i
ABSTRACT
AISI 316 is stainless steels that commonly used in industries. AISI 316 is the most favored construction material of various components required in chemical, petrochemical and nuclear industries. It is important to study the corrosion properties of AISI 316 due to the mechanical properties, microstructure and corrosion attack. Two types of corrosion attack that commonly found on AISI 316 stainless steels are intergranular corrosion and pitting corrosion. Intergranular corrosion is occurring when AISI 316 is sensitized to the heat at temperature 500ºC to 800°C leads to the grain boundary precipitation of chromium rich carbides (Fe,Cr)23C6 and the
ii
susceptibility to intergranular corrosion in AISI 316. Moreover, the effect of solution annealing and sensitizing of AISI 316 on hardness is evaluated by microhardness test. Five indentations were taken from each sample to obtain a representative average. In corrosion studies, when AISI 316 is exposed in 3.5% NaCl solution, based on hysteresis loop analysis, the corrosion resistance of AISI 316 on the sensitized is decreased due to the Cr23C6 form. In morphologies studies, after
solution annealing and quenching by cold water in order to homogenized all austenite (γ) phase. Further investigation, X-ray diffraction (XRD) analysis is applied to support the existence Cr23C6 on the sensitized and heat treated sample. Theoretically,
solution annealing process is done to dissolve Cr23C6 as to form homogeneity of alloy
in a sample, leaving on desired phase which in this case involve that austenite. However, Cr23C6 still present this is due to the time of growth for Cr23C6 has
iii
ABSTRAK
AISI 316 adalah keluli tahan karat yang paling biasa digunakan di industri. Keluli tahan karat jenis AISI 316 adalah dari siri kumpulan T 300 baja keluli. AISI 316 merupakan bahan pembinaan yang paling banyak digunakan dari berbagai-bagai komponen yang diperlukan dalam indusri kimia, petrokimia dan nuklear. Adalah penting untuk mempelajari sifat kakisan AISI 316 kerana sifat mekanik, mikrostruktur dan serangan kakisan. Dua jenis serangan kakisan yang biasa ditemui pada AISI 316 adalah kakisan intergranular dan kakisan bopeng. Kakisan intergranular terjadi ketika AISI 316 terdedah panas pada suhu 500ºC hingga 800°C dan menyebabkan batas butir kaya kromium karbida (Fe,Cr)23C6 dan pembentukan
daerah pengurangan kromium (Uhlig, H. H. 2008). Proses larutan anil diikuti penyepuh lindapan cepat menggunakan air dapat merawat kakisan intergranular pada AISI 316. Sementara itu, kakisan bopeng terjadi kerana serangan dari lingkungan kakisan terhadap komposisi bahan heterogen pada permukaan AISI 316 di mana permukaan, tompok hitam yang mengandungi kromium (Cr) yang tinggi akan memudahkan pelokalan serangan kakisan bopeng dan berlaku pada kawasan setempat. Pembentukan kakisan masing-masing dipelajari dengan menggunakan analisis elektrokimia, manakala ujian calar oxalic untuk untuk mengklasifikasi struktur calar dalam menentukan kesesuaiannya dengan kakisan intergranular. Potensiodinamik Putaran Polarisasi seperti yang dinyatakan dalam standard ASTM G 61 digunakan untuk menganalisis pembentukan kakisan bopeng dan perilaku AISI 316 dalam melawan kakisan. Sementara ASTM A 262-01 amalan A, pemendakan terhadap serangan kakisan batas butir pada AISI 316 digunakan. Jenis-jenis kakisan yang telah tersebar pada permukaan AISI 316 dianalisis dengan Mikroskop Optik. X-Ray Diffraction (XRD). Analisis ini digunakan untuk menganalisa tahap bentuk pemendakan kromium karbida (Cr23C6) dalam struktur AISI 316. Formasi ini telah
iv
AISI 316. Selain itu, Penyepuhlindapan dan pemekaan AISI 316 pada kekerasan diukur dengan ujian mikrokekerasan. Ujian mikrokekerasan digunakan untuk menentukan peringkat pembentukan kristal dan memberikan anggaran bahan ketahanan terhadap deformasi AISI 316. Sebanyak 5 lekukan diambil dari sampel untuk mendapatkan purata. Dalam kajian ini, ketika AISI 316 direndam dalam 3.5% larutan NaCl, berdasarkan analisis histeresis loop, rintangan karat AISI 316 pada peka adalah kerana bentuk Cr23C6 menurun. Dalam kajian morfologi, selepas
penyelesaian pemanasan dan pendinginan segera dengan air sejuk untuk menyamakan struktur aloi pada fasa austenit (γ). Penyelidikan lebih lanjut dengan X-ray pembelauan (XRD) analisis untuk menyokong kewujudan Cr23C6 pada sampel
diperlakukan peka dan panas. Secara teori, penyelesaian proses anil dilakukan untuk melarutkan Cr23C6 untuk membentuk homogenitas gabungan dalam sampel,
berangkat pada peringkat dikehendaki yang dalam hal ini melibatkan austenit itu. Namun, Cr23C6 masih ada ini kerana apabila pertumbuhan Cr23C6 telah melebihi
v
DEDICATION
vi
ACKNOWLEDGEMENT
Alhamdulillah, thank to ALLAH for HIS blessing, provide me a good health and thinking during progress this project.
Special thanks to my supervisor Dr. Zulkifli Bin. Rosli, because of his commitment and supported throughout my final year project and report writing with his patience and knowledge that he give to me in this research.
I would also like to thanks to the Faculty of Manufacturing labs technicians, Mr. Azhar, Mr. Farihan, Mr. Hisham and Madam Nurhafizah who encourage me in laboratory during my PSM project.
Finally, I thank my parent for supporting me throughout all my studies at University. Thank for being a wonderful parent of mine and thank for providing a space for me in which to complete my writing up.
I hope, through their guidance will completing my Final Project in my final year study. These experiences are also made me be more matured than before and increasing my self-confident.
vii
2.1.2 Effect of Alloying Elements on the Corrosion Behavior of Stainless Steels
8
viii
2.1.2.2 Nickel (Ni) 9
2.1.2.3 Molybdenum (Mo) 9
2.1.2.4 Manganese (Mn) 10
2.2 Scanning Electron Microscope 10
2.2.1 Chemical and Microstructure Analysis 11
2.3 Corrosion Resistance of Stainless Steels 13
2.3.1 General Corrosion 13
2.4.2 Cyclic Potentiodynamic Polarization Methods 18 2.5
Previous Studies on the Microstructure of Steel 20
2.6 Microstructure Analysis 21
2.6.1 Microstructure of Wrought Stainless Steels 25
2.7 Hardness Test 27
3.0 METHODOLOGY 28
3.1 Introduction 28
3.2 Raw Material 28
3.3 Sample Size preparation 28
3.3.1 Sample Preparation 30
ix
4.2 Effect of solution annealing of sensitized AISI 316 stainless steels on microstructure
37
4.2.1 X-Ray Diffraction (XRD) Analysis and Interpretation 42 4.2.1.1 X-Ray Diffraction Pattern for Chromium Carbide
(Cr23C6)
43 4.3 Effect of solution annealing of sensitized AISI 316 stainless steels on
corrosion behavior
45
4.3.1 Effect of Heat Treatment 45
4.3.2 Result and Discussion of Electrochemical results 46 4.4 Effect of solution annealing of sensitized AISI 316 stainless steels on
x APPENDICES
xi
LIST OF TABLES
2.1 Properties of AISI 316 Stainless Steels 8
2.2 Chemical Composition of AISI 316 Stainless Steels 8
3.1 Summary of sample preparation for heat treatment effect 31
3.2 Preparation Method (ASTM E3) 33
4.1 Critical potentials and protection intervals obtained for stainless steels after exposure in 3.5 wt% NaCl solution
47
4.2 Analysis ofCritical potentials and protection intervals obtained for stainless steels after exposure in 3.5 wt% NaCl solution
48
xii
LIST OF FIGURES
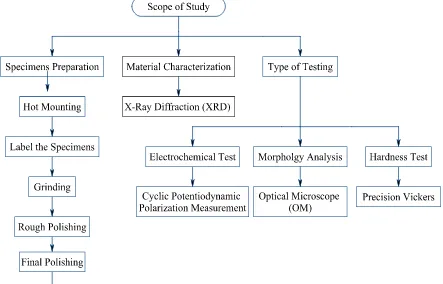
1.4 Scope of study flowchart 4
1.5 Research flowchart 5
2.1 Effect of Molybdenum content on the anodic polarization curves of Fe 18% Cr alloy in H2SO4
9
2.2 Morphology of AISI 316 before and after electrochemistry test 11 2.3 SEM analysis of a pit formed around a MnS inclusion of AISI 304
polarized in 3.5 wt.% NaCl solution until a potential value close to Epit
12
2.4 Anodic polarization curve of stainless steel in sulphuric acid solution 13 2.5 Schematic diagram of apparatus for the determination of polarization
curve of a stainless steel in a solution using a potensiostat
16
2.6 Tafel extrapolation of measured potential-current density curves. 17
2.7 Schematic diagram of breakdown potential 19
2.8 AISI 304 behavior when polarized in 3.5 % NaCl solution 20 2.9 SEM microstructural (A) austenitic mode contains 0Mo - 18Cr - 17Ni
and (B) austenitic-ferritic mode 0Mo - 18Cr - 12Ni
23
2.10 SEM microstructural (A) ferritic-austenitic mode contains 2Mo - 18Cr - 12Ni (B) ferritic mode contains 2Mo - 17Cr - 8Ni
22
2.11 Microstructure of the AISI 304 A) after solution annealing – 0% CW, B) after solution annealing – 40% CW, C) after aging at 650 °C/0.5 h, 0% CW, D) after aging at 650 °C/0.5 h, 40% CW
2.13 (A) AISI 304 sensitized 700°C/1 h furnace (B) AISI 304 after Solution Annealing at 1200°C /1 h (water quenching)
xiii
2.14 Type 304 stainless steel strip, annealed 5 min at 1065°C (1950°F) and cooled in air
3.1 Flowchart of research study on AISI 316 29
3.2 Project specimen of AISI 316 30
3.3 Flow Chart of preparation metallographic specimens 32
3.4 Mounting machine 32
3.5 Optical microscope provided in UTeM’s laboratory 34
3.6 Gamry Potential Stat 35
4.1 20 X Magnification microstructure, (A) AISI 316 before exposed to heat, (B) AISI 316 after exposed to heat in two hour at temperature 700 ℃
38
4.2 Microstructure of samples after etched with oxalic acid at 50X magnification (A) and 100 X magnification (B)
39
4.3 20 X Magnification microstructure, (A, B, C, D) AISI 316 after solution annealing, 1130 ºC (24, 48, 72, 120 minute) and rapidly quench in cold water
40
4.4 Reference XRD patterns for Cr23C6 (01-085-1281) 42
4.5 Reference XRD patterns for Martensite(00-044-1290) 42 4.6 Reference XRD patterns for Austenite(00-031-0619) 43 4.7 X-ray diffraction pattern for sample of AISI 316 43 4.8 Cyclic polarization curves obtained as a function of Sensitizing
Temperature and Solution Annealing various soaking time after exposure in 3.5 wt % NaCl solution
47
4.9 Analysis of high potential stress for AISI 316 specimens with
xiv
4.10 The comparison of hardness value between specimens produced by different process
xv
LIST OF ABBREVIATIONS
A - Austenitic
AF - Austenitic-Ferritic
AISI - American Iron and Steel Institute
ANSI - American National and Standard Institute ASTM - American Society for Testing and Materials
CW - Cold Working
EBDS - Electron Backscatter Diffraction
EDS - Energy Dispersive X-Ray Spectroscopy
FCC - Face Centre Cubic
F - Ferritic
FA - Ferritic-Austenitc
GBs - Grain Boundaries
HRB - Hardness Rockwell B-scale SAE - Society of Automotive Engineers SEM - Scanning Electron Microscope UNS - Unified Numbering System
xvi
LIST OF SYMBOLS
Ecorr - Corrosion Potential
Epit - Pitting Potential
Erep - Repassivation Potential
iapp - Applied Current Density
icorr - Current Density
βa - Anodic Tafel Slope
βc - Cathodic Tafel Slope HCl - Hydrochloric acid H2SO4 - Sulfuric acid
NaCl - Sodium Chloride
C - Capacitance pH - -log a (H+)
V - Voltage
T - Temperature (oC)
M - Molar
1
CHAPTER 1
INTRODUCTION
1.1 Background of Study
Austenitic stainless steels 300 series contain chromium (Cr) and nickel (Ni) as major alloying elements. The steels from this group have the highest corrosion resistance, weldability and ductility. Austenitic stainless steels retain their properties at elevated temperatures. At the temperatures 500ºC-800ºC chromium carbides (Cr23C6) form
along the austenite grains (Uhlig, H. H. 2008). This causes depletion of chromium from the grains resulting in decreasing the corrosion protective passive film. This effect is called sensitization. It is particularly important in welding of austenitic stainless steels. Stabilization heat treatment of such steels results in preferred formation of carbides of the stabilizing elements instead of chromium carbides. These steel are not heat treatable and may be hardened only by cold work.
High corrosion resistance of austenitic stainless steels is primarily attributed to the passive oxide film formed on its surface when exposed to an aqueous solution. However, the resistance of this passive film is determined by the environmental conditions which stainless steel is exposed to, as well as by the alloy composition (Pardo, A., et al., 2008). Ronald (2007) stated that a very large number of phases can be present in the microstructure of austenitic stainless steels, mainly carbides and intermetallic phases. Cr23C6 is carbides that are frequently presence in the
2
1.2 Problem Statement
It is well-known that austenitic stainless steels are the most favored construction material of various components required in chemical, petrochemical and nuclear industries. The selection of these is made basically due to a good combination of mechanical, fabrication and corrosion resistance properties. However, problems which often arise when welding process applied on stainless steel type AISI 316, it will cause chromium carbide precipitation at boundary. So, the area of chromium carbide deflection or is impecunious of chromium, that is less than 12 wt% as steel condition become to hold up to corrosion. If residing in corrosive environment tend to happened item boundary corrosion. Seen the problems, hence carbide precipitation representing cause of failure of function result of steel stainless welding represent matter which need to be studied by more circumstantial. In this study, AISI 316 will be sensitized at 700°C temperature for two hours then heat treated by solution annealing followed by water quench. The corrosion resistance of AISI 316 is analyzed by study the effect of solution annealing by using electrochemical test and oxalic acid etch test. Therefore, the present work attempts to provide a further understanding of the effects of solution annealing of sensitized AISI 316 on microstructure, corrosion and mechanical properties.
3
1.4 Scope of Study
The scope of this project is to study the effect of solution annealing on microstructure, corrosion behavior and mechanical properties of sensitized AISI 316 as shown in Figure 1.1. The aspects that should be study on this material are structure and microstructure of AISI 316 such the present of inclusion and formation of chromium carbide by using optical microscope and X-ray diffraction respectively. Hardness test is conducted to investigate the hardness of AISI 316 by varying soaking time use in solution annealing process. Precision Vickers a hardness measurement with load 0.5 is used to measure the hardness. The corrosion property of the material is studied by using electrochemical technique and oxalic acids etch. The purpose of electrochemical technique is to investigate corrosion properties of AISI 316 due to sensitizing effect and heat treated by solution annealing process. The oxalic acid etch technique is to investigate susceptibility to intergranular attack due to heat sensitization effect. The result of the corrosion behavior of AISI 316 in sensitized conditions analyze as to provide the corrosion control and prevention method.
1.5 Report Organization
4
discussion of the investigation on corrosion behavior of AISI 316 based on data result. Chapter 5 discusses the conclusion and recommendation based on the study objective and finding the effect of solution annealing on microstructure, corrosion behavior and mechanical properties of sensitized AISI 316.