Prapti Sedijani
LETTER OF DECLARATION
Herewith I declare that the dissertation entitled “CLONING TREHALOSE SYNTHASE GENE AND ITS EXPRESSION IN Arabidopsis thaliana” is originally my work under supervision of the Supervision Committee. This manuscript has not been submitted to any University at any occasion.
Any information mentioned in the text cited from published or unpublished of other parties are mentioned in the text and listed in the list of references.
Bogor, 11 June 2007
ABSTRACT
PRAPTI SEDIJANI. Trehalose is a well known as biological stabilizer functioning as stress protecting agent. It is ubiquitously found in bacteria, fungi, invertebrates, and certain desert-adapted resurrection plants such as Selaginella. In most flowering plants, trehalose is synthesized in amounts nearing the detection limit. Gene(s) encoding trehalose-metabolizing enzymes from E.coli, trehalose synthase and trehalose phosphate phosphatase (OtsA and or OtsB) and the same gene from yeast (TPS1 and or TPP) have been introduced to plants and increased stresses resistance, yet resulted in phenotypic defect, due to accumulation of its intermediate product, trehalose-6-phosphate (T6P). T6P increases along with the increase of trehalose level. This research was aimed to find out if introducing trehalose synthase gene (TreS) which does not produce T6P which prevent phenotypic defect resulted from trehalose accumulation, and whether the presence of TreS in plant improves plant resistance to stresses. The use of TreS as an alternative among non toxic selectable markers was also assessed. For this reason, genes encoding trehalose synthase originated from Thermobifida fusca (TfTreS) and originated from Mycobacterium tuberculosis (MtTreS) had been cloned into plant expression vector (pBIN1935S). TfTreS was isolated from T. fusca using PCR and was inserted to pGemT for sequencing. DNA sequent analysis of the 2 clones, TfTreS within pGemT were identical to the published data yet several silent point mutations occured. TfTreS from these clones were inserted into pBIN19 with CaMV35S promoter. It has been successfully transferred to Arabidopsis thaliana via Agrobacterium mediated transformation. MtTreS readily available in pBlunt TOPO II, was exiced from the plasmid and was inserted to pBin1935S, and transferred to Arabidopsis in the same manner, but it has not been successfully transferred to the plant.
Selection of putative transgenic seedlings was carried out either on 50 mg/L kanamycin or on 125 mM trehalose containing media. Comparison of selection efficiencies using kanamycin or trehalose selection, it can be concluded that trehalose/TRES selection system allows for a tight selection yet yielded fewer (1/3 – 1/10) of transgenic lines than kanamycin selection system. Selection using a combination of trehalose and Validamycine A yielded transgenic lines yet at an even lower frequency (1/10) than trehalose selection. PCR analysis amplified TfTreS from all 5 transformed plants tested, and trehalose resistance was inheritable to the next generation. By growing transgenic plant on trehalose (100 mM) in combination with validamycin A (10 µM), it can be conclude that TfTreS can be used as an alternative of non-toxic selectable marker among the existing non-toxic selectable markers.
ABSTRAK
PRAPTI SEDIJANI. Trehalose dikenal sebagai agen stabilator terhadap materi biologi, berperan dalam perlindungan terhadap beberapa stres. Trehalose ditemukan pada bacteria, fungi, invertebrata serta tanaman yang telah beradaptasi dengan kondisi gurun, seperti Sellaginella. Kebanyakan tanaman berbunga,mensintesis trehalose dalam jumlah yang hampir tak terdeteksi. Gen penyandi enzyme metabolisme trehalose dari E. coli, trehalose phosphate synthase 1 and trehalose phosphate phosphatase (OtsA dan atau OtsB) and gen yang sama dari yeast (TPS1 dan atau TPP) telah diintroduksikan pada tanaman, namun dihasilkan kelainan fenotip, disebabkan oleh akumulasi trehalose 6 phosphate (T6P). Sebagai produk antara, T6P meningkat seiring dengan akumulasi trehalose. Penelitian ini bertujuan untuk mengetahui apakah introduksi gen trehalose synthase (TreS) yakni jalur yang tidak menghasilkan T6P sebagai produk antara, dapat mencegah timbulnya masalah yang disebabkan oleh akumulasi trehalose, dan apakah keberadaan TreS dapat meningkatkan ketahan terhadap beberapa stres. Kemungkinan penggunaan TreS sebagai salah satu alternative selectable marker juga di uji. Untuk itu, gen trehalose synthase dari Termobifida fusca (TfTreS) dan dari Mycobacterium tuberculosis (MtTreS) telah dikonstruksi dalam vektor expressi (pBIN1935S). TfTreS diisolasi denagn PCR. Analysis sikuen terhadap 2 klon TfTreS pada pGemT identik dengan data yang dipublikasi (), namun terjadi beberapa mutasi titik yang tidak bermakna. Gen tersebut telah dikonstruksi pada pBIN1935S, dan berhasil diintroduksikan pada Arabidopsis dengan bantuan Agrobacterium. MtTreS yang telah tersedia pada plasmid pCR Blunt II TOPO, dipotong dari plasmid tersebut, dikonstruksi pada pBIN1935S, dan ditransfer pada Arabidopsis dengan cara yang sama, namun belum berhasil terintegrasi pada tanaman tersebut.
Seleksi tanaman transgenik putative dilakukan pada medium yang mengandung 50 mg/L kanamisin atau 125 mM trehalose. TRES/Trehalose merupakan system seleksi yang ketat dengan jumlah perolehan tanaman transgenik lebih rendah (1/3-1/10) dibanding dengan seleksi kanamisin. Sistem seleksi kombinasi trehalose dan validamisin A menghasilkan jumlah tanaman transgenik lebih rendah (1/10) dari kedua sistem seleksi tersebut. Analisis PCR menunjukkan bahwa TfTreS telah terintegrasi pada genom Arabidopsis pada semua (5) tanaman transformant yang di test. Resistensi tanaman transgenik terhadap trehalose bersifat menurun. Dengan tumbuhnya tanaman transgenik pada medium mengandung trehalose (100 mM) atau trehalose dan validamycin A (10 µM) menunjukkan bahwa TfTreS dapat digunakan sebagai alternatif selectable marker diantara non-toksik selectable marker yang telah ada.
© Copy right belong to Bogor Agricultural University, the year of 2007 Copy right is protected
It is forbidden to copy and multiply partially or the whole of the dissertation in any form either reprint, photo copy, microfilm etc, without permission from Bogor
CLONING TREHALOSE SYNTHASE GENE AND ITS
EXPRESSION IN
Arabidopsis thaliana
Prapti Sedijani
DISSERTATION
Prepared to fulfill the requirement to obtain a doctoral
degree at the Department of Biology
Title of dissertation : CLONING TREHALOSE SYNTHASE GENE IN ITS EXPRESSION IN Arabidopsis thaliana
Name : Prapti Sedijani
Registration number : P17600002
Department : Biology
Approved by Supervision Committee
Prof Dr. Ir. Edi Guhardja, M.Sc Chairperson
Dr. Dwi Andreas Santosa Prof. Dr. Maggy T. Suhartono Member Member
Chairperson of The Study Program of Biology Dean of Postgraduate School
Dr. Ir. Dedy Duryadi S., DEA Prof. Dr. Ir. Khairil A. Notodiputro, MS
Acknowledgement
AlhamduliLlah wa syukuriLlah, I would humbly express my deepest gratitude I addressed to Allah SWT for the life and all things supporting along with it, all the good will, the guidance and blessing that I always enjoy. The accomplishment of this dissertation is a part of His gives. He has chosen and allowed such many constructive and kind parties to contribute for the accomplishment of my study. Hence, via this opportunity, allow me to express my great gratitude and high appreciation to:
Prof Dr. Edi Guhardja as The Chairperson of Supervision Committee for the guidance, knowledge, skills, all very kind sincere supports either material or immaterial all a long of my study. For the precious time, concerns and specifically for the outstanding wisdom that you always perform, I do thank you very much indeed. Dr. Dwi Andreas Santosa as Member of Supervision Committee for the guidance, knowledge, skills, all kind supports particularly the great laboratory supports with all the facilities that I have been used freely for such a long time. Without kind supporting laboratory, it would be nearly impossible to do research. Thank you very much indeed for the uncountable supports. Prof. Dr. Maggy T. Suhartono as Member of Supervision Committee for the guidance, knowledge, and skills that have been shared, I have been enjoyed the discussions that were very alive, criticisms, constructive, encouraging and the fruitful inputs that kept the work on the right trek. Dr. Henriette Schluepmann as Training Supervisor at Department of Molecular Plant Physiology (MPF), The Trehalose Project Leader Utrecht University, for the guidance, knowledge, skill and all kind supports in term of material and immaterial, the sincere concerns and very kind attention, during the laboratory works. I have been enjoyed the nice quality working together, that was very alive, criticism, constructive, encouraging and the fruitful. Thank you very much indeed.
Ma’sum, MSc. PhD the current Rector of Mataram University who allowed me to be free from my duties in the university as well as for all kind supports so far for my study either material or immaterial supports.
DUE-like Program of Mataram University, which provide scholarship enable me to conduct study at Bogor Agricultural University.
Laboratory of Indonesian Centre for Biodiversity and Biotechnology (ICBB), The Chairperson (Dr. Dwi Andreas Santosa), The Head of Technical Laboratory (Mbak Sulastri) and all Staff members of laboratory that allowed me to do research conveniently with such nice and warm circular friendship. Laboratory of Molecular Plant Physiology Utrecht University and all the staffs that have been so helpful during my training in the department, thank you very much. Laboratory of BBBiogen, Chimanggu, and staffs that have been so helpful in trial of growing Arabidopsis, I do appreciate all the helps.
Dr Yayuk Andayani, Dr Fauziah Harahap, Heru Kusdiyanto MSi/family, Zessi Menursita MSi, you all have been very supportive in term of material and immaterial all along of my study. Thank you very much for being nice and giving a warm circular friendship. Great appreciation also I addressed to friends and all parties that cannot be mentioned one by one, thank you very much for all the kind supports and warm circular friendship.
Ibunda Siti Asiyah (the late) and Ayahanda Ismail (the late) there is no word can ever describes, no action can ever represents for all the scarifying, all the life, love and care, all sincere concern, all tears in all prayers that always been giving not only during my study but all along of my life. I always fill your presence in every moment of my time that keeps my spirit to fruit my life.
All my brothers/sister (Mas Isnen M/the family, Mas Wiachto TM/the family, Mbak Triwahyuni/the family, Mas Terbit S/the family and Mas Sapto/the family) thank you very much indeed for your uncountable supports, sincere concern love and care, long life prayers for me that make my life easier, nicer, and better.
With no means to discount the contribution of all parties, due to the limitation I own, this dissertation still lots of rooms for improvement. I invite the genuine constructive suggestions to make the dissertation better and useful.
Curriculum Vitae
LIST OF CONTENT
Title Page
LIST OF TABLE ... xiii
LIST OF FIGURE ... xv
LIST OF APPENDIX ... xviii
I. INTRODUCTION 1.1 Rationale ... 1
1.2 Objectives of the research ... 2
1.3 Research Benefit ... 3
1.4 Hypothesis ... 3
II. LITERATURE CITATION 2.1 Damaging effects of stress ... 4
2.2 The character of trehalose and its role in several organisms 6
2.3 Trehalose, its role as stress protecting agent ... 6
2.4 Other compatible solutes ... 11
2.5 Trehalose biosynthesis ... 12
2.6 Characterization of trehalose synthase (TRES/TSASE) on plant ... 16
2.7 Foreign trehalose synthase gene on plants ... 17
2.8 Trehalose induce starch biosynthesis in Arabidopsis thaliana ... 18
2.9 Flow chart of the research ... 21
III. CLONING GENES ENCODING TREHALOSE SYNTHASES from Thermobifida fusca and Mycobacterium tuberculosis 3.1 Introduction ... 23
3.2 Materials and methods ... 23
3.2.1 Place and time of research ... 23
3.2.2 Material of research ... 23
3.2.3 Cloning of TreS genes from Thermobifida fusca and from Mycobacterium tuberculosis ... 24
Growth condition of Thermobifida fusca and DNA isolation ... 24
Verifying TreS from Mycobacterium ... 25
Insertion of TreS gene to Plant Expression Vector ... 25
3.3 Result and discussion 3.3.1 Cloning a gene encoding Trehalose synthase (TreS) from Thrmobifida fusca ... 26
3.3.2 Cloning a gene encoding Trehalose synthase from Mycobacterium tuberculosis (MtTreS) ... 33
3.4 Conclusion ... 36
IV. INTRODUCING TREHALOSE SYNTHASE GENE INTO Arabidopsis thaliana 4.1 Introduction ... 37
4.2 Material and methods ... 37
4.2.1 Place and Time of Research ... 37
4.2.2 Material of Research ... 37
4.2.3 Preparation Agrobacterium solution for floral dipping ... 38
4.2.4 Floral Dipping ... 38
4.2.5 Selection of transgenic lines ... 38
4.2.6 Inheritability of TreS Expressing Lines ... 39
4.2.7 PCR analysis of TreS lines ... 39
4.3 Result and discussion ... 39
4.3.1 Frequency of putative mutants ... 39
4.3.2 The presence of TfTreS within putative mutants ... 42
4.3.3 Inheritability of TreS Expressing Lines ... 43
3.4 Conclusion ... 43
V. PRELIMUNARY TEST OF THE USE OF TRES AS A SELECTABLE MARKER 5.1 Introduction ... 45
5.2 Material and methods ... 46
5.2.1 Place and time of research ... 46
5.2.2 Material of research ... 46
5.2.3 Assessment of TreS plants on trehalose or in combination with valydamycin A ... 46
5.3 Result and Discussion ... 47
VI. CHARACTERIZATION OF TRES EXPRESSING LINES
6.1 Introduction ... 55
6.2. Material and method ... 55
6.2.1 Place and time of research ... 55
6.2.2 Material of research ... 55
6.2.3 Characterization of TreS line ... 55
Growing Arabidopsis thaliana ... 55
Enzymatic trehalose determination ... 56
Determination of enzyme activity ... 56
TfTreS expressing lines in response to drought ... 57
Leaf water retention and leaf recovery test ... 57
In planta TfTreS expressing plants in response to drought ... 57
6.3 Result and discussion 6.3.1 Growing Arabidopsis thaliana in tropical climate ... 57
6.3.2 Trehalose content and enzyme activity of TfTreS expressing lines ... 60
6.3.3 Enzyme of TfTreS Expressing lines ... 60
6.3.4 Trestf expressing lines in response to stress ... 62
Leaf Water Retention ... 62
Cluster Analysis based on parameters observed ... 65
Leaf Recovery After 16h RT incubation ... 69
TfTreS expressing lines in response to drought ... 71
6.4 Conclusion ... 76
VI. GENERAL DISCUSSION ... 77
IX. CONCLUSION AND RECOMMEDNDATION ... 81
XI. LIST OF REFERENCE ... 82
XII. APPENDIXES ... 92
LIST OF TABLE
Title Page
Table 1. Frequency of putative mutants selected on kanamycin or trehalose 41
Table 2. Frequency of trehalosa resistant seedlings of T1 dan T2 ... 43
Table 3. Frequency of resistant seedling to 125 mM trehalose and 10 mM validamycin A ... 47
Table 4. Number of T3 seedlings resistant to 100 mM trehalose ... 60
Table 5. Trehalose content of published some transgenic plants ... 62
Table 6. Leaf water lost at interval of time after detachment ... 63
Table 7. Detached Leaf Total Water Loss and Leaf Dry Weight ... 65
Table 8. Observed characters on leaves after detachment ... 66
LIST OF FIGURE
Title Page
Figure 1. The damaging effect of heat to membrane and
the role of trehalose to decrease the effect ... 10
Figure 2. Trehalose biosynthesis ... 14
Figure 3. Trehalose pathways in organisms ... 15
Figure 4. Starch breakdown in chloroplast and carbon export at night ... 19
Figure 5 PCR Product at various annealing temperature ... 27
Figure 6. Schematic Diagram of pGemT reS ... 28
Figure 7. Restriction analysis of plasmid isolated from E. coli colonies expected to bear TreS gene ... 29
Figure 8. Restriction analysis based on computer simulation of TreS sequence ... 30
Figure 9: Sequence of TreS from Thermobifida fusca cloned in E.coli ... 31
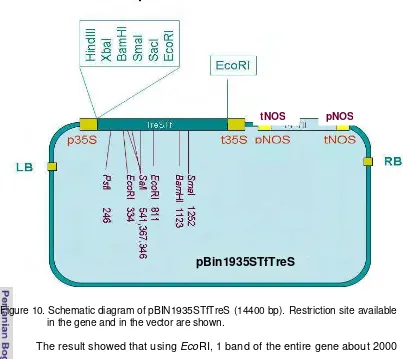
Figure 10. Schematic diagram of pBIN1935STreS ... 32
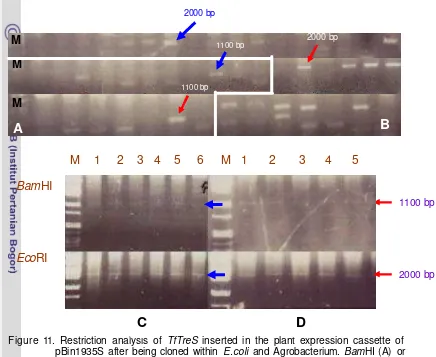
Figure 11. Restriction analyses of TreS inserted in the plant expression cassette of pBin1935S ... 33
Figure 12. Restriction analysis of TreSMtbc within pCR Blunt II TOPO (A) 34
Figure 13: Schematic diagram of pBin1935SMtbc ... 35
Figure 14. One-week-old putative TfTreS transformed seedlings selected on selection medium ... 40
Figure 15. Seedling resistant to kanamycin or trehalose ... 42
Figure 16. PCR product of putative mutant ... 42
Figure 17. Selection of putative mutants on trehalose/validamycin A ... 42
Figure 18. Seedling growth on validamycin A ... 47
Figure 19. TfTreS lines plants grown on various medium ... 51
Figure 20. Rice callus grown on sugars ... 52
Figure 21. Grown Arabidopsis in tropical area ... 58
Figure 22. T3 plants selected on trehalose ... 59
Figure 23. Trehalose concentration and enzyme activity of TfTreS expressing lines and its control plants ... 61
Figure 24. The average of excised leaf water loss at pointed time ... 63
Figure 25. Drought tolerance test using leaf water retention measurement method ... 64
Figure 27. Leaf recovery test of dehydrated leaves ... 67
Figure 28. The trehalose content / enzyme activity of recovered leaves from drought ... 68
Figure 29. TfTreS expressing lines against drought ... 72
Figure 30. TfTreS expressing lines against drought (2nd experiment) ... 73
Figure 31 Plants recover from drought ... 74
Figure 32. Fresh and dry weight of plant recovered from drought ... 75
LIST OF APPENDIXES
Appendix 1. TreS from several organisms ... 92
Appendix 2. Codon usage of AtTPS1 of Arabidopsis thaliana and TreS of Thermobifida fusca ... 93
Appendix 3. Trehalose content and enzyme activity of TfTreS expressing plants and Wt plants ... 93
Appendix 4. Regression analysis of standard curve: glucose versus optical density and graphic plot ... 94
Appendix 5. T-Test of Trehalose Content or Enzyme Activity between Wt and TfTreS plants ... 95
Appendix 6. Box lot analysis of Trehalose content or Enzyme activity between Wt and TfTreS plants ... 96
Appendix 7. Site mutation of TfTreS found in the gene after being cloned in E. coli ... 97
Appendix 8: Single factor analysis of water loss at pointed time ... 98
Appendix 9. Leaf Total water loss ... 100
Appendix 10. Single factor ANOVA of leaf Dry Weight ... 100
Appendix 11. Fresh and Dry Weight of Tested plants ... 101
Appendix 13. Single Factor Anova of Frequency of Putative Mutants Selected on Kanamycin or Trehalos ... 102
1.1 Rationale
Trehalose is a disaccharide that is well known as a stress protecting agent for many organisms particularly microbes, insects, nematodes and some plants. It belongs to the unhydrobiotic group of organisms (Crowe et al. 1982). The concentration of trehalose within the tissue of a certain organism correlates with the capability of the organism to face stresses such as osmotic and oxidative stresses (Franco et al. 2000; Benaorouj 2002), heat stress (Reinders 1999; Kandror 2003) and pressure (Iwahashi et al. 2000) as Elbein (2003) suggested that trehalose is a multifunctional molecule. The presence of gene encoding trehalose synthesizing enzyme converted drought, cold, and salt plant resistance (Garg et al. 2002; Jang et al. 2003, El-Bashiti 2003). The destructive effect of abiotic stresses often resulted from protein/enzyme denaturation, membrane disintegration, and/or resulting ion balance disturbance within the cells. As a non-reducing sugar, trehalose has many unique features; it is very stable in a very wide range of conditions such as pH, temperature, and water status. This sugar further remains amorphous in completely dry conditions (Hagen 1995). These unique features make trehalose is able to stabilize the surrounding compounds and structures and so minimize the destructive effect of stress; hence, it is also called as biological stabilizer (Shinohara et al. 2002).
enzyme converts maltose directly to trehalose (Smet et al. 2000). This enzyme is also capable to convert trehalose to maltose with equilibrium state at about 60% trehalose concentration (Wei et al. 2004).
Research on trehalose concerning stress resistance of many organisms has been done. Expression of either foreign OtsA or OtsB or both gene(s) on plant caused stunted growth, such as on tobacco (Romero et al 1997; Gidijn et al 1998; Almeida et al. 2005,). This event is resulted from accumulation of its intermediate product, trehalose-6-phosphate (T6P) that leads to feedback inhibition of trehalose phosphate phosphatase (trehalose immediate enzyme synthesis). T6P accumulation depletes P inorganic that disturbs glycolysis influx and in turn disturbs energy conversion. The stronger effect on plant development, however, T6P induces starch immobilization leading to root starvation. In a certain level, however, T6P is needed for seedling growth at least it was proved in Arabidopsis (Schluepmann et al. 2004). This problem has been solved by fusing both genes OtsA-OtsB into a molecule; hence, fused protein translated from the genes is obtained. Consequently, T6P resulted from TPS activity is directly converted into trehalose and T6P accumulation can be minimized. This work has been reported on rice contained a fused genes of OtsA-OtsB from E. coli. This rice transgenic showed more resistant to drought, salt and cold, as well as produced more yield than the control (Garg et al. 2002; Jang et al. 2003).
TreY-TreZ and TreS pathways, as mentioned above, do not produce T6P as intermediate product. Hence, questions rise, 1) whether introducing TreS that converts maltose to trehalose without producing T6P could overcome the problem resulted from T6P/trehalose accumulation, 2) whether TreS can be used as a selectable marker and 3) whether TreS plant lines withstand various stresses (e.g. drought, salt and pH). This research was addressed to answer those questions. When positive result is obtained, the gene will be transferred to crop plants. To be able to answer these questions, TreS from Thermobifida fusca and Mycobacterium tuberculosis were cloned separately, and were introduced into Arabidopsis thaliana.
1.2 Objectives for the research are:
1. To clone TreS gene from Thermobifida fusca and Mycobacterium tuberculosis.
3. To evaluate whether TreS can be used as an alternative selectable marker in cloning strategy.
4. To charachterize the TreS expressingplants under stresses condition
1.3 Research Benefit
The result of this research would provide:
1. TreS gene inserted within pGemT and constructed within expression cassette
2. TreS expressing Arabidopsis thaliana as plant model for further research concerning on plant responses to stresses.
3. Information whether TreS can be used as an alternative of non toxic selectable marker in cloning strategy
4. Information about the expression of TreS gene from T. fusca on plant under stresses.
1.4. Hypothesis
1. Clones of TreS gene from T. fusca that has been characterized by Wei et al. (2004) would be available and could be used to develop transgenic plant for crop improvement.
2. Transgenic Arabidopsis which express trehalose synthas gene from Thermobifida fusca would be available
3. TreS can be used as an alternative among existed non toxic selectable markers in cloning strategy.
II. LITERATURE CITATION
2.1. Damaging effects of stress.
Stresses could result in biological damages such as cell membrane disruption, protein denaturation, and disturbance of ion balance (Garcia et al. 1997). Cell membrane is very important part of the cell to maintain cell integrity and cell function. There are many physiological processes that involve membrane such as chemiosmosis, selective transport molecules, membrane polarization, and compartment separation that taken together called as cellular homeostasis. Cell membrane is very susceptible to stress. Crowe and Clegg (1978); Leopold (1986) for instance, suggested that the primary site of drought effect is membrane that is intolerant to desiccation, since drought deforms membrane and protecting membrane component from drought is likely feature of desiccation tolerance.
The membrane deformation occurs due to dessication such as removal of integral membrane protein, changes of transition phase from fluid to gel phase or to hexagonal non-bilayer phase, lateral separation and membrane fusion (Crowe and Crowe 1982). This is due to membrane bilayers are composed from many different lipids as well as proteins with different composition at any part of the membrane. Each compound of the membrane has its own transition phase at different temperature and different water status. Hence, when dehydration or rehydration occurs at a given condition (the same status of water status and temperature), membrane disintegration could occur. Phospholipids consisted of head part, which is semi fluid hydrophilic lipoprotein, and tail that is a long phospholipids hydrophobic chain. The phospholipids chain become rigid when it encounters to water stress or freezing condition, which lead to the damage at rehydration and thawing state (Crowe and Crowe 1990).
Removing water resulted in hydration force (suction/ negative force) that lead to reduction of membrane surface. This event makes membrane favor to be in the gel phase; hence, melting temperature (Tm) increases. In other word, membrane is in gel phase at such temperature which in fully hydrated situation it should be in liquid phase (for review see Bryant et al (2001).
further that this decrease is caused by the activation of lypolytic genes that degrade membrane.
The damage resulted from drought might be irreversible (Mundree et al 2002), and many fundamental roles in living organism, such as solvent for biological reactions, and transport medium and particularly for photoautotroph organism in which water has another crucial role as an electron donor for Photo system II (PSII). Photosynthesis disturbance, shutting down carbon assimilation, free radical production, and cell turgidity lost. Drought also increases salt concentration that in turn induces biological damage.
Any stresses such as oxidative stress causes biological damage. Any stress that disturbs cellular homeostasis leads to the enhancement of reactive oxygen intermediates (ROIs). Oxidative damages caused by ROIs are membrane lipid peroxidation, protein oxidation, enzyme inhibition, and DNA/RNA breakdown. During stress, ROIs may increase resulted from photorespiration, mitochondrial respiration, pathogens, wounding and environmental stresses such as drought or osmotic stress (Hammond and Jones 1996). Protein is directly oxidized by oxygen species, or superoxide, particularly 4Fe-4S clusters within the enzymes such as mitochondrial aconitase that in turn release iron. This event caused cell limitation in using non-fermentable carbon source accelerating the aging process (Halliwell and Gutteridge 1999). The iron released when it pooled in vacuole implicated to vacuole fragmentation, consequently, the cell become sensitive to various stresses such as pH, nutrition limitation, and metals (Corson et al. 1999; Srinivasan et al. 2000). ROIs also oxidize nucleic acid and lead to bases and sugar damage, single strand break and damage the crosslink between abases site of protein-DNA. Modification bases are important effect that caused mutagenic or lethal effect of ROIS. The failure to maintain the genomic integrity has been associated with ageing, degenerative diseases. ROIs damaging effect to lipid is autocatalytic process leading to production of fatty acid hydrogen peroxide that causes fragmentation.
Heat stress on plant reduces photosynthesis and yield. Sharkey (2005) suggested that this effect is due in activation of ribulose bisphosphate carboxylase oxygenase (rubisco), the enzyme responsible for carbon dioxide fixation by moderate heat stress. While Ortiz and Cardemil (2001) found that heat stress, cause dissociation of (LHC) and (PSII) reaction centre. While damage due to ice is well known as irreversible damage of cellular micro architecture that happen when transition phase from liquid to ice (Bryant et al 2001).
2.2 The character of trehalose and its role in several organism
Trehalose (α-D-glucopyranosyl[1-1]- α-D glucopyranose) is a non reducing disaccharide consists of 2 glucose joined with glycosidic bond. This sugar is ubiquitously found in biological world, such as in bacteria, yeast, fungi, some animals such as insects and worms, and, in lower kingdom of plant (e.g. in fern (Selaginella lepidophylla) called “resurrection plant” (Zentella et al 1999). Recently, however, trehalose is also found in higher kingdom of plants of in a very small amount. Trehalose is commonly found in spores, fruiting body, seeds, and vegetative tissue. Calaco et al (1995) suggested that this compound is stable in high temperature and low pH. It is stable and stabilizing sorounding compound against heat (Singer and Lindquist 1998; Reinders 1999; Kandror 2003), cold, drought and salt (Garg et al 2002, Jang et al 2003, El-bashiti 2003), oxidative stress (Franco et al. 2000; Filinger 2001; Benaorouj et al. 2002) and pressure (Iwahashi et al. 2000). More detail is discuss in the nex subheading.
number of trehalose molecules bridging three or more lipid molecules (Pireira et al. 2004).
This compound has several roles in organisms; however, it may has different function among various organisms (for review, Albein et al. 2003). Trehalose function as energy reservation, e.g on Neurospora tetrasperma which contains 10% of it’s dry weight on germination while in adult insect, trehalose is also used as energy such as for flying (Albein, 2003). On plant material, despite of osmotic protecting agent, trehalose is also functioning as regulator for glucose, abscisic acid and stress signaling (Avonce et al. 2004) that implicated on carbon allocation and involve in sugar metabolism (Vogel et al. 1998; Muller et al. 1999; Garg et al. 2002; Jang et al. 2003). This also has a role in growth and development of Arabidopsis thaliana (Shluepmann et al 2003; 2004). Arabidopsis thaliana trehalose phosphate synthase (AtTPS1) is up regulated along with seed developmental stage and required for full expression of seed maturation marker gene (Eastmond et al. 2002; Schluepmann et al. 2004) and knocked out AtTPS1 is embryo lethal.
Trehalose metabolism also affects biosynthesis and starch degradation. Feeding with trehalose induced starch accumulation and ADP-glucose pyrophosphorylasegene (ApL3) expression (Wingler et al. 2000), which lead to metabolically available carbon immobilization from source to sink that in turn cause growth arrest. The starch induction in chloroplast occurred via posttranslational redox activation of ADP-glucose pyrophosphorylase by accumulation of trehalose-6-phosphate (T6P) (Kolbe et al. 2005). This growth arrest is relieved when sugars are added simultaneously together with trehalose (Schluepmann et al. 2004). Trend reduction of hexose phosphates activity was also shown on plant with accumulation of T6P in Arabidopsis (Schluepmann et al. 2004). While in yeast, this accumulation causes significant growth reduction (Bonini et al. 2003). Its intermediate product of trehalose in Arabidopsis (T6P) is needed in small amount for embryo development but in high amount resulting in growth arrest. Abolish of its synthesis cause embryo lethal (Schluepmann et al. 2005).
2.3 Trehalose and its role as stress protecting agent
situation to avoid starvation, and is not used as energy reserve (Wiemkem A, 1990). Mutants of E. coli that unable to synthesis trehalose were osmotically sensitive to glucose medium (Giaever et al. 1988). While Alarico et al. (2003) reported that disrupted gene for trehalose synthase in Thermus thermophylus reduced the capability to survive on salinity condition with maximum concentration of 5%. This situation was relieved when trehalose was added into the medium, while it was not releaved when glycine betaine, manosylglycerate, maltose, or glucose was added to the medium. Trehalose content on stressed sensitive wheat species has lower level than the resistant one either in normal conditions or in drought and salt (El-Bashiti, 2003).
Trehalose is the most effective compatible solute among sugars (Sampedro 1988). The effectiveness of trehalose in preserving biomoleculs is related with the flexibility between the two monomers compared with other sugars such as sucrose and maltose (Crowe et al. 1983) by which it conforms to irregular polar groups of macromolecules provided a better interaction with them. Hence, trehalose could reduce these effects of multiple stresses such as water status and temperature (Kandror et al. 2002), salinity (Alarico et al. 2005), oxidative stress (Peral et al. (2002), osmotic and oxidative stress (Giaever et al, 1988, Franco et al 2000; Fillinger et al. 2001; Benaorouj 2002) and nutrient starvation. Membrane damage for instance, has been effectively protected from temperature (Macdonald and Johari 2000; Pereira, 2004; Hincha et al 2004; Patist et al. 2005); from freezing and hydration force (Yoon et al. 1998) and from water stress (Chen et al. 2001; Bryant et al. 2001) by trehalose. Shinohara et al. (2002) found that trehalose is also involved in cicardian regulation of stress responses and development.
suggestion, however, it is depending on the condition of biological compound when vitrification occur (Bryant et al. 2001).
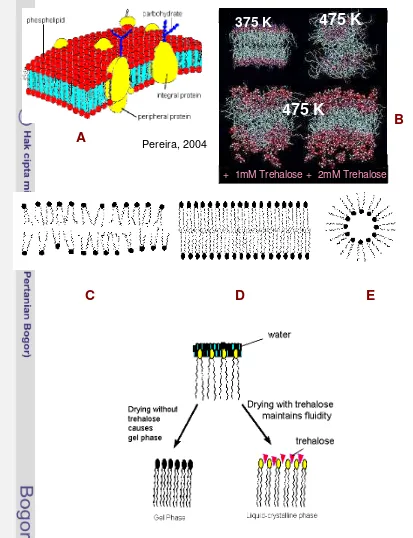
There are many suggestions supporting the theory. Trehalose has a tendency to make a direct interaction with biological compounds, such as membrane, protein and nucleic acid and prevents their biological damage (Luzardo et al. 2000; Ekdawi, 2001; Bordat et al. 2004). This is due to the capability of trehalose to adsorb water and reduces its dynamic, replaces the hydrogen bond, reduces molecule expansion, reduces over vibration specific site of molecules. Furthermore, trehalose is very stable of its own molecule and stabilizes its surrounding compounds, as known as biological stabilizer. Trehalose interacts with phosphate group laid between the head and the tail, keeping the membrane fluidity (Crowe et al. 1987; Rudolf et al. 1990). Pirera et al. (2004) have reported interaction simulation between membrane bilayers (Figure 1). The increase temperature from 325K to 475K increase structural disorder of the membrane. The presence of trehalose 2 mM much reduced the degree of disorder.
Bryant et al. (2001) suggested that the main effect of cytoplasmic small solutes is simply related with water relation. Solutes increases the osmotic pressures that reduce water removal from the cell and solute also have volumetric effect that intercalate between lipids, hence, force of hydration is reduced. However, they agree that there is no controversial suggestion about the first and second theory of biological compounds stabilization by sugars, especially membrane via this manner. For the third theory, however, they suggest that it is still confusing since the effect of vitrification differs in different situation and different solute used (such as the size). The ability of trehalose in stabilizing the biological compound from freeze is related with the capability of trehalose to disrupt tetrahedral hydrogen bond network of water, and reduce the freezable water (Crowe et al. 1983).
Such interesting though is suggested by Alpert (2006), suggesting that stress tolerant organism is very rare found on multi cellular organism but it does widelyspread on those < 5 mm in size. He suggested that there is a trade-off between desiccation tolerance and growth. Recent molecular and biochemical research shows that organisms tolerate desiccation through aset of mechanisms, including sugars that replace water and formglasses that stabilize
F
Figure 1: The damaging effect of heat to membrane and the role of trehalose to decrease the effect. Diagram of membrane bilayer (A). Effect of heat to membrane structure (B). Schematic diagram of liquid phase of biological membrane (C). Schematic diagram of membrane in gel phase when subjected to stress (D). Schematic diagram of lipid as part of membrane when it subjected to severe stress (E). A model of trehalose that is suggested to interact with membrane at hydrophyllic part of lipid (F). This picture is cited from Web (www.agronomy.psu.edu, 07/25/2004).
475 K
+ 1mM Trehalose
Pereira, 2004
+ 2mM Trehalose
375 K
475 K
A
B
macromolecules such as proteins and membrane, and via production of anti-oxidants that counter damages resulted from reactive oxygen species. These protections are often induced by drying, and some of the genesinvolved may be homologous in microbes, plants, and animals.
As mentioned above that trehalose is also involve in preserving biomolecules from cold, hence it is well used as cryopreservation agents. Additional of sugars or salts to the solution distort the water structure where which these compounds may enhance the tetrahedral coordinated hydrogen bond structure or reduced it that respectively called as water structure maker and water structure breaker. Preservation of biological membrane would be effective with additional of water solute act as water structure breaker that reduced freezable water (Branca et al 1999). Including in water structure breaker are ClO4-, MnO4-, Br-, Cl-, K+, Cs+, sugars and I-. Those belong to water structure maker are Li+, Cu+, Al+, Mg+, and OH-. While Na+, Ag+ and Ba+ are in borderline (Bryant et al. 2001). Miller and Pablo (2000) suggested heat solution value of trehalose, maltose and sucrose as water structure breakers. The conformation of sugars in the solution release heat (enthalpy), where 19.1, 15.6 and 5.95 kJ/mol of trehalose, maltose and sucrose respectively that in turn reduce freezable water.
Trehalose is also associated with resistance of organism to oxidative stress, such to super oxide. Yeast cell that exposed to mild heat stress induced accumulation of trehalose and its resistance to hydrogen peroxide. Conversely, those lacking of trehalose synthase were sensitive to hydrogen peroxide as its protein oxidation run faster (Benarouj et al. 2001). Trehalose is also found to be essential for mycolic acid biosynthesis in Corrynebacterium glutamicum. The absence of mycolic acid in defective trehalose synthase mutant caused cell wall disorder, excretion of amino acids and impairment with bacterial growth (Wolf 2002).
There is no discussion about trehalose in combating the deleterious effect of high salinity has been reported. However, Garg et al. (2002) and Jang et al. (2003) found that transgenic rice bearing gene for trehalose synthase (fusion of TPS1-TPP) were also tolerant to salt and cold, despite to drought.
2.4 Other compatible solutes
processes; hence, they are called as compatible solutes. The osmolytes can be synthesized or taken from medium. They interact to biological compound accordingly, such as via making hydrogen bonding or electrical interaction (Bryant et al. 2001). Beside their function for osmolytes these compound also stabilize biological compounds. Ion channel, membrane protein involves in sensing external and internal differences, such as salt concentration, cell volume and turgor pressure changes. They may not act exclusively in particular organism and particular stress, yet they may be accumulated at different stresses, and act together that called as cocktail solutes.
There are many compatible solutes that can be categories into in several ways. Anonyl (Anonim, www.sbu.ac.uk) suggested that it can be devided into inoprganic and organis compatible solutes. Including in inorganic solutes are such as: K, Mg, SO4, HPO4, Ca, Li, Na, H, and OH. While organic osmolytes include polyol and derivates, amino acid and derivates, betaine, ectoin and occasionally peptides (Robert MF for review).that can be sub categories into three groups: zwitterionic solutes, (ii) noncharged solutes, and (iii) anionic solutes. The first group includes glycine-betain, ectopin and Na-acetyl b-lysine and b-glutamin. The second group includes carbohydrates (glycerol, myo-inositol, trehalose, sucrose etc) and uncharged amino acid and peptides such as carboxamine and acetylated neutral glutamine dipeptides, glutamate, ß-hydroxybutyrate and derivatives. The unionic solutes are anionic polyols and carbohydrates. Neutral osmolytes usually accumulated by bacteria, while archea tend to accumulate negative charged osmolytes.
2.5 Trehalose biosynthesis
Trehalose
G6P
UDP-G
trehalose 6-phosphate
OtsA
OtsB
(1-O-α-D-glucopyranosyl-α-D-glucopyranoside)
inhibites
1. OtsA–OtsB pathway
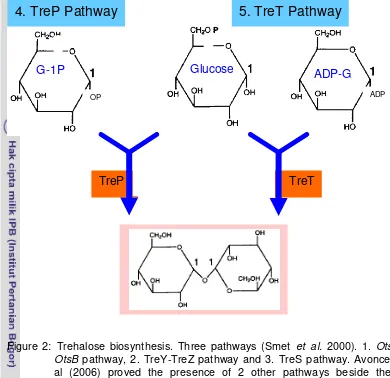
Figure 2: Trehalose biosynthesis. Three pathways (Smet et al. 2000). 1. OtsA-OtsB pathway, 2. TreY-TreZ pathway and 3. TreS pathway. Avonce et al (2006) proved the presence of 2 other pathways beside the 3 mentioned, distributed in diverse organisms called as TreP (4th pathway) and TreT (the 5th pathways).
oligomere. Third pathway called TreS pathway, one enzymatic reaction involves in the pathway, by which enzyme trehalose synthase (TRES) convert maltose to trehalose. Similarly to the second pathway, this enzyme also rearrange intermolecular of maltose from α1-4 glycosidic linkage to α-α, 1-1 glycosidic linkage of trehalose, hence maltose is converted to trehalose. This enzyme is also capable to convert trehalose to maltose (Smet et al. 2000) with equilibrium state at 60% trehalose and 40% maltose (Wei et al. 2004). Avonve et al (2006) proved 2 others pathways beside these pathways, they are called as TreP and TreT pathways (Figure 2). TreP pathway, converts glucose-1P and glucose to trehalose and release Pi catalysed by trehalose phosphorylase (TreP). This pathway mainly occurs in eubacteria and small portion occurs in archaea. TreT pathway converts ADP-glucose and glucose to produce trehalose and ADP is released. This conversion is catalysed by trehalose glycolsiltransfering synthase,
4. TreP Pathway
TreP
OP ADP
TreT
5. TreT Pathway
[image:31.595.106.496.86.464.2]and is found mainly in archaea with small event in eubacteria (Figure 3).
[image:32.595.103.511.95.783.2]The first pathway is widely spread in many organisms, E. coli (Murata et al. 1971) and S. cerevisiae (Smet et al. 2001); S. pombe (Franco et al. 2000); Aspergillus nidulan (Fillinger et al. 2001); A. niger (Wolschek and Kubicek, 1997; Soto et al. 1999) and also in Arabidopsis thaliana (Leymann et al. 2001). The second pathway can be found such as in Brevibacterium helvolvum (Kim et al. 2000), Arthrobacter (Nakada et al. 1995), Rizobium (Murata et al. 1996) and Archeon Sulfolobus acidocaldarius (Murata et al. 1996). While the third pathway can be found in Thermus aquaticus (Tsusaki et al. 1997), Agaricus bisphorus (Wannet et al. 1998), Pimelobacter (Nishimoto et al. 1995), Thermobifida fusca (Wei et al. 2004), Thermus acidophyllus and Grifola frondosa (Saito et al. 1998). Some organism has more than one pathways, it may be two or three pathways. Mycobacterium tuberculosis (Murphy et al. 2005), M. smegmatis, Mycobacterium bovis and M. leprae have those three pathways in trehalose synthesis (Pan et al. 2004).
2.6 Characterization of Trehalose Synthase (TRES/TSASE)
Enzyme with optimum condition similar to that plant cellular is favorable for generating transgenic plant. Information was gathered regarding to enzyme characters (pH, temperature, Km) of trehalose synthase from few organisms that has been reported, summarized at appendix1. It is shown that among he reported trehalose synthase, TSase with optimum temperature of 20oC, pH 6.5 with low Km and high maximum conversion, seems to be most possible to be active in plant cellular. Saito et al. 1998 found trehalose synthase from Grifola frondosa that is annotated as TSase has a molecular mass 120 kDa consisted of a dimmer protein 60 kDa. This enzyme is very interesting, since it converses a-D glucose and a-D glucose 1P. While other TSae used maltose as substrate.
TSase from G. frondosa also catalyses trehalose phosphorylation into its substrate; however its equilibrium is favor to trehalose synthesis. The temperature optimums of trehalose synthesis and trehalose phosphorylation were 32.5-35oC and 35-37.5oC respectively. The rate conversion of maltose to trehalose by trehalose synthase is in optimal rate when it occurs at pH of 6.5, while the opposite direction is optimal at pH of 6.5-6.8. In the optimal condition, the conversion rate of trehalose synthesis was 18mM h-1 with maximum conversion rate was 90% of the substrate added. Interestingly, this enzyme also can produce trehalose from sucrose when 1.05 U of glucose isomerase and 20 mM MgSO4 is added to the reaction mixture. The rate production of the second way of synthesis was 4.5 mM h-1.
The same enzyme from Pimelobacter, is annotated as TreS. TRES from Pimelobacter sp. was shown to catalyze an intramolecular rearrangement of maltose to convert the α-1,4-glycosidic linkage of this disaccharideto the α,?α 1,1-glycosidic linkage of trehalose (Nishimoto et al. 1995). TreS of Pimelobacter consisted of a 1719-bp gene, codinga 573-residue amino acid sequence. It has homologues area with maltases from Saccharomyces carlsbergenesisand Aedes aegypti at 220 N-terminal residues. The same enzyme from Thermus acidophyllus (annotated as TS) has a molecular mass of 110 kDa. At pH of 7.0 this enzyme has optimum temperature at 40oC with Km of 0.9 mM. While at pH 6.5, it has optimum temperature at 45oC with Km of 4.2 mM.
about 68 kDa, however in the active form it has 390 kDa. Hence it is an hexamere of 6 identical monomers. This enzyme catalyzes maltose to trehalose and capable of interconversion with equilibrium state at 42-45% of each that can be reached at incubation for 6h with 0.5 mM maltose as the substrate. Higher concentration reduced the conversion rate. When trehalose was used as substrate, 30% maltose was obtained after et least 12h incubation with 8-10% of glucose was obtained. The optimum pH was 7 and experiment was conducted at 37oC. When maltose is used as substrate it has Km was 10 mM but he Km for trehalose was 90 mM.
The character of the enzyme TRES from Thermobifida fusca was also reported (Wei et al. 2004) reported. It has a molecular weight of 66 kDa with optimum condition of pH at 6.5 and temperature at 25oC. This enzyme converts matose into trehalose, and has capability to reverse back trehalose into maltose at equilibrium state, about 60% trehalose concentration. At higher temperature (37oC), or when the enzyme concentration is high, the enzyme also diverts matose into glucose up to 15% of the substrate. Reaction mixture with15% maltose, it converts substrate about 55-60% at optimal condition. Heavy metal (Cu2-, Mn2- and Zn2-) at 5 mM did not inhibite its activity. The catalytic sites of this enzyme laid at 223-257 amino acids and 400-439 amino acids.
TreS is also found in Mycobacterium tuberculosis (Murphy et al. 2005). In this bacteria, there 3 pathways are present, and TreS is not the used major pathway compared to the two others. While Cardoso and Castro (2007) reported 2 pathways (OtsA-OtsB and TreS) found in Propionibacterium freudenreichii. This bacteria accumulated high level of trehalose especially in response to stresses (osmotic, oxidative and acid stresses). The first pathway is suggested to function in trehalose synthesis while the second is functioning as trehalose degradation.
2.7 Foreign genes encoding for Trehalose Synthase on plants
Trehalose 6 phosphate synthase (OtsA) and trehalose 6-phosphate phosphatase (OtsB) showed larger leaves and better growth on drought. Phenotypic alteration of tobacco expressing trehalose synthase was corrected by expressing both genes of yeast (Tps and Tpp) and tolerant to drought was conserved (Welin et al. 2001). One-step further was done by Garg et al. (2002) and Jang et al. (2003), they introduced fused of both genes OtsA-OtsB into rice, and the transgenic rice was tolerant to drought, cold and salt with higher yield. They suggested further that the increase of trehalose migt not be enough to act as osmolytes, instead as regulator of sugar sensing and expression of genes involve in carbon metabolism.
Unlike the previous report by Paul et al. (2001) suggested that the increase of photosynthetic capacity was modulated by T6P, while Garg et al. (2002) suggested that it was due to trehalose, since introducing fusion OtsA-OtsB ( low T6P) resulted in high photosynthetic capasity. Using the third pathway of trehalose synthesis genes proved to overcome high trehalose induced morphological defect as well as increased the resistance of transgenic plant to drought and salt, such as tobacco and sugarcane bearing TSase from Grifola frondosa (Zhang et al. 2005; Zhang et al. 2006).
2.8 Trehalose induces starch biosynthesis in Arabidopsis thaliana
Starch is the main storage carbon in plant. In chloroplast starch is synthesized during the day as a temporary store, and it is degraded during the night and exported to non-photosynthetic tissue. For long-term storage such as in tuberous plant, e.g. potato tuber, starch is resulted from sucrose conversion. ADP-glucose pyrophosphorylase (AGPase) is the first enzyme in starch formation. Glucose-P and ATP is catalyzed to produce ADP-glucose and PPi. ADP-glucose then is used to form starch by elongating glucan chain of starch granule.
chloroplast, activation thioredoxin f and m increase monomerisation of AGPase that lead to its activation by 3PGA (Geigenberger et al. 2005). In leaves, AGP-ase is regulated by light-dependent signal, by which transfer electron from photo system I to feredoxin via feredoxin: thioredoxin reductase to thioredoxin f and m that leads to activation of the enzyme (Balmer et al. 2006). AGPase also respond to sucrose level in cytosol. Trehalose has been proved to induce starch accumulation by promoting reductive activation of AGPase in the plastid (Kolbe et al. 2005).
Figure 4. Starch breakdown in chloroplast and carbon export at night. Starch breakdown and the fate of its derivates (A), α-amylase (1), disproportionating enzyme (DPE1) (2), glucose transporter (3), maltose transporter (MEX1) (4), disproportionating enzyme 2 (DPE2) (5), Hexokinase (6) and phosphorylase (7). Cited from (Chia et al. 2004). Enzyme involve in granule breakdown (B).
Trehalose feeding increases T6P level within the tissue, which induces APL3 that encodes AGPase, the first enzyme of starch synthesis. Beside the induction, T6P also reduces activation of AGPase through redox reaction. This deduced from the study of Kolbe et al. (2005), over expression of TPS1 that increase T6P eccelerated the monomerization of AGPas (the active form of AGPase). Conversely, over expression of TPP that converts T6P to trehalose reduced the monomerization of AGPase. They further proved that adding T6P to isolated
A
B
α-amylase
Branched oligosaccharides
Β-amylase
[image:36.595.91.519.123.524.2]choloroplast resulted in AGPase activation. Yet when T6P was added to disrupted chloroplast, the enzyme was not activated. Addition of other sugars did not activate the enzyme, but sucrose activated the enzyme in slower/latter than T6P. They suggested further that T6P and not trehalose or other sugars senses the sucrose status within cytosol to chloroplast.
Flow Chart of the Research
Mycobacterium tuberculosis Thermobifida fusca
Arabidopsis
Seeds
Putative transgenic selection
Gene isolation – PCR
TfTreS
pGemT
pBIN1935S
Agrobacterium
Arabidopsis
Seeds
Putative transgenic
selection
Check the presence of
TfTreS
Inhetitability
Chooped from
pCR-Blunt II -TOPO
MtTreS
pGemT
pBIN1935S
Agrobacterium
Preliminary test
TreS
–
as an alternative
Selectable marker
Characterization of
TreS expressing
lines
TRES - SELECTABLE MARKER TRES EXPRESSING LINES CHARACTERIZATION
Kill curve of Wt plants
of Trehalose and
Validamycin A
Grown
TfTreS
plant
on Trehalose and
Validamycin A
• Leaf water
retention test
• Leaf water
recovery test
• Drought
resistance test in
planta
• Trehalose content
III. CLONING TREHALOSE SYNTHASE GENE from Thermobifida fusca and Mycobacterium tuberculosis
3.1 Introduction
This chapter concentrated to provide the clone of the gene (TreS) within cloning vector and within plant expression cassette as a preparation to express the gene in Arabidopsis thaliana to assess the effect of the gene in plant responses to stresses.
There are many TreS bearing organisms, such as bacteria and fungi. The gene that is going to be cloned, would be expressed in the plant, hence, enzymes that active in the condition about similar to the cellular plant condition would be favorable. Few enzymes with their condition for optimum activities are presented in Table 1. Codon usage is also necessary to be considered to maximize the expression of the gene. Some comparisons of codon usage are presented on appendix 1.
Information was gathered regarding to the characterisstics (pH, temperature, Km) of trehalose synthase from few organism that has been reported. It is shown on appendix 1 that among trehalose synthases reported, those with low Km and high maximum conversion rate at the condition suitable in the plant cells would be favourable to be express in plant. TreS gene from Thermobifida fusca, has optimum temperature of 25 and pH 6.5 and it’s activity is not affected by the concentration of substrate (Wei et al. 2004). Hence, this enzyme was chosen to be expressed in Arabidopsis thaliana. It was also cloned TreS from Mycobacterium tubercolusis (Murphy et al. 2005) as it’s sequence is very similar to that of Thermobifida fusca.
3.2 Materials and Methods 3.2.1 Place and time of research
This experiment was conducted in The Department of Molecular Plant Physiology, Utrecht University, Netherlands, started from September 2004-March 2005.
3.2.2 Materials
plasmid (pCR Blunt II TOPO) was kindly donated from B. Rebertson (Imperial Collage, London, UK).
3.2.3 Cloning of TreS genes from Thermobifida fusca and from Mycobacterium tuberculosis
Growth condition of Thermobifida fusca and DNA isolation
Lyophilized cells of the bacteria received in tablet was dissolved in sterile water and spread on top of solidified 65 medium pH 7.2 [glucose 4 g/L; yeast extract 4 g/L; malt extract 10 g/L; CaCO3 2g/L (omit if liquid medium is needed) and 12 g/L agar]. The culture was incubated at 55oC as suggested by DSMZ. When grown cells were obtained, DNA was isolated using plant DNA extraction kit, Pure Nucleophyton (Qiagen).
Cloning strategy
mg/L amphycilin. The rest of suspension cells were centrifuged and about 100 µL of supernatant was kept for resuspension before plating on the same medium. The culture was incubated at 37oC, and colony number was recorded after 24 h.
To verify whether the colonies bring the right plasmid with the right gene, 6 colonies were reanalyzed. These colonies were grown on 3 ml liquid culture supplemented with 100 mg/L amphycilin and incubated at 37oC for overnight. Plasmid was then re-isolated using alkali lysis (Sambrook and Russel, 2001) and digested using EcoRI to see the right size of the gene, or using PstI to see the orientation of the gene and also to give another prove for the right gene. Two clones among those that contained the right TreS gene were re-grown in the same medium and the plasmid were re-isolated using Jet Quick Miniprep Spit Kit (Qiagen) to provide a high quality of plasmid DNA. DNA concentration was determined using spectrophotometer at 260 nm. The DNA then was sequenced with the sequencing mixture as follow: DNA plasmid 1.2 µg, 10 pmol Primer forward (as used to obtain the gene) or 10 pmol M13 primer and then adjusted volume using deionized water to 16 µL final volume. To obtain the whole gene sequence forward or reverse internal primers were also used. This mixture then was sent to commercial sequencing company at Utrecht University.
Verifying TreS from Mycobacterium
TreS gene from Mycobacterium that has been inserted in plasmid pCR Blunt II TOPO was kidly donated from B. Robertson. The plasmid was retransformed into E.coli, and after overnight culture the plasmid was re-isolated from cells. Restriction analysis was performed to recheck whether it bears the right gene. Digestion using NdeI/XhoI was done to check the size of the gene and BamHI or EcoRI to see the orientation of the gene and give more prove for the right gene. The digestion mixture was then run on 0.8% gel electrophoresis at 90 volt for about 30 minutes.
Insertion of TreS gene to Plant Expression Vector
and klenow fragment was subjected to this fragment to obtain blunt ended fragment. pBin19 fragment and 35S promoter-multiple cloning site from PUC19 was then ligated to create pBIN1935S plasmid.
TfTreS that was available in pGemT easy vector system I was digested using EcoRI and was eluted from gel using QIAEX II (Qiagen). At the same time, pBIN1935S was digested using EcoRI and then was treated with alkaline phosphatase for 30 minutes. The gene fragment and the vector was ligated for overnight, followed by transformation to CaCl2 E.coli competent cells using heat shock transformation method as the same method mentioned before. The cell suspension then was plated on agar solidified LB supplemented with 50 mg/L kanamycin. Colonies obtained was observed after over night culture and 20 colonies were chosen to be re-analyzed whether they have the right plasmid with the right gene at the right orientation. Plasmid isolated from over night liquid culture then was digested using EcoRI to see the size of the gene, and using BamHI to see the orientation of every clone. Plasmids that produced about 2000 bp fragment when digested with EcoRI and about 1100 bp fragment when digested using BamHI were the expected one.
MtTreS was exciced from plasmid PCR Product Blunt II TOPO using XbaI/SacI. The digestion mixture was verified on 0.8% gel electrophoresis. Fragment DNA about 2000 bp was eluted from gel, and ligated with pBIN1935S that has been digested with XbaI/SacI and treated with alkaline phasphatase for 30 minutes. The ligation mixture was transformed into E.coli, and the clones obtained were reanalyzed using BamHI.
3.3 Result and Discussion
3.3.1 Cloning trehalose synthase gene (TreS) from Thrmobifida fusca
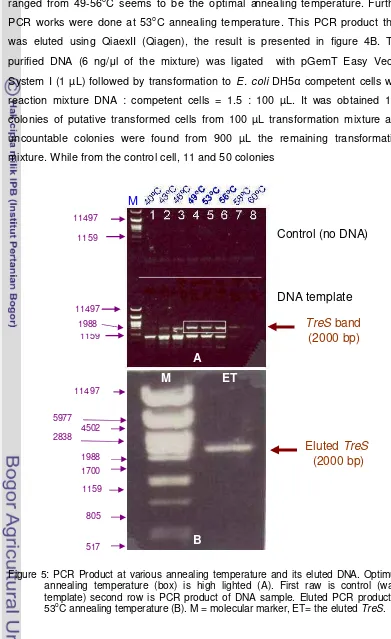
This research was started by growing Thermobifida fusca that was purchased from DSMZ Germany. The lyophilized material was dissolved in water and spread at solidified 65 medium at 55oC. DNA was isolated from mycelium and PCR was done in various annealing temperature to obtain the optimal annealing temperature, the result is presented at Figure 5.
size shown in the figure should be the TreS gene. It is shown that TreS can be amplified at 43oC up to 58oC annealing temperature, however, the temperature ranged from 49-56oC seems to be the optimal annealing temperature. Further PCR works were done at 53oC annealing temperature. This PCR product then was eluted using QiaexII (Qiagen), the result is presented in figure 4B. The purified DNA (6 ng/µl of the mixture) was ligated with pGemT Easy Vector System I (1 µL) followed by transformation to E. coli DH5α competent cells with reaction mixture DNA : competent cells = 1.5 : 100 µL. It was obtained 100 colonies of putative transformed cells from 100 µL transformation mixture and uncountable colonies were found from 900 µL the remaining transformation mixture. While from the control cell, 11 and 50 colonies
Figure 5: PCR Product at various annealing temperature and its eluted DNA. Optimum annealing temperature (box) is high lighted (A). First raw is control (water template) second row is PCR product of DNA sample. Eluted PCR product at 53oC annealing temperature (B). M = molecular marker, ET= the eluted TreS.
Control (no DNA)
DNA template
TreS
band
(2000 bp)
Eluted
TreS
(2000 bp)
B
M ET
M
A
805 517 1159
1700 1988 2838
4502 5977
11497 11497
1159
[image:44.595.105.496.126.765.2]were obtained from a 100 µL and 900µ reaction mixture respectively. From the results, we can see that the efficiency transformation/ligation was approximately 100 Colonies / ng DNA, and, each colony has 90% probability to contain TreS. The plasmid with the insert then was annotated as pGemTreS with schematic diagram shown in Figure 6. The non-transformed colonies (˜ 10%) possibly resulted from T overhang of the vector that has been broken and self ligation occurred.
Figure 6: Schematic diagram of pGemTreS (pGemT with TreS gene as the insert). Cloning sites of pGemT are indicated. Restriction sites of TfTreS are also indicated. T7 and SP6 are the promoters and arrows indicate the direction of promoters.
T7
Apa
I
Aat
II
Sph
I
BstZ
I
Nco
I
BstZ
I
Not
I
Sac
II
EcoR
I
Spe
I
Eco
R I
Not
I
Bst
Z I
Pst
I
Sal
I
Nde
I
Sac
I
Bst
X I
Nsi
I
SP6
1
Start
14
20
26
31
37
43
43
49
64
70
77
77
88
90
97
109
118
127
141
119 Sac II 200 Pst I
766 Sac I 829 Sac I
1103 BamH I
1136 Sma I
1680 Sma I
1830
pGemT
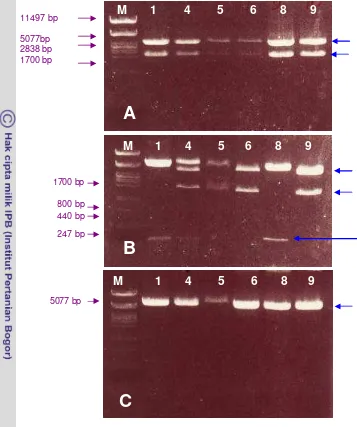
[image:45.595.108.471.232.662.2]Figure 7. Restriction analysis of plasmid isolated from E. coli colonies expected to bear
TreS gene. Plasmid isolated digested with EcoRI (A), using PstI (B) and using
BamHI (C). Number of each well is an annotation for the clone used. Blue arrows indicated the fragments that were expected to be the right size when cut using the corresponding enzymes.
From the putative transformed colonies, six colonies were analyzed to verify the presence of TfTreS The right size of the gene was verified using EcoRI. In order to have idea of the right sequence and to see their orientation they were digested using BamHI and PstI (see analysed Figure 7). These enzymes were chosen based on the sequence of TfTreS gene analyzed with computer analysis in term of enzymes that may or may not cut as well as those that cut frequently (figure 7). Hence, as it was expected that using EcoRI the original size about a
A
B
C
11497 bp 5077bp 2838 bp 1700 bp
800 bp 440 bp 247 bp
5077 bp
M
1 4 5 6 8 9
1 4 5 6 8 9
M
1700 bp
[image:46.595.110.467.94.521.2](2000 bp) would be obtained, while using BamHI, single band of about 5000 bp was produced, since this enzyme cut once only within the gene.
Figure 8. Restriction analysis based on computer simulation of TreSTf sequence.
Cutting with PstI, 2 profiles of bands were obtained, 1) bands of about 1800 bp and of about 3300 bp were shown when the start codon spanned near T7 promoter, and, 2) a band of about 300 bp and of about 5000 bp were obtained if the start codon spanned near SP6 promoter. From the picture, it can be seen that the resulted bands from any restriction enzyme appeared to be the right size as expected. Hence, this gene should be the TreS from Thermobifida fusca. This result confirmed with sequence analysis (Figure 9). We found, however, there was a point mutation both clones at base 27th (the third base of the 9th codon) from GGC TO GGG and at base 1406 from GCT to GCC, fortunately, those codons encode for alanine. The clone number 9, however, another point mutation occurred at base of 1257 from GGA to GGG (both for glycine), and, hence these silent mutations should not alter properties of the protein. Regarding to codon usage, there were slight increase of the first point mutation (from 6.5 to 8.1%), and the third mutation shift the codon usage from 8.1 to 20.2%. While the second mutation, there was dramatic decrease of codon usage from 31.8 to 8.5%. Whether these mutations affect protein activity, it would be discussed in session 6 (characterization of transgenic line).
mix mixture then was transformed into E .coli. Uncountable colonies were found and 20 of each clone were verified using EcoRI and BamHI (Figure 11) to see if the right gene was inserted and if insertion was in the correct orientation.