic Liquid Crystals
- Mesogenic Materials
The phase known as "plastic crystal" as the positional order intact and absent of the orientational order. Smectic liquid crystal possesses positional order which means that the position of the molecules are correlated in some ordered pattern. In each layered structure of a smectic A liquid crystal, the molecules are in random position, but directionally ordered with their long axis perpendicular to the plane of the layer (Collings, 2002).
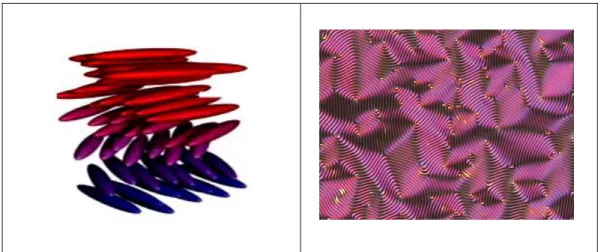
The structure of smectic C is broken fan-shaped under POM observation as shown in Figure 1.8b. As in the nematic, the smectic mesophase C has a certain chiral state C* in smectic C*, the direction of the molecules changes continuously from layer to layer forming a helix as shown in Figure 1.8c. As shown in Figure 1.9, the rigid core of calamitic molecules is formed by the connecting group A together with the ring structures O1, O2.
Liquid Crystals with Schiff Bases
Influence of Linking Group on Mesomorphic Properties
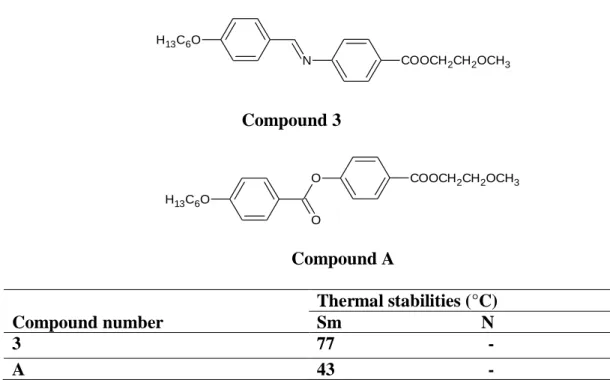
One of the useful methods to lower the transition temperature and destabilize the mesopach is the use of a bridging group, as it initiates the flexibility of the material. The effect of Schiff base linkage on mesomorphism was discussed by Prajapati et al. 19 central linkage is more coplanar than the ester (-COO-) central linkage and allows more efficient packing of the molecules.
For example, high liquid crystal transition temperatures can be achieved when the entire system is linked through central linking groups that include multiple bonds such as -CH=N- or -CH=CH- (Prajapati et al., 2004). The use of a sufficiently long hydrocarbon chain, which is usually alkyl or alkoxy, has become one of the most successful routes in the formation of liquid crystals. In the series of Schiff base esters with benzothiazole and aromatic cores, 2-(4-alkanoyloxybenzylideneamino)benzothiazole, nBABTH, Ha et al.
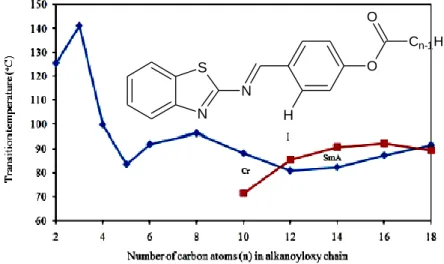
The mesophase begins to manifest as a monotropic (metasable) smectic A phase, beginning with the n-decanoyloxy derivative. The onset of the enantiotropic (stable) smectic A phase is observed as the carbon chain length increases from the n-dodecanoyloxy to the n-hexadecanoyloxy derivative. The smectic phase for 18BABTH changes to a monotropic phase as the carbon chain length is further increased.
This information indicates that the flexibility provided by the carbon chain has a significant influence on the mesophase. As the length of the terminal chain increases, the molecule becomes more flexible and therefore promotes a monotropic mesophase in a certain. However, the lowering of the stability of the mesophase will be induced as the chain length continues to increase.
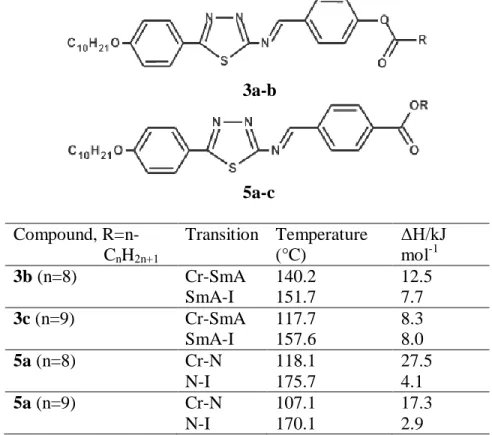
22 Table 2.4: Structure, transition temperatures (°C) and associated enthalpy changes (kJ/mol) of nBABTH upon heating and cooling, Ha et al.
Influence of Lateral Group on Mesomorphic Properties
22 Table 2.4: Structure, transition temperatures (°C) and associated enthalpy changes of (kJ/mol) of nBABTH on heating and cooling, by Ha et al. 23 is significantly higher than compounds of series B. This indicates that the introduction of hydroxyl group in the ortho position of the aldehyde fragment increases the degree of anisotropy of the molecular polarizability of compounds of both series. Therefore, the degree of molecular order can be increased, causing the stability of the smectic phase to increase.
By studying the transition temperature of both series, the introduction of hydroxy group at the ortho position in the aldehyde fragment causes the degree of anisotropy of the molecular polarization of compounds of both series to increase. This increases the van der Waals forces and therefore contributes to a high degree of stability on mesophase. isonicotinoylcarbonohydrazonoyl) phenyl 4-alkoxybenzoate, by Thaker et al. Four mesogenic homologous series containing three rings in the main core and replaced by a lateral acetyloxy group or hydroxy group on the central benzene core were studied although Vora et al.
Based on Table 2.7, series 1 has lower average nematic stabilities compared to that of series A. The molecules of series 1 have greater width due to the lateral acetyloxy (CH3COO-) group on the central benzene ring. The low average nematic thermal stability of series 1 is probably caused by the suppression of lateral substituents on thermal stabilities of the compounds.
Due to the intramolecular connection of the side hydroxyl group with the azo central bond, it is less effective in widening the molecules of series B. 25, while the effect of increasing the width is pronounced in series 1 due to the side acetyloxy group (Vora et. al., 2002). The molecules of the C series are arranged in lamellae, characterized by parallel molecular alignment with interweaving of neighboring molecules with their extensive branches and intercalation of the alkyl chain (Vora et al., 2002).
But the lateral motif in series 1 induces the formation of nematic phase, which prevents the more ordered in plane molecular association necessary for smectic arrangement, as observed in the number of homologous series.
![Table 2.5: Structure, transition temperature (°C) of 3-hydroxy-4-[(4-1, 2, 4- triazol-4-ylimino)methyl] phenyl 4-alkoxybenzoate of series A, by Thaker et al](https://thumb-ap.123doks.com/thumbv2/azpdforg/10250037.0/39.918.210.816.711.1013/structure-transition-temperature-hydroxy-triazol-ylimino-alkoxybenzoate-thaker.webp)
Chemical
Instruments
All chemicals and reagents were used without further purification unless stated in the synthesis section. To identify the mass and structure of compounds by observing the fragmentation of the compound.
COOH
- Synthesis of p-n-(Dimethylamino)benzylidene-p-hexanoyloxyaniline, DBA-O6
- Synthesis of p-n-(Dimethylamino)benzylidene-p – decanoyloxyaniline, DBA- O10
- Synthesis of p-n-(Dimethylamino)benzylidene-p- dodecanoyloxyaniline, DBA- O12
- Synthesis of p-n-(Dimethylamino)benzylidene-p- octadecaboyloxyaniline, DBA- O18
4-{[4-(Dimethylamino)benzylidene]amino}phenol (0.24 g, 1 mmol) was dissolved in acetone and then subjected to Willamson etherification with 1-bromohexane (0.17 g, 1 mmol). The solution is then heated to reflux in the presence of potassium hydroxide for three hours. The same procedures in synthesizing DBA-06 were repeated, except that 1-bromohexane was replaced with 1-bromooctane (0.19 g, 1 mmol).
The same procedures for the synthesis of DBA-O6 were repeated, except that 1-bromohexane was replaced by 1-bromodecane (0.22 g, 1 mmol). The same procedures for the synthesis of DBA-O6 were repeated, except that 1-bromohexane was replaced by 1-bromododecane (0.25 g, 1 mmol). The same procedures for the synthesis of DBA-O6 were repeated, except that 1-bromohexane was replaced by 1-bromotetradecane (0.28 g, 1 mmol).
The same procedures for the synthesis of DBA-O6 were repeated, except that 1-bromohexane was replaced by 1-bromohexadecane (0.31 g, 1 mmol). The same procedures for the synthesis of DBA-O6 were repeated, except that 1-bromohexane was replaced by 1-bromooctadecane (0.33 g, 1 mmol).
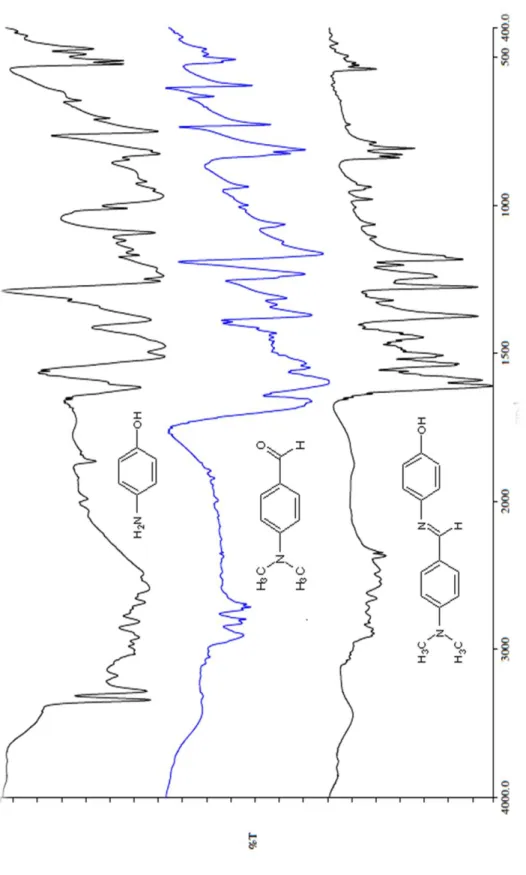
Infrared Spectral Analysis
Differential Scanning Calorimetry (DSC)
The sample was analyzed using aluminum-backed silica gel plates and examined under short-wave ultraviolet light. Chloroform mixture of ethyl acetate and chloroform which acted as mobile phase was prepared with the ratio of 1:1 to dissolve the sample. Initially, sample was dissolved in the chloroform and applied to 1 cm from the bottom of the TLC plate.
The TLC evaporation chamber was lined with a folded piece of filter paper to create a uniform and saturated atmosphere of solvent vapor. CDCl3 solvent was used to dissolve the sample at room temperature (298 K), while tetramethysilane (TMS) was used as the internal standard.
Mass Spectroscopy Analysis
Liquid Crystal Texture and Transition Temperature Studies
Synthesis and characterization of
- Synthesis Route of 4-{[4 (Dimethylamino)benzylidene]amino}phenol
- Synthesis Route of Etherification
- Polarizing Optical Microscope Analysis
This has the effect of reducing the concentration of the nucleophile required in the first step. In the first stage of the reaction, the alcohol is deprotonated, usually by the addition of a strong base. The analysis of the results for compound DBA-O16 is considered as representative of the DBA-On series.
In ether, the hydrogens on the carbon next to the oxygen are stripped because of the electronegativity of the attached oxygen. The structure and purity of the synthesized compound is confirmed using 13C NMR analysis techniques. Both C18 and C19 have higher intensity than C20 and C17 due to the NOE effect of heteronuclear interaction between C-H atoms.
The structure of the respective compound, DBA-O16, is chosen in the discussion as representative of the DBA-On series. In addition, intense Brownian motion is observed, a feature of the nematic phase due to the high degree of disorder of the phase structure. DBA-O12 exhibits an endotherm corresponding to the direct melting of the crystal phase to the isotropic liquid phase during the heating cycle.
The graph of the melting temperature in relation to the number of carbon atoms in the alkyl chain is shown in Figure 4.14. Based on the graph, it can be concluded that the mesophase behavior is strongly influenced by the length of the final chain. In this work (DBA-On), the influence of the dimethylamino group is compared with other end substituents.
For the compound DBA-O12, the polar dimethylamino group increases the polarization of the molecule and the intermolecular interactions, which leads to the formation of the nematic phase. As the length of the alkyl chain increases, the increase in intermolecular Van der Waals attraction leads to an increase in the melting point. Both ether groups are attached in the para position of the Schiff base compound.