A thesis submitted in fulfillment of the requirements for the degree of Master of Science (Physical Chemistry). I declare that this project, which was submitted in fulfillment of the requirements for the title of Master of Science in Chemistry (M.Sc) at North-West University. I am grateful to you for the support you have provided me throughout this project; I was not stressed about any financial problems.
Density functional theory (DFT)-based quantum computation was used to investigate the reactivity and selectivity of the four (4) studied dyes. Estimation of Fukui functions on atoms for the molecule Allura Red (AR) - 84 Estimation of Fukui functions on atoms for the molecule Tartrazine (TZ) - 85.
INTRODUCTION
- Definition of corrosion
- Types of corrosion
- Rate of corrosion
- Effects of corrosion
- Kinetics and thermodynamics of corrosion .1 Kinetics of corrosion
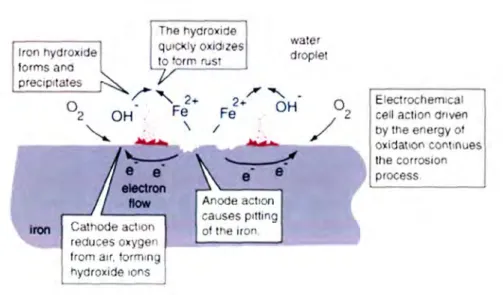
- Mechanism of metal corrosion
- Corrosion cost and control
- Research aim
- Research objectives
Erosive corrosion is often the result of the wearing away of a protective layer or coating on the metal surface [I. It can also take place by redeposition of the noble component of the alloy on the metal surface [8.9. Water Velocity: This is also one of the factors that influence the corrosion rate.
In each of the countries studied, the cost of corrosion was found to be approx. 3-4% of the gross domestic product (GDP) [3 I. The corrosion resistance properties of the metal can vary appreciably due to the adsorption of the surfactant on the metallic surface [40.

LITERATURE SURVEY
- Dyes
- Description and background
- Classification of dyes
- Properties of dyes
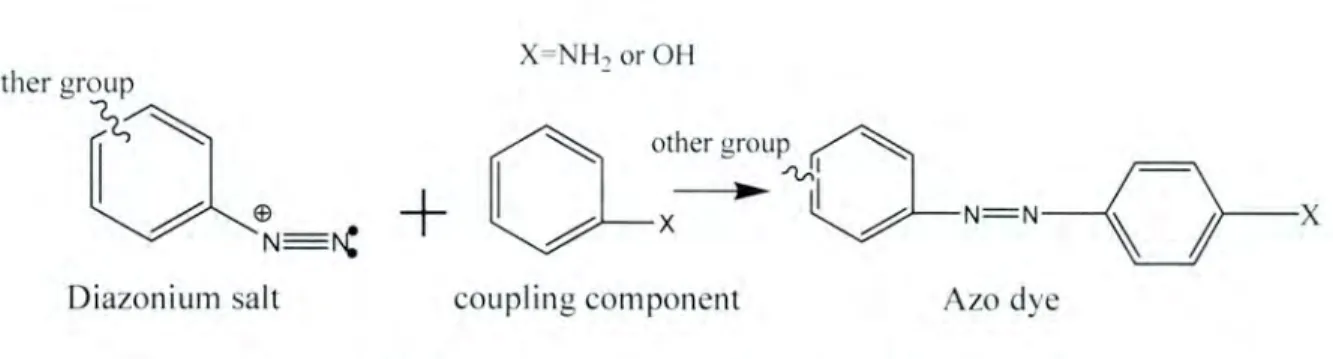
- Synthesis of dyes
- Applications of dyes
- Dyes as corrosion inhibitors
- Materials
- Reagents
- Inhibitors
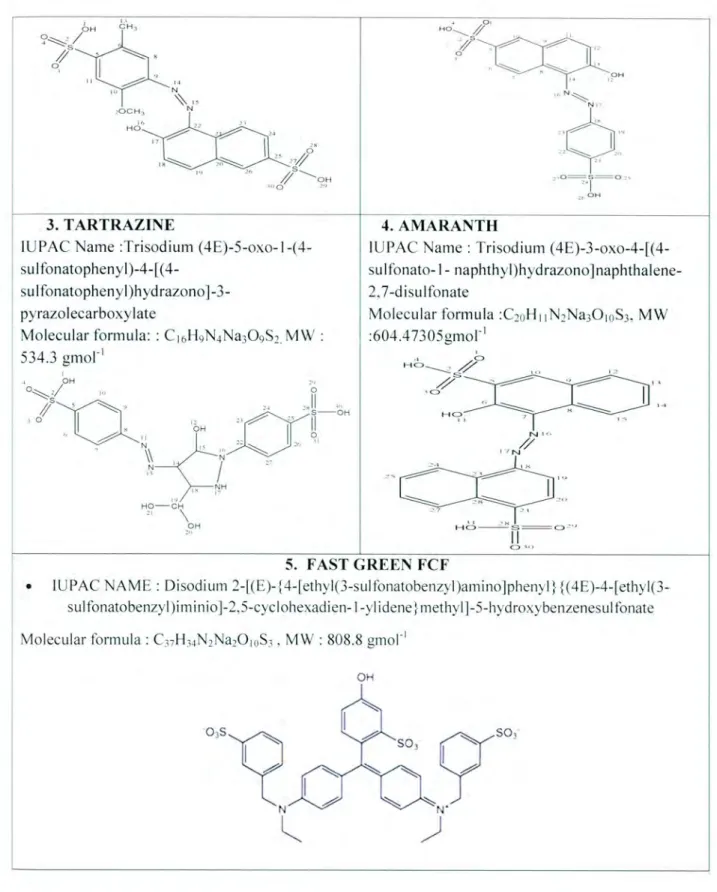
- SUNSET YELLOW FCF
- FAST GREEN FCF
- Gravimetric methods
- Electrochemical techniques
The adsorption of the FD (food coloring) inhibitor on the metal surface during the corrosion process is influenced by the electronic structure of the inhibitor molecules and also by the steric factor, aromaticity, electron density at the donor atoms and also by the presence of the functional group. The results also indicated that the inhibition mechanism of the inhibitors involved chemisorption interaction between the inhibitor and the mild steel. Langmuir adsorption isotherm was also found to provide an accurate description of tile adsorption behavior of the investigated azo compound.
The results obtained also showed that the adsorption of the inhibitors on the metal surface obeyed Langmuir adsorption isotherm. The Vieth-Sladek isotherm explained the adsorption of the three dyes very well, and the maximum adsorption amounts (Q1) were found for AY-6. The first set of experiments was done on the acid solution in the absence of the dyes and the second set of experiments was performed by fully immersing the metal plates in the solution containing the dye (corrosion inhibitor) in different concentrations and KI (potassium iodide) (Fig. 3.2 ).
Weigh the metal plate again Figure 3.3 Flow diagram of the experimental method followed in this work. W= the average weight loss of mild steel (ill grams. g) S= the total area of the mild steel (cm). From the values of the corrosion rate, the inhibition efficiency (%IE) and the degree of surface coverage (0) were calculated for the mild steel.
P2 = corrosion rate of the metal sheet in the presence of the inhibitor The equation for the degree of surface coverage is given by;. The corrosion behavior of the sample was investigated in 0.5 M HCl solution using the linear potentiodynamic electrochemical measurement technique. The DFT method is commonly used in the analysis of the properties of the inhibitor/metal surface mechanism and in the description of the nature of the inhibitor on the corrosion process [71].
RESULTS AND DISCUSSION
Gravimetric method
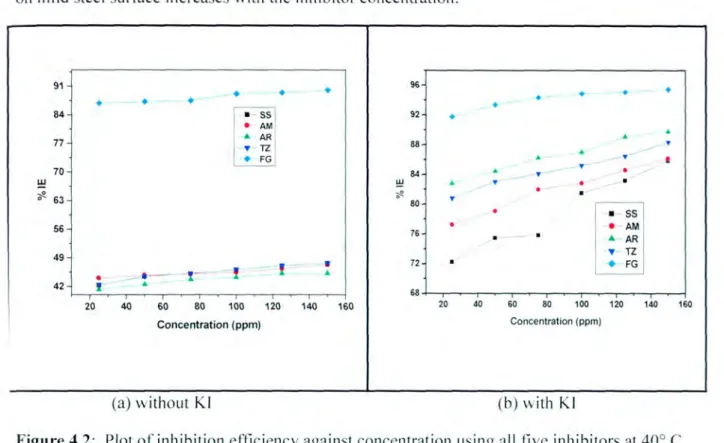
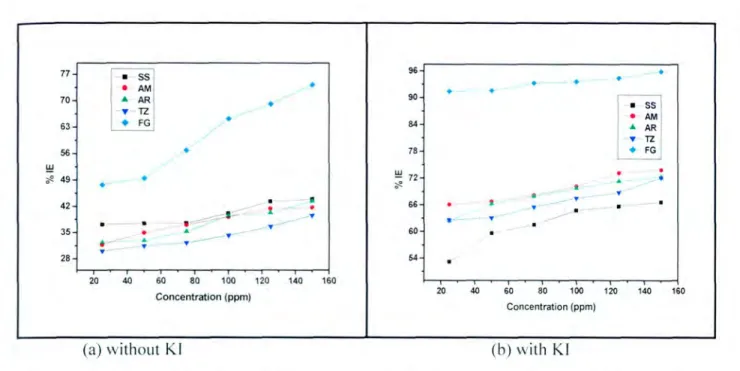
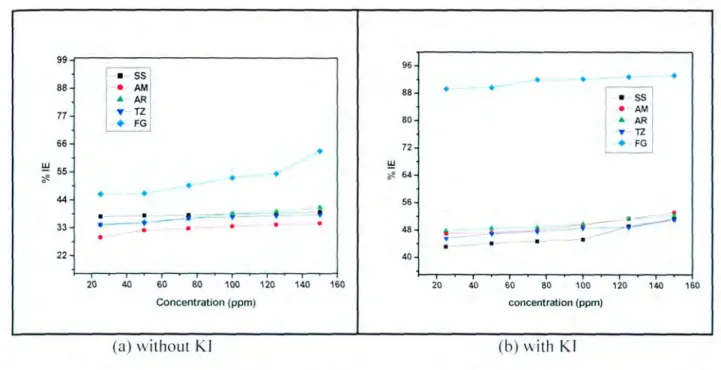
- The effect of the inhibitor concentration on the inhibition efficiency for mild steel The efficiency of an organic compound as corrosion inhibitor does not only depend on the
- The effect of temperature on the inhibition efficiency for mild steel metal
- Adsorption isotherm and thermodynamic parameters
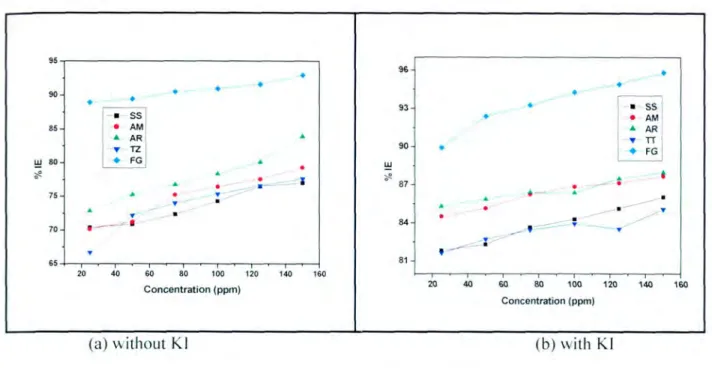
It has been reported in the literature that the presence of the halide ion ([) in acidic media increases the inhibition efficiency of some organic compounds. The plot in Figure 4.3(h) shows that as the concentration of the inhibitor increases, the inhibition efficiency also increases. These results suggest a poor inhibition on mild steel, which means that the inhibitor molecules are desorbed on the metal surface at higher temperatures [58].
Corrosion of mild steel in an acidic medium strongly depends on the reaction temperature. The results show that Ea values increase with increasing inhibitor concentration. Other reports [79] explained that the increase in apparent activation energy is also due to a significant decrease in inhibitor adsorption on the mild steel surface.
The data obtained from the above graphs with the addition of KI show that the rate of mild corrosion in the absence and presence of the studied dyes increases with increasing temperature. Some studies [77] pointed out that the decrease in apparent activation energy at higher inhibition is due to the shift of the net corrosion reaction. Some research [80, 81] showed that in the presence of inhibitors, the apparent activation energy was lower than in the absence of the inhibitor.
The higher value of activation energies in the presence of the inhibitor suggests higher inhibition efficiency and indicates physisorption or weak chemical bonding between the inhibitor molecules and the metal surface [20. AH*and AS t are derived from Arrhenius plots in the absence and presence of different concentrations of the studied food colors. Csokent is known as the molar concentration of the solvent, which, normally in the case of water.
VIZ FG
Electrochemical measurements
- Potentiodynamic polarization (PDP)
- Summary of the electrochemical measurements findings
- Influence of molecular structure and the correlation of inhibition action for the studied food dyes
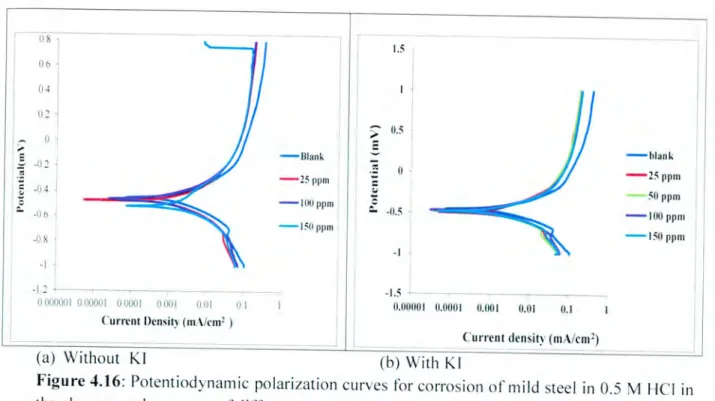
The results show that in the presence of an inhibitor without the addition of KI, the cathodic and anodic reactions were suppressed by the addition of the studied dye, implying that the food dye molecule reduces the anodic dissolution and also hinders the hydrogen evolution reaction. In addition, hydrogen evolution is actively controlled and the reduction mechanism was not affected by the presence of the inhibitor [77, 84]. It should be noted that the anodic curves for mild steel in 0.5 M HCl in the presence of an inhibitor showed that the dyes studied do not have much effect at a potential higher than E01- and this may be the result of significant mild steel dissolution. leading to a desorption of the inhibitory layer.
In this case, the desorption rate of the inhibitor is said to be higher than the adsorption rate, and studies have shown that the inhibitor is of a mixed type mechanism, but mainly acts as a cathodic inhibitor for the mild solution at 0.5 M HCl. The cathodic Tafel slope (bc) showed a slight change with the addition of an inhibitor, suggesting that the inhibitory action occurred simply by blocking the available cathodic sites on the metal surface leading to a decrease in the exposed area required for evolution and a decrease in hydrogen. rate of digestion with increasing concentration of food coloring. The corrosion current density (Icrr) values obtained with the addition of KI were smaller compared to those without the addition of Kl and were also found to decrease with increasing inhibitor concentration.
It was found that in all the food coloring compounds studied, the calculated inhibition efficiency increased with increasing inhibitor concentration. 91] showed that the adsorption process is influenced by the chemical structure of the inhibitors, the nature and the charged surface on the metal, and the charge distribution throughout the entire molecule of the inhibitor. To understand the mechanism of inhibition, a complete knowledge of the interaction between the protective compound and the metal surface is required.
In the presence of halide ions (1, in this present study), the mechanism of the anodic is given by [93];. The results obtained are presented in Table 4.2 for different concentrations of the inhibitors and are found to be greater than one. Stabilization of the adsorbed iodide ions with cations leads to a greater surface coverage and therefore greater inhibition.
Quantum Chemical Calculations
Results of the theoretical studies on selected food dyes as potential corrosion inhibitors
- Introduction
- Computational methods used in corrosion inhibition studies
- The molecular properties related to the reactivity of food dyes The highest occupied molecular orbitals
- Summary
- Chapter 5
The various Fukui functions (ie the f(r) and thef(r) functions) can also be defined in terms of charges on the inhibitor's atoms. I = - q(NI) for an electrophilic attack (43), where q(N+I), q and q_ are the charges of the atoms on the systems with N+l, N and N—I electrons, respectively. There are several computational methods used in the study of the properties of molecules.
Allura Red (AR) and Tartrazine (TZ)) and the corresponding atom numbering is shown in Figure 4.22 only for studies (4) of the dyes studied. One of the naphthalene units (where the sulfonic group is in the meta position relative to the C atom attached to the aza group) also has a keto group (C=O) in the ortho position relative to the C atom attached to the aza group. S atoms are suitable for adsorption on the metal surface due to the presence of a lone pair of electrons.
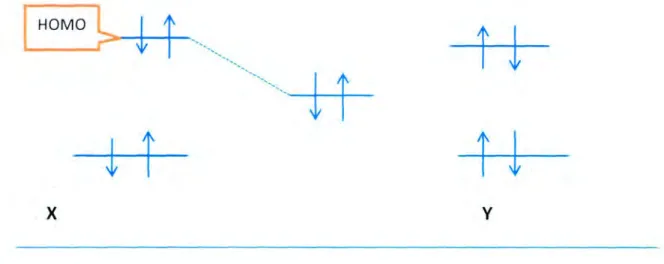
E110\j() provides information about the molecule's tendency to donate electrons to an electron-poor species. in a series of homologous sets of molecules. It is believed that the molecule with the highest E}R)\j() has the greatest tendency to donate electrons to an electron-poor species. The sites on the Allura Red molecule that have a greater tendency to donate electrons have already been discussed in the section on optimized structures of the dyes. The trend in the results obtained here is somewhat consistent with the results of the experimental inhibition efficiencies (FZ > AR > AM > SS > 17).
The electrophilicity values provide the information about the nucleophilic or electrophilic nature of the molecules. Of the studied compounds. the order of electrophilicity, value c shows that Allura red has the lowest value of w while Amaranth has the highest. In this study, the HOMO and the LUMO regions of the molecules were identified and explained in terms of their possible interaction with the metal surface.
CONCLUSIONS
The results were true for protonated and unprotonated species and in both environments, in vacuo and in solution. Protonated species are said to have weak electron-donating character and higher electron-accepting ability. In comparison to non-protonated species, protonated species have less tendency to chemisorb to the metal surface.
Tables
AJI 0