2s65
"{swrnal
af
Food
ISSN: 0362-028XProtectiorua
Ofiicial Publication
lnternational Association for
Fsod
Protection.
Fleg. U.S. Pat. Off.
Vol.
69November
2006
No.11
Microbiological Contamination
of
Pig Carcassesat
Different
Stagesof
Slaughter in Two
EuropeanUnion-Approved
Abattoirs
C. Spescha, R. Stephan, and C.Zweitel'
...
2568Prevalence
of
Salmonella
in
Diverse Environmental
FarmSamples
Andres Fodriguez, Philipus Pangloli, HaroldA.
Richards, John R. Mount, and F. AnnDraughon....
...2576
Microflora
of
Minimally Processed
Frozen VegetablesSold
in
Gaborone,
Botswana
TinnaA.
Manani, ErnestK. Collison, and Sisai
Mpuchane*...
...
2581Prevalence
of
Potentially Pathogenic Bacillus cereus
in
Food Commodities
in
The
Netherlands
L. M.Wijnands,-
J.
B. Dufrenne, F. M. Rombouts, P. H.in't
Veld, and F. M. vanLeusden
...2587
Salmonella and Shigella
in
Freshly
Squeezed OrangeJuice, Fresh Oranges, and Wiping Cloths Collected
from
Public
Marketsand Street Booths
in
Guadalajara,Mexico: lncidence
andComparison
of
Analytical
Routes
A. Castillo, A. Villarruel-L6pez, V. Navarro-Hidalgo,N.
E.Martinez-Gonzllez,and M.
R.Torres-Vitela-...2595
U.S.
Food
Safetyand lnspection
ServiceTesting
tor
Salmonella
in
Selected
Raw Meatand Poultry Products
in the United States,
1998through
2003:An Establishment-Level
Analysis
Denise R. Eblen, Kristina E.Barlow.- and Alecia Larew
Naugle
....
2600U.S.
Food
Safetyand lnspection
ServiceTesting
tor
Salmonella
in
Selected
Raw Meatand Poultry Products
in
the
United
States, 1998through
2003:Analysis
of
Set
Results
Alecia Larew Naugle,- Kristina E. Bariow,Denise R. Eblen, Vanessa Teier, and Robert
Umholtz
...
2607Distribution
of
Virulent and
PandemicStrains
ot
Vibria parahaemolyticus
in
Three Molluscan Shellfish
Species lMeretrix meretrix,
Pernaviridis,
andAnadara granosal
andTheir Association
with
Foodborne
Disease
in
Southern
Thailand
Varaporn Vuddhakul, Supatinee Soboon, Wattanee Sunghiran, SukhonKaewpiboon, Ashrafuzzaman Chowdhury, Masanori Ishibashi, Yoshitsugu Nakaguchi, and Mitsuaki
Nishibuchi"...2615
Lethality
of
Chlorine, Chlorine Dioxide,
and aCommercial Produce Sanitizer
lo
Bacillus cereus
andPseudomonas
in
a Liquid
Detergent,on
Stainless
Steel,and
in
Biofilm
AudreyC.
Kreske, Jee-Hoon Ryu,Charles
A.
Pettigrew, and Larry Fl.Beuchat.
...
2621A
Comparative Study of Two Food
ModelSystems
To Testthe Survival
ot
Campytobacterjejuni at -18'C
Tina Birk, Hanne Rosenquist, Lone Brsndsted, Hanne lngmer, Anette Bysted, and Bjarke
BakChristensen*...
2635Growth and Stress
ResistanceVariation
in
Culture Broth among Listeria manocytogenes Strains
of
VariousSerotypes and
Origins
Alexandra Lianou, Jarret D. Stopforlh, Yohan Yoon, Martin Wiedmann, andQuantifying Nonthermal lnactivation
of
Listeria monocytogenes
in
European
FermentedSausages
UsingBacteriocinogenic Lactic
Acid
Bacteria or Their Bacteriocins: A
CaseStudy
for
Risk
Assessment
EleftheriosH. Drosinos,* Marios Mataragas, Slavica Veskovic-Moracanin, Judit Gasparik-Reichardt, Mirza HadZiosmanovi6, and
lntragastric lnoculation
with
aCocktail
of
Listeriamonocytogenes Strains
DoesNot Potentiate
the
Severity
of lnfection in
A"/J Mice Comparedto
lnoculation
with the lndividual
Strains Comprising the
Cocktail
NancyG.
Faith, Luke D. Peterson, John B. Luchansky, and CharlesJ.
Czuprynski-...
....2664
Effects
of
Microbial
lnhibitors
and Modified Atmosphere Packaging
on
Growth
of
Listeriamonocytogenes
and
Salmanella
entericaTyphimurium
andon
Quality
Attributes of
lniected Pork Chops and Sliced
CuredHam
Andrew R. Michaelsen, JosephG.
Sebranek,* and JamesS.
Dickson
...2671
Survival, Elongation, and
Elevated Toleranceof
Salmonella enterica Serovar Enteritidis
at
Reduced WaterActivity
Jasper Kieboom, Harshi D. Kusumaningrum, Marcel H. Tempelaars, WilmaC.
Hazeleger, Tjakko Abee,. Asierisk
indicates author ior corresponcience.
The publishers do not ularrant, either expressl)/ or by implication, the factiJal accuracy of the afticles or descriptions herein. nor dc they so warrant any views or
2-566
Heat Tolerance
of
Sa/rnonellaenterica Serovars Agona, Enteritidis,
andTyphimurium
in
PeanutButter
DinaShachar and Sima Yaron. 2687
Spread
of
a GreenFluorescent Protein-Tagged
Pseudomonasputida in
a Water Pipefollowing
Airborne
Contamination
Severine Gagnidre, Fi'6deric Auvray, and BrigitteCarpentier*
....
2692Systematic Environmental Evaluations
Toldentity
Food Safety
Differencesbelween Outbreak
andNonoutbreak
Restaurants
CraigW.
Hedberg," S. Jay Smith, Elizabeth Kirklanci, Vincent Radke,Tim
F. Jones,Carol A. Selnran, and the EHS-Net Working Group
...
....
2697Efficacy
of
Candida sake CPA-1Formulation
for Controlling
Penicillium expansum
Decayon
Pome Fruit from
Different
MediterraneanRegions
R. Torres," N. Teixid6,l.
Vinas, M. Mari, L. Casalini, lvl. Giraud, andJ.
Usall...2703
Optimization of
aFluorescence Polarization lmmunoassay
for
RapidQuantification
of
Deoxynivalenol
inDurum
Wheat-Based
Products
Vincenzo Lippolis, Michelangelo Pascale, and AngeloVisconti"
...
2712A
DetailedStudy of
Therm:l
Decomposition, Amalgamation/Atomic Absorption Spectrophotometry
Methodotogy
for
the Ouantitative Analysis
of
Mercury
in
Fish and
Hair
StevenJ.
M. Butala,- Larry P. Scanlan,and Sanwat N. Chaudhuri 2720
Occurrence
of
Proteolytic
Activity
and N-Acyl-Homoserine Lactone Signals in
the
Spoilage
of
Aerobically
Chill-Stored Proteinaceous
RawFoods
M. Liu,J.
M. Gray, and tr4. W. Griffiths- 2729Research Notes
Survey
on the Hygienic Status
of
Plastic Doors
of
a Pig
Abattoir
Robin GroBpietsch,* Kathrin Einschuiz,Dorothea Jaeger, and Reinhard
Fries...
....2738
Occurrence and
Densityof
Vibrioparahaemolyficus
in
Live Edible Crustaceans
from
Markets
in
ChinaYutaka Yano,- Masaki Kaneniwa, Masataka Satomi, Hiroshi Oikawa, and Shun-Sheng
Chen
...2742
Morphological and Physiological Responses
of
Campylobacterjejuni to
Stress
Pussadee Tangwatcharin,Sugan;ra Chanthachum, Prapaporn Khopaibool, and Mansel W.
Grifiiths-
...2747
Assessment
of
Environmental Factors
on
Listeria monocytagenes Scott
A,inlA
GeneExpression
by
RelativeQuantitative
Taqman Real-Time ReverseTranscriptase
PCR
Scott E. Hanna and Hua H.Wang-
....2754
lnactivation of the Crp/Fnr Family
of
Regulatory
Genesin
Listeria manocytogenes Strain
F2365Does
NotAlter
lts
Heat Resistanceat
60"C
Darrell O. Bayles" and GaylenA.
Uhlich
...2758
Surrogates for the Study
of
Norovirus Stability and lnactivation in
the
Environment:
A
Comparison
of
Murine
Norovirus and
FelineCalicivirus
Jennifer L. Cannon, Efstathia Papa{ragkou, GeunwooW.
Park, Jason Osborne, Lee-Ann Jaykus. and JanVinje-Comparison of the
Reveal Test,the
U.S.Food and Drug Administration Culture
Method,and Selective
Mediafor
Recoveryol
Salmonella Enteritidis
from
Commercial Egg Layer Flock
Environments
Lei Zhang, Zhinong Yan, and ElliotT.
Ryser"Evaluation of
anAlkaline Phosphatase-Labeled Oligonucleotide
Probefor
the Detection and Enumeration
of
the
Thermostable-RelatedHemolysin (trh)
Geneof
Vibrio
parahaemolytrcus
Jessica L. Nordstrom,- RachelRangdale, Michael C. L. Vickery, Andrea M. B. Phillips, Shelley L. Murray, Sariqa Wagley, and Angelo DePaola
...2770
Measurement
of
T-2and
HT-2Toxins
in
Eggs
by
High-Performance
Liquid
Chromatography with
Fluorescence
Detection
Chris M.Maragos.
...2773
Reviews
Fish
Mealin Animal
Feedand
HurnanExposure
to
Persistent Bioaccumulative and Toxic
Substances
Jos6lnactivation
of
Protozoan Parasites
in
Food, Water,and Environmental
Systems
MarilynC.
Erickson. and2761
J. Food Prot.. Vol. 69' No. I l
Scientific Editors
P Michael Davidson, Fh.D.. Departnrent of Food Sciencc and Technology. Universitv of
Tennessee,2-509 Rjver Drive, Knoxvilie, TN i7996-4539, USA; Phone 865.974.0098; Fax 865.91 1.'1 332 E-mail : pmdavidson @ utk.edu
Joseph Frank, Ph.D., Fcod Science Building. Roorn 2l l, Cedar Street, Universitl,
ofGeor-gia. Athens, GA 30(102-7610. USA: Phone 70(r.5.12.0994; Fax 706.542.1050; E-mail:
crrsjoe@ uga.edu
Elliot T. R1,ser, Ph.D.. Depanment of Food Science and Hunran Nutrition.334,4 C-M.
Trout, Michigan State University. East Lansing,
MI
48824- 1225, USA; Phone517.3-55.8474 ext 185t Fax 517.i5i.8963: E-irail: rvser@nrsu.edu.
Jol.rn N. Sofbs, Ph.D., Departnient of Aninral Science, Colorado State Univcrsity. Fort Collins. CO 80523-i 171. USAI Phone 970.491.7703; Fax 970.49 1.0278: E-mail: john.sofos @ colostate.edu
Journal l\Ianagement Committee Chairperson
Roger L. Cook, Ph.D., tr-erv Zealand Food Safetl- Authority. ,86 Jervois Quay. South Tower
PO. Box 2835, Wellington, Ncw Zealand: Phorre 64.4.4(;3.2523; Fax 61.4.463.2510:
E-mail: rogercook@nzfsa.govt.nz
Journal
Editorial
StaffDavid W Tharp, CAE, Executive Director
Lisa K. Hovel', CAE, N{anaging Editor
Tarnara P Ford. Administrative Editor Didi Loynachan. Adnrinistrative Assistant
Journal
Bditorial
Offi ceInternational Associalion for Food Protection. 6200
Ii4oines,
IA
5032?-2861, USA: Phone 515.276.33.1,1:foodprotection.org Executir.e Board
President, Frank Yiannas. M.PH-, Walt Disney \!'orld. I-ake Buena Vista. FI-President-Elect, Gary R. Acufl', Ph.D.. Teras A&l\,1 University, College Starion, TX
Vice President, J. Stan Bailey. Ph.D.. USDA-ARS-BEAR. Arhens, GA
Secretary, Vickie Lewandorvski, M.S.. Krall Foods. Glenview,
II-Past President, Jeffi-ey J\,1. Farbr.r, Ph.D.. l-lealrh Canada, Ottau,a. Onrario, Canada Afnliate Councii Chairperson. Maria Teresa Desho. Ph.D.. University of Sio Paulo, Slio
Paulo. Brazil
Executive Director. Dar,id \V. Tharp, CAIi. Inrerntrional Association lor Food Protecrion, Des lr{oir.res. IA
Jounrul of Food Pruttectirnt (lSSN,0362-023Xt is published rronrhly by the lnrernational Association for Food Prorection. 6200 Aurora Avenue. SuiLe 200W, Des Moines. lA 50322, 2864. USA. Each volunre consists oi'12 issues. Periodical postage paid ar Des N{oincs, Iou'a 50318, and additional entrv offrccs. Claims tirr missing issues must be submitted ro
the Association rvithin 30 days (US. Canada. and N,lexico). International claints rrust be
submitted within 60 days.
Postmaster: Send address changes to Journal of Food Prot€ctiott, International Association
for Food Protection, 6200 Aurora Avenue. Suite 200\!'. Des Moines, lA 50322-286,1, USA. Scope of the Journal: The Journal
rf
Food Prorectiou is intended for publication ofresearch and review anicles on al) aspects of food protection and safety. Ma.ior emphascs of JFP rre placed on studies dealing with (i) causes (microoreanisms, ciremicals. natural toxicants) and control of all lbrrns of foodborne illness; (ii) contamination (microorgan-isms. chemicals. insects. rodents) and its control in rarv fixld and in fbods during process-ing. distribution, preparation. and service to consumers: (iii) causes of tbod spoilage and
its control through processing (low or high tcnrperatures. preservatives, drying.
fermen-tation. irradiation. pressure, and other innovative rechnologies); (iv) food quality and
mi-crobiologrcal. chemical. and physical methods to assay food qualit_v; and (v) wastes from
tl.re food industry and means to use or treat the wastes.
S_ubmission of Manuscripls. All manuscripts must be submitted at http://fbodprotection. allentrack.net. [-etters to the Editor must bJ submitred to Didi I-oynachin, Administrative
Assistant, International Association for Footl Protection. 6200 Aurora Avenue. Suite 200w. Des Moines.
1A
50322-2864, USA. Instructionsfor
Authors are available arwww.foodprotection.org or from the Journal of FOotl prorection llditorial oflice. Jounrul of Food Prorection is available by institutional subscription for $345 US, $36-5
Canada.Mexico. and $395 Inrernarional. JFP Online subscriptiori rate is $600 per
'olume
1"ear. Call the Associarion lor individual membership information. Single copies are
avail-able for $39 US and S48 orher counrries.
All
ratcs include shippin! and handling. No cancellations accepted. I\fenrbers of the International Association ftii Foori Protection havethe option of receiving JFP and, JFP Online at a substantial discount. Membership
infor-Ination can be obtained fiom our \fo'eb site ar rvwrv.fbodprorection.org.
Copyright@ 2006 by the International Association for Food Protection. No pan of the
publication rnay be reproduced or transmitted in any tbrrl, or by any means. eiectronic or
mechanical, including photocopy, recording. or an1, 1p16.n.,"1ion stoiage and retrieval sys-l!m. txctpt in limited qu"lntities fbr the non-comnter.cial purposes of scientific or educa-uollal advancenrent. wirhour perntission in writing tl-oni the International Association for
Food Prorecrion Edirorial olfiee.
Request single reprints of afiicles publishe,C in the Journat tiom rhe corresponding author
at the address listed in the tbotnote of each article. Elcctronic reprints ire available at
wwrv.ingentaselect.conr. l\,[icrofiim o1' Jounu! o;f food Protectio, is available iiom Bel] and Howell.300 N. Zehh Road. Ann Arhor. I\4i,tSl06-1346. IISA. Ali righrs reserverl.
Aurora Avenue. Suitc 200W, Des Fax 515.27(;.u655; E mail: tford@
Bditorial
BoardS. N'1. Alz-amora, ARC (06)
\\i I'1. Andrews. MD (071
T. J. Banett, GA (07) S. E. Beattie, lA (07) N{. Berrang, CA (07)
E. D. Berry, NE (06)
R. R. Beumer NLD (08)
A. K. Bhunia, IN (0?)
P Bodnaruk. lr'lN (07)
V Bohaychuk, CAN (08)
L. Bohra, OH (06)
D. J. Bolton. lRIr (0O K. J. Boor NY (06)
R. E. Brackcu, MD (08)
lv'I. M. Brashears. TX (06)
R. 1.. Buchanan, MD (08) S. Buncic, UK (07)
S. L. Burnctt. MN (07)
J. A. Byrd, TX (08)
T. R. Callaway. TX (06)
B. Carpentier FRA (07)
A. Castilio. TX (08) .i. G. Cervcny. WI (06) J. Chen, CA (061
D. O. Clivcr. CA (0S)
R. Cook. r'Z (07)
K. Cooksey-. SC (06)
A. Cookson, NZL (07)
A. Darra, MD (07)
T Deak. HUN (07)
E. Decker. l\4A (08)
P Dclrquis. CAN (07)
A. Denrirci, PA (08)
P Dcsmarchelicr, AUS (08)
J. S. Dickson, IA (0E)
l-i Diez. it'IN (07) \'1. Drake. NC {07)
D. D'Souza. NC (06)
C. Dykes. AUS (0li) E. E Escanin. [4EX ()7)
J. I{. I--aLbcr. CAN (06)
P Feng, IvID (08;
C. Franz, GER (06)
P N{. Fratanico. PA (08) S. Garcia-AIvarado, MEX (0?) S, M. Gendel. tL (07)
I. Gcornaras. C0 (08)
c. o. Gilr, cAN (06)
D. A. Golden. TN (08)
L. Goodridge, WY (0ti) L. G. M. Goris. UK (08)
L. Gorski. CA (06)
L. Gram, DEN (07) 1l{. W Gdffiths, CAN (08)
\'--ll D. Hao. MD (08)
1\,1. A. I{anison, CA (06)
A. Hassan. SD (06)
C- Hedberg. MN (07.;
S. L. Helle. NE (06)
R. P Herwig. WA (06)
K. L. Hier. CA (07)
B. H. Himelbloom, AK (07)
R. Holle1,. CAN- (07)
D. G. Hoover, DE (06)
l. G. Hotchkiss. NY (06)
A. Hwang. PA (06) S. C. Inghani. WI (06)
K. Isshiki. jPN 107)
X. Jianr, SC (06)
it{. G. Johnsor. AR {06)
R. JorCano Salinas. SPA (07) V. J. Juneja. PA (08)
D. H. Kang. WA (07)
2561
(2006-2008)
S. Kathariou, NC (06)
S. E. Katz. NJ (07)
I{. Korkeala, FIN (06)
K. Koutsoumanis, GRE (08)
R. G. Labbe, MA (07)
K. A. Lampel, MD (08)
A. Leclercq, FRA (08) S. J. Lchotay, PA (08) J. T I-cJeuBc. OH (07)
R. E. Levin, MA (06)
Y Li, AR 08)
R. H. Linton, IN {06)
A. L6pez-Malo, I\'IEX (06)
B. Magnuson, MD (07)
R. Mandrell. CA (08)
D. L. Marshall, MS (07)
R. T Mamhall, MO (06) S. E. Martin, lL (07)
K. R. Manhcws, NY (06)
A. Mazzona, IL (08) S. A. Mccarthy, AL (08)
P McDermott, MD (08)
L. Mcllefont, AUS (07)
C. Michiels. BEL (08)
L. J. N{oberg. NY (0E)
C. L. Mw, CA (06)
R. Molins, FL (07)
D- lt4omcilovic, VA (08)
1l J. N'lontville, NJ rc7) R. N4urphy, AR (08) T. Nesbakken, NOR (06)
C. Nguyen-The, IiRA (0(,
B. Niemira, PA (08) J. S. Novak, IL (06)
G.-J. E. Nychas, CRE (08) J. Odumeru, CAN (08)
S. T Oinayc, NV (08)
Y R. Oncga. GA (06)
T P Oscar. MD (06)
F Pagono, CAN (08) S. A. Palumbo, IL (07)
M. Parish, MD (08)
N,f. \V. Peck, UK (08)
M. Peleg, MA (06) J. J. Pestka, MI (07) S, Pillai, TX (08)
M. E. Pouer GA (06)
P Prueu. OH (06)
K. J. Rajkowski, PA (07)
J. Rocourt, CAM (07) S. Salminen, FIN (08; J. Samelis. GRE (07)
C. Santene, IN (08)
D. W. Schaffner, NJ (07)
D. Sepulveda, MEX (08)
G. E. Skinncr: IL {06) D. M. Smith, ID (08)
C. H. Sommers, PA (06)
P J. Taormina, GA (07)
R. Thippareddi, NE (08)
E. C. D- Todd, MI (06)
Ir4. L. Tonorello, lL (07)
K. Venkitanamyanan, CT (08)
A. r,on Holy. S AIrR (08)
l. T. walis, DC (08)
H. W'ang, OH (07)
M. M. Wekcll, N,tD t06) R. C. Whitiog. MD (07) 14. Wiedmann, NY (06)
C. E. \\blf-Hall. ND (07)
H. Xue, NC (08) S. Zhao. MD (06)
2681
Jounral of FooC Protection. VoL.69, No. 11,2006, Pages 2681-2686
Copyright O, lnternationai Association for Food Protection
Survival, Elongation,
and
Elevated Tolerance
of
Salmanella
enterica Serovar
Enteritidis
at
Reduced Water
Activity
JASPER KIBBOOM,-i HARSHI D. KUSUMANINGRUM,+
MARCEI,
H. TEMPELAARS,WILMA
C. HAZBLEGER,TJAKKO
ABEE, aNoRIJKELT
R. BEUMER.*laboraton,of Food Micxil:irLlogr, Delrurinrcti of Agrotcchnologt and Food Stiences, Wageningen Universitl,, P.O. Box 8129, 6700 EV Wugenirtgcn. The Nctherlands
MS 05-578: Received l5 Novernber 2005/Accepted 27 May 2006
ABSTRACT
Growing microorganisms on dry surl'aces. which results in exposure to lorv water activity (a*), may change their nolmal nrorphology and physiological activity.
In
this study, the morphological chanses andcell viabiiity of
Salmonella etiericaserovar Enteritidis challenged to low ao. were analyzed. The results indicated that exposure to reduced a* induced filamentation
of
the cells. The amount of filamentous ceils at a*, 0.94 was up to 9OVc of the total number of cells. Surviving filamentous cells maintained their membrane integrily afier exposure to lor.v ao. for 21 days. Furthermore, cells prechallenged to low a*,, obtained with an ionic humectant. demonstrated higher resislance to sodium hypochlorite than control cells. These resistantcells are able to survive disinl'ection more efhciently and can therefore cause contamination
ol
foods coming in contact u,ith surfaccs. This points to the needibr
incrcased attention to cleaningof
surlaces in household environments and disinfectionprocedures in processing plants.
Salmonellu enteric{t sero\/ar E,nteritidis is the most int-portant cause
of
Salntonellu infections associatedu,ith
theconsumption
of
shelled eggsand
poultry
in
Europe (25Jand
the United
States (217. Cross-contantination directlyfrom raw poultry
to
ready-to-eatproducts
or
indirectly
throu-eh contanrinated surfaces
or
nichesin
the
householdkitchen
is
the predominant modeof
infectior.r(9).
In
gen-eral,
microorganisrns
often
experience environmental
stresses, such as nutrient starvation, osmotic shock, or tem-perature variation durin.q transmission. The risks associated
with
cross-contaminationof
Salntonelfu.rEnteritidis from
surfaces have been recognized because this microorganism
has
the
abiiity
to
survive
on
stainless steel surfacesfor
hours or days, depending on the
initiai
counts and the pr-es-enceof
food
residues(11,
l3).
On
surfaces, thecells
areexposed
directly to
theair
that rnay leadto
water removalfrom
the cells and adjustrnentof
cytoplasrnic soh,entcom-position. Osmotic
stressis
one consequenceof
the
initial
stage of the air-drying of cells on surfaces (4, 20, 22).
Little
is
known
aboutthe
responseof
SalmonellaEnteritidis
todry or drying
surfacesor
surfaceswith iow
wateractivity
'(a*)
(e.g., a,"
<
0.96) and the consequencesof celluiar
ad-* Author for correspondence.Present address: Laboratory of Food
Micro-biology, Department of Agrotcchnology and Food Sciences, Wageningen University. PO. Box 8129, 6700 EV Wageningen, Tl.re Netherlands- 'lel: +31 (317),18 32 13; Fax: + 31 (317),+8 49 78: E-maii:
rijkeit.beunter@ wur.nl.
i
Present address: TNO Deicnce. Security and Safety, Business UnitBi-ological and Chemical Protection. PO. Box 45. 2280 AA Rijswijk. The
Netherlands.
i
Present address: Laboratory of Food Microbioiogy, Department of FoodTechnology and Human Nutrition, Faculty of Agricultural Engineering and Technology. Bogor Agriculturai Universiry, PO. Box 220, Bogor 16002. Indonesia.
aptation
on
these surfacesto
subsequent stress exposure,such as
disinfection u,ith
sodiumhypochlorite.
Hypochlo-rite is
generally used aschemical
sanitizer becauseof
itsefficient
action against awide variety
of
microorqanisnts.Ii{attick et al. (/8J
have shownthat
filamentous ceilswere formed
hy
threewild-type
strainsof
SalmonellaEn-teritidis in
lorv a*, broth.Lowering of
a*
r,alues to 0.95 was achievedby
additionof
sucrose,glycerol,
and sodiumchlo-ride (NaCI).
With
thelatter
compoundin
the medium, theelongated
cells
appearedto
be longer and more numerous(18).
Furthermore,in
another study,it
has been shown thatair-dried Salntonella
ceils
become more toierant
to
heat (12).In
this
study, asa
rnodeilfor dry or drying
surfaces,we
investigated the morphological changes andcell
r'iabil-it.v
of
eight wild-type
strainsof
SalnronellaEnteritidis
of
different
phage typesafter
exposureto
reduced aw atdif-ferent
temperatures.Agar
surfacesrvith
reduced a*,,ob-tained
with
NaCl. were
used asa
model
to
study
the re-sponseof
bacteriato low a*.
The
cross-protection a-vainsthypochlorite solutions as a resuli
of
prechallenge to reduced aw was studiedin
suspension (esls.MATERTALS AND METHODS
Bacterial strains and grou'th conditions. Six human iso-lates
ol
Salmonel.la Enteritidis (1438 [phage type,Pl
13], 1.139IPT 41, 1444 IPT 25], 139i IPT 21], i389
IPT
il
and 1514 [PT281), a chicken isolate (1138, PT 28), and a chicken meat isolate
(1448, PT 4) were obtained from the National Institute of Public Health and the Environrnent, The Netherlands. The stock cultures
':'.'
rr,h.
2682 KIEI]OONI ET
AI-broth rvith 257o (vot/vol) glvcerol (Fluka-Chemica, Buchs, Swit, zerland) and glass beads (dianreter, 2 mm; Emerso, Landsmeer,
The Netherlands). Strains were precultured
by
transferring oneglass bead
to l0
ml of
BFtl broth. lbllorved by or,ernight incu-bation (20 ro 22 h) ar 37'C.Survival in environments u'ith reduced a*.. Survir,al at low
a*
was studied on tryptone soy agar (TSA; Oxoid, Basingstoke,UK) containing 4, 6. :rnd 87r NaCl (N{erck. Darmstadt. Germany) in petri dishes, resulting in ar" of0.97.0.95, and 0.94. respectively. The a,u was measured with a water activit)' rrreter (Novasina, Zu, rich, Switzerland) that rvas based on the relative vapor pressure.
A
totalof
50pl
of the appropriate dilutions of the preculture inpeptone saline solution (PSS: NaCl R.5 g/liter and neutraliz-ed
bac-teriological peptone [Oxoid]
I
g/]iter) was apnlied on agarsur'-faces rvith a spiral inocuiation apparatus (Eddy Jet; IUC,
Barce-lona, Spain). and the piates u,ere sealed r.r,ith parafilrn to avoid evaporation of water during incubation at 25 and 37"C. Thc col-ony counts were determined when the visible colonies were
ob-served: after 2, 4. or 6 days. depending on the as,
of
the rnediaand the incubation ternpelature. The recovery percentages were
calculated as quotients
of
the coiony couilts at reduced a,u andthose on TSA without additional huntectanls.
On sterilized
-elass surl'aces (2 by 7 cm2;,0.2 ml of overnight
crrlture of Salntotrcllo Enteriridis diiuted
in
fieshBHI
broth to aconcentratioll
of
approximetel_r,lUj
CFU/ml u,as appiied andspread r.vith a sterile loop, then incubated at 25 and 37"C in perri
dishes. Microscopic exan.iination of the glass surfaces with a Zeiss standard 20 light microscope rvas perlormed 24.48, and 72 h after
incubation-Morphology changes. The nrorphttlog), changes
ol
the cells (i.e., cell eiongation) rverc' observcd b1, vierving the cells fr-om agar plates prepared on a rnicroscopic slide witha
X100 phase contrast objective of a Zeiss standard 20 light microscope- Imagesrecorded by a Son1, H1'per HAD. CCD-lrisiRGB color yideo cam-era.
A
Protocol computer system (Synoptics l-td-, Cambridge.UK) was used to -qenerate digitai photomicrographs. The percent-ages
of
elongated cells, calculated fl"om the total cells, rverede-termined by the direct microscopic count procedure with a
Br"irker-Tiirk countins chamber (Schreck. Hof'heirn. Germany,). Results are displal,ed as averages from trvo experintents rvith {rve observa-tions fbr each experiment. Because under optinral growth condi-tior-t Sd.ntonella Enteritidis cells rvere found to be bet*,eerr
)
to 3pn-r in length. ce)ls longer than t\\,o times the uraximal length (i.e.,
6 pm) were considered to be elongated cells.
Cell
viability.
The cells srown at a.., 0.95 anda*
0.94 andincubated for 6 days at 25"C were transf-erred rvith a sterile loop into an Eppendorf tube (Greiner Bio-One) containing 1 rnl of PSS. The
cell
suspensions were centrifuged (BHG-Hermle, Goshein.r, Gerrnany) at 4,000X
g
at4'C fbr 5
min, and the pellers were resuspended in PSS to a concentration ranging fi'orn 1.0to
1.5 X 10s CFU/ml. The culturabilitvof
these cells was deterrnined onTSA
and
mannitol lysinecrystal violet brilliant
green agar(MLCB; Oxoid), a selective mediunl fbr isolation of Salntonell.a.
The membrane inte-erity of the cells grown at reduced aw was detern.rined
with
LIVE/DEAD Bacl-ight Bacterial Viability Kits (l\4oiecular Probes. Inc., Eueene, Oreg.) according to the protocols provided rvith the kit. Thiskit
uses ruixtures of 3.34 mM SYTO 9 green fluorescent nucleic acid stain and 20 rnM of the redfluo-rescent nucleic acid stain propidiurn iodide (PI). With a
l:1
rnix-ture of the SYTO 9 and PI stains. bacteria u,ith ilttact cell mem-branes stain fluorescent green, whereas bacteriawith
damageci membranes stain fluorescent red. The cell suspensions wererni-J. Food Prot., Vol. 69. No. 11
croscopicaliv analyzed with an Axioskop epifluorescence micro_ scope equipped rvith
a
50W
rnercury arc lamp,a
fluoresceinisothiocyanate filter set (excitation wavelength, 450 to 490 nrx; emission r-vavelerigth, >515 nm), a X100 1.3-numerical apefture Plan-Neofluar objective lens, and
a
camera (Carl Zeiss, Ober-kochen, Germany). Photomicrographs were madewith
simulta-neous light and epifluorescence microscope, a low light intensity,a n-ragnificarion
of
x1,000, and an exposure timeof
15 to 45 son Kodak 400 ASA color fihn. In these photon.ricrographs. both the SYTO 9 and Pl-labeled celis could be counted. Depending on
the number of cells, 10 to 20 microscopic fields were counted. Flow c1'tometry. The cells -qrown at reduced a*, labeled with
Baclight bacterial viability kits, were analyzed u,ith a
FACSCaI-ibur flow
c-ytometer (Becton Dickinson Immunocytometry Sys-tem. San Jose. Calif.) equipped with al5
mW blue light at 488nm. air-cooled argon ion laser. The side scatter signal was used as a tr-igger signal. The green fluorescence fronr SYTO 9-stained
cells *,as detected through a 515- to 545-nm band-pass filter (FLl channel), and the red ffuorescence of the PI signal was collected
in the FL3 channel (>670 nm long-pass filter). FACSFIow
solu-tion (Becton Dickinson) was used as the sheath fluid. The cells
\\'ere measured at a
low
flow rate con-espondingto
150 to 500cells per second, and 10,000 events were collected
for
furtheriuraiysis. A conrbination of forward scatler (FSC) and side scatter (SSC) signals rvas used to discrinrinate bacteria ir-om the back-ground and to characterize the morphology of the cells.
All
signals s'ere collected by logarithmic amplifications. Data fiom the flou,cytometer u,ere analyzed by WinMDI (Joseph Tbtter; Salk Institute
for
Biological
Studies,La
Jol1a,Calil'.:
availableat
http:// l-acs.scripps.edu/softu'ar e.html).Cross-protection to sodium hypochlorite challenges. The
cell suspensioils \\rere prepared fiom the population on TSA u,ith
rcduced a.r. and incubated at ,5oC
tbr
6 days to a corlcentrat.ionof
,l.0to
1.5x
lOs CFU/m1, as described f<rrexamination of thecell
culturability. Sodium hypochlolite (Acros Organics, Morris Plains. N.J.) solutions were prcpared at chlorine concentrations of 4.2 mM (300 ppm) and 5.6 mM (400 ppm). The available chlorineconcentrations were confirmed by titration (1). The preparation of the solutions and the suspension tests was carried out following European Norrlr 1276 14). Bovine serur.n albumin (3 giliter from
Si-sma-Aldrich. Steinheim. Gerrr-rany) was used as an interfering substance to simulate dirty conditions. The reductions of the log
numbers were determined at 10, 30, and 60 min
of
exposure to hvpochlorite.Statistical analvses. Each experirnent was carried out at least tu'ice on dift'erent days, and no fewer than two replications were performed for each experirnent. Except flow cytometry data. data
analvses were performed on the statistical software package SPSS
tbr
Windows 95/98AJT/2000, release 10.I
(SPSS Inc.. Chicago'Il1.).
A
P valueof
<0.05 was considered to be statistically sig-nificant.RESULTS
Survival
in
reduced
au.environments.
In BHI
broth(a,.
0.999).
all
strainsformed visible
coionies2
days afterincubation
at 37'C
at
a*
0.97
and0.95,
whereas at 25'C, the colonies appeared after 4 and 6 days, iespectiveiy.Strik-ingli,.
at
a,.,-0.94no
separate colonies werie formed, but athin
layer
of
bacterialgrowth
appeared. The recoveryper-centages, calculated as quotients
of
the colonies at reduced,ii
2682 KIEI]OONI ET
AI-broth rvith 25Vc, (volJvol) glvcerol (Fluka-Chemrca, Buchs, Swit,
zerland) and glass beads (dianreter, 2 mm, Emerso, Landsmeer,
The Netherlands). Strains were precultured
by
transferring oneglass beaC
to l0
ml of
BFil broth. follo',vcd by overnight incu-bation (20 Lo 22 h) ar 37'C.Survival in environments lvith reduced a*,. Survival at low
a*
was studied on tryptone soy agar (TSA; Oxoid, Basingstoke,UK) containing 4, 6. and 87r NaCl (N{erck. Darmstadt. Germany) in petri dishes, resulting in ar" of 0.97. 0.95, and 0.94. respectively. The aru was measured with a water activit)' rrreter (Novasina, Zu, rich, Switzerland) that rvas based on the relative vapor pressure.
A
totalof
50pl
of the appropriate dilutions of the preculture inpeptone saline solution (PSS: NaCl R.5 g/liter and neutraliz-ed
bac-teriological peptone [Oxoid]
I
g/]iter) rvas apolied on agarsur-faces rvith a spirai inocuiation apparatus (Eddy Jet; IUC,
Barce-lona, Spain). and the piates u,ere sealed q,ith paralihn
to
avoid evaporation of water during incubation at 25 and 37"C. Thc col-ony counts were delermined when the visible colonies wereob-served: after 2, 4. or 6 days. depending on the as,
of
the rnediaand the incubation ternperature. The recovery percentages were
calculated as quotients
of
the coiony couilts at reduced a,u andthose on TSA without additional hurnectanrs.
On sterilized
-qlass surfaces (2 by 7 cm2;,0.2 ml of overnisht
culture
ol
Salntotrclla Enteritidis dilutedin
fieshBHI
broth to aconcentratioll
of
approximetel_r,lUj
CFU/ml u,ils appiied andspread r.vith a sterile loop, then incubated at 25 and 37"C in perri
dishes. Microscopic exan.iination of the glass surfaces with a Zeiss standald 20 light microscope \\/as perlormed 24.48, and 72 h after
incubaticln-Morphology changes. The nrorpholog), changes
ol
the cells (i.e.,cell
eiongation) rvere observcd b1, vier.ving the cells froni agar plates prepared on a rnicroscopic slide witha
X100 phase contrast objective of a Zeiss standard 20 light microscope. Imagesrecorded by a Son1, H1'per HAD, CCD-lrisiRGB color video cam-era.
A
Protocol computer system (Synopticsl-td.,
Cambridge.UK) was used to -qenerate digital photoniicrographs. The percent-ages
of
elongated cells, calculated fl"orn the total cells, rverede-termined by the direct microscopic count procedure witlr a
Br"irker-Tiirk countins chamber (Schreck. Hof'heirn. Germany,). Results are displayed as averages from trvo experintents rvith {rve observa-tions fbr each experinrent. Because under optinral growth condi-tior-t Sdnnnella Enteritidis cells rvere found to be bet*,een
)
to 3pn-r in length. ce)ls longcr than t\\,o times the rnaximal length (i.e.,
6 pm) were considered to be elongated cells.
Cell
viability.
The cells srown at a., 0.95 and ao, 0.94 andincubated for 6 days at 25"C were transl'emed rvith a sterile loop into an Eppendorf tube (Greiner Bio-One) containing 1 rnl of PSS. The
cell
suspensions were centrifuged (BHG-Hermle, Goshein.r, Gerrnany) at 4,000X
g
at4'C fbr 5
min, and the pellets were resuspended in PSS to a concentration ranging frorn 1.0 to 1.5 X 10s CFU/ml. The culturabilitvof
these cells was deterrnined onTSA
and
mannitol lysine cry'stalviolet brilliant
green agar(MLCB; Oxoid), a selective medium fbr isolation of Salnnnell.a.
The membrane inte-erity of the cells grown at reduced aw was
determined
with
LIVE/DEAD Bacl-ight Bacterial Viability Kits (l\4olecular Probes, Inc., Eueene, Oreg.) according to the protocols provided rvith the kit. Thiskit
uses ruixtures of 3.34 mM SYTO 9 green fluorescent nucleic acid stain and 20 mM of the redfluo-rescent nucleic acid stain propidiurn iodide (PI). With a
l:1
rnix-ture of the SYTO 9 and PI stains. bacteria u,ith intact cell mem-branes stain fluorescent green, whereas bacteriawith
dan-ragecimembranes stain fluorescent red. The cell suspensions were
tni-J. Food Prot., Vol. 69, No. 11
croscopicaliy analyzed with an Axioskop epifluorescence micro_ scope equipped rvith
a
50W
rnercury arc lamp,a
fluoresceinisothiocyanate filter set (excitation wavelength, 450 to 490 nrx; emission r-vavelerigth, >515 nm), a X100 I.3-numerical aperture Plan-Neofluar objective lens, and
a
camera (Carl Zeiss, Ober-kochen, Cerrnany). Photomicrographs were madewith
simulh-neous light and epifluorescence microscope, a low light intensity,
a magnification
of
x1,000, and an exposure timeof
15 to 45 son Kodak 400 ASA color fihn.
In
these photon.ricroeraphs, both the SYTO 9 and Pl-labeled celis could be counted. Depending onthe number of cells, 10 to 20 microscopic fields were counted. Flow c1'tometry. The cells -qrown at reduced a*, labeled with
Baclight bacteriai viability kits, were analyzed rvith a
FACSCaI-ibur flow
c-ytometer (Becton Dickinson Immunocytometry Sys-tem. San Jose. Calif.) equipped with al5
mW blue light at 488nm. air-cooled argon ion laser. The side scatter signal was used as a tr-igger signal. The green fluorescence fronr SYTO 9-stained
cells *,as detected through a 515- to 545-nm band-pass filter (FLl channel), and the red ffuorescence of the PI signal was collected
in the FL3 channel (>670 nm long-pass filter). FACSFIow
solu-tion (Becton Dickinson) was used as the sheath fluid. The cells
\\'ere measured at a
low
flow rate con'espondingto
150 to 500cells
pel
second, and 10,000 events were collectedfor
furtheranaiysis. A cornbination of forward scatler (FSC) and side scatter (SSC) signals rvas used to discriminate bacteria irom the back-ground and to characterize the morphology of the cells.
All
signals u'ere collected by logarithmic amplifications. Data fiom the flou,cytometer u,ere analyzed by WinMDI (Joseph Tbtter, Salk Institute
for
Biological
Studies,La
Jolla, Calil'.:
availableat
http:// l-acs. scripps.edu/softu'ar e. html ).Cross-protection to sodium hypochlorite challenges. The
cell suspensions \\rere prepared fiorn the population on TSA u,ith
rcduced a*. and incubated at 25'C
tbr
6 days to a concentrat.ionof
,l.0to
1.5x
lOs CFU/m1, as described tbrexamination of thecell
culturability. Sodium hypochlorite (Acros Organics, Morris Plains. N.J.) solutions were prepared at chlorine concentrations of 4.2 mM (300 ppm) and 5.6 mM (400 ppm). The available chlorineconcentrations were confirmed by titration (1). The preparation of the solutions and the suspension tests was carried out following European Norrlr 1276 14). Bovine serum albumin (3 giliter from
Si-sma-Aldrich. Steinheim. Germany) was used as an interfering substance to simulate dirty conditions. The reductions of the log
numbers were determined at 10, 30, and 60 min
of
exposure to hvpochlorite.Statistical analvses. Each experiment was carried out at least tu'ice on dift'erent days, and no fewer than two replications were performed for each experirnent. Except flow cytometry data. data
analvses were performed on the statistical software package SPSS
tbr
Windows 95/98AJT/2000, release 10.I
(SPSS Inc.. Chicago'Il1.).
A
P valueof
<0.05 was considered to be statistically sig-nificant.RESULTS
Survival
in
reduced
au,environments.
In BHI
broth(a,.
0.999).
all
strainsformed visible
colonies2
days afterincubation
at 37'C
at
a*
0.97
and0.95,
whereas at 25'C, the colonies appeared after 4 and 6 days, iespectively.Strik-ingli,.
at
a,..-0.94no
separate colonieswerc
formed, but athin
layer
of
bacterialgrowth
appeared. The recoveryper-centages, calculated as quotients
of
the colonies at reduceda\\ and those on
TSA
without additional
hurnectants, were iiJ. Food Prot.. \rol. 69. No. I I
TABLE
l.
Partetttoges o.f ektngated cells(>6
p,n1)et
reducecl a,,. afler6
days at 25oC, deterntirted bv direct n1.icro.\(ofiL'Lt)utt'tinq''
pcreenruLt' ur er(,nrJreJ cerr. at:
Strain a,, 0.95 a.., 0.94
PHYSIOI-OGICAI- BEHAVIOR OF SAL\4ONEI-LA ENTERITIDIS AT REDUCED a*, 2683
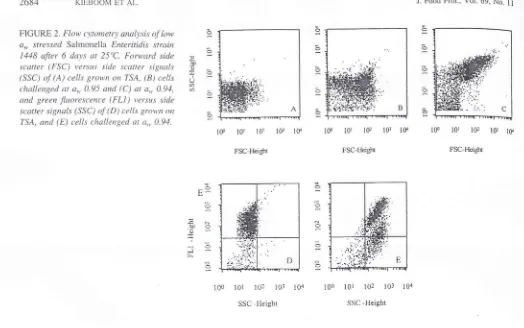
monella-contarninated wet surfaces after a
slow-drying
pro-cess.
Analysis
of
data obtainedby
flow
cytometry
indicated that cells challenged to aw 0.95 showedslightly
higher sig-nals on FSC and SSC(Fig.
28)
comparedwith
the controlcells grown
on TSA (Fig. 2A).
A
noticeable increaseof
FSC
and SSC
signalswas observed
in
cell
populations-srown at
a*
0.94,which
resultedin
a completeshift of
apopulation
on
both detector signals(Fig.
2C).
Thesefind-in.qs indicated
that particularly
at
a,,0.94 cells
with
largedimensions
were
observed,which
was confirmed
by
themicroscopy analysis.
Cell
viability.
Theculturability
onTSA
and N{LCBof
cells prechalienged at
a*
0.95 and 0.94 at 25"Cfor
6 daysare shorvn
in
Figure3.
h.ra control
experiment,we
dem-onstrated
equal colony
countson MLCB
andTSA,
indi-cating that
the viability
of
cells
was
not
affected
by
theselective agents present
in
MLCB.
Epifluorescence
microscopy
of
the
stressedcells
re-vealed
the
existenceof
four
subpopulations composedof
viable short and elongated celis aswell
as nonviable sl'rortand
elongatedcells (Fig. a).
The
viabilit;'
was
based on assessmentof
intact
or
damaged membraneof
individual celis. Deadcells
with
darnaged uembrane accumuiatedPl
and rvere stained fluorescent red.At
a,, 0.95 at 25oC, about 80 and 7jc/oof
the total nutnberof
the celis werestill
\'iableafter
6
and
2l
days,
respectively.Antong the
elongatedcells. the percentage
of
theviable
cells was approximately75%
after6
days and decreasedto
507cafter
2l
daysof
exposure
(Fig. a).
At a*
0.94,
about 50'loof
thecell
pop-ulation
kisttheir
viability
after 6 days. Exposure at a$, 0.94resulted
in
morerapid
lossof
viability
thanat higher
a,u.Fuithermore, u,hen
the
elongatedcells were
recoveredin
BHI
broth
and incubated at 37"C, themajority
of
the fila-mentssplit
up, and the separation was completewithin
ap-proxirnately
3
h,
as was
observedunder
the
microscope [image:8.595.42.571.577.826.2](data not shown).
Figure
2
shorvsdot plots
of
eventscollected
by
flow
cytometry
of
thecontrol cells
(D)
andcells
challenged at ao,0.94
(E),
subsequently stainedrvith
the
Baclight
kit.
FL1
is a [leasure for green fluorescence of SYTO 9-stainedcells. The control celis
demonstratedlow
signalson
SSCI l -)d
I 389 i 391
t444
I 448
1,138
I 439
I 51.1
iiti
t
4 (ll +')90+3
91
+2
93+2
82+6
87+3
90+6
29+6
24*5
:10
+
-5
38+6
l/ + <
34+4
aoi
35+3
., Data are expressed as a\/erage
-
SD percentages.in
a rangeof
70 to
95c/c at a,,. 0.97. and 30to
707oat
a,"0.95. The overali recovery at 25 or 37"C was not dependent on the ten'rperature
(P
-
0.45) but wasoniy
affectedby
thea* (P
:
0.01).Morphologl'
changes. Microscopy
revealedthat
alltested strains
of
SolnrcnellaEnteritidis
fbrn.red elongated ce1ls(longer than
6
prl)
at
a,*,0.95
ancl a,n,0.94.
At
a",0.97,
no
elongatedcelis wele found. Dilect
rnrct'oscopiccounting
indicatedthat
at
a,, 0.95, the
pel'centageof
the eiorrgatedcells
u'as betrveen24
and 40ch. andat
a,n 0.94between
82
and 90cr'cof
the total
cell
nurrbers (Table
l).
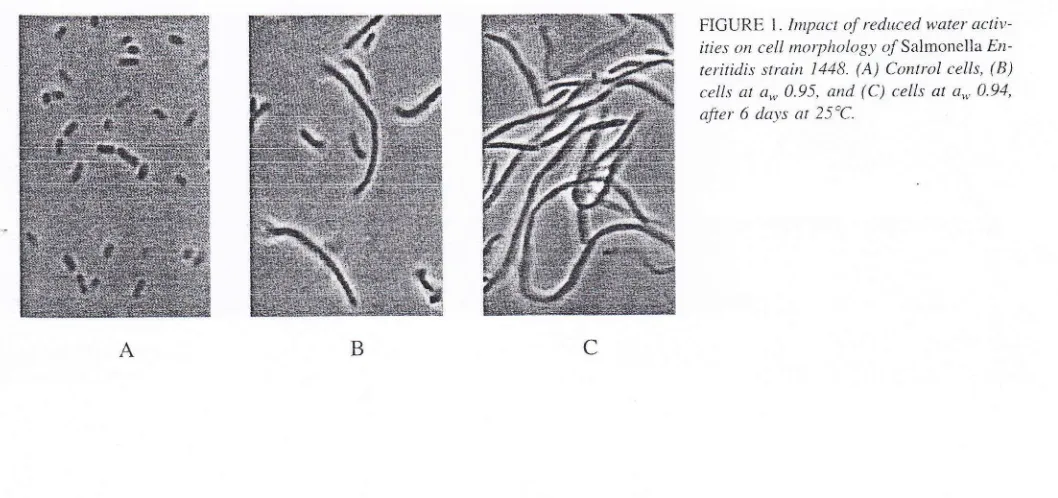
Particularll, at 25"C and a,, 0.9.1. elongated cells rvith a size
of
50pm
or more were found(Fig.
l).
Microscopicalanal-ysis
of
cellsin liquid
lou,a*
rnedium, obtainedwith
NaCl and glucose (datanot
shoivn).botli
sl.rowed the formationof
elongated cells.This
sug-tested that reduced a.., resultedin
fllamentation and that this was not dueto
surface effectsor
the presenceof
high NaCl
concentrations.Our
study also indicatedthat
when analiquot
of
bac-terial
suspensionin
freshBHI
broth
u,as appliedon
glass surfaces, elongated cells were nricroscopically observedaf-ter
slow
air-dry.'ingfor
24h at
37'C
andfbr 48 h at
25'C. The elongatedcells
were found. on these glass surfaces inlow
percentages (approxirnately 37cof
thecell
population), indicating that filamentationof
the cells may occur onSal-FICURE 1. Impact o.f reduced w-ater acti\t-ities on cell ntorpltology oTrSalmonella En
-teritidis srrain 1448. (A) Control cells, (B) cells at
a*
0.95, and (C) cells ata*
0.94,after 6 da-ys at 25'C.
2684 KIEBOO\,I ET AI-.
FIGURE 2. Flow c\tonietr\t arutlysi5 pf
1.*
a*
stressed Salmonelia Enteritidi.s strain 1448 after6
days at 25"C. Forward sidescaner (FSC) versus side scatter signals
(SSC) of (A) cells grown ort TSA, (81 cells
<:hallenged at
a,
0.95 and (C) at a., 0.94,and green fiuorescenr:c (FLl
)
rtersus si.descatter signals (SSC)
(f
(D) cells grou'nrn
7'SA, and (E) c'ells challenged at a,,. 0.94.
5
o
O
J. Food Prot., Vol.69, No.
ll
FSC-Height
E
(J q q_
a
c
1S tot 10r lG lff
FSC-Height
100 101 102
,103 104SSC -Height
.i ij, . -..a-\.i
-.".:,i:i
lffi&r'
(1#'i
:'j' E l0l
t0
IE IO]
Iff
FSC-Height
o
E-:
dO .6
:E
_O 9.
c
O
o
O
O
O
100
I0r 102 l0l
104SSC -Height
with high
signalson
FL1,
indicating that almosi
all
cellswere viable. Cells challenged at a\\, 0.94 revealed a
shift
inpopulation
with
hi-sher SSC signals, indicating lbrn.rationof
elongated cells as also sho\\,n belbrein
Figure 2C, and thisconsisted
of
two
populations(approximately
507cof
thetotal
countsfor
each population) eitherwith
high
andlow
signais
on
FLl
(Fig.
2E).
Moreover,the population with
the
low
FLI
signals also displayedhigh FL3
signals (datanot
shown), indicating that these Pl-stained cells arc dead. These resultsconfirm
the findin-es obtainedby
thefluores-cence microscopy analysis,
rvhich
indicatedthat after
ex-posure at 0.94for
6 days at25'C,
thernajority
of
the cellswere elongated, and approximately 50Vo
of
thecells
were viahle.1448 1144 1439 1438 '1391 1389 1 138 1514 Strarns
ETSA,pre-chalienged atAw0.95 EMLCB,pre-challenged atAw0.95 nTSA,pr€-challe.Eed atAw0.94 IMLCB,pre-challenged atAw0.94
FIGURE 3. Cu.lturabilit_t on TSA and MLCB agar o.f Salmonella Enteritidis strains prccha.llenged
for
6 datsat
25"Cat
a,, 0.95 and a-,4.94(n
:
2).Cross-protection
to
sodium
hypochlorite.
The toler-anceof
cellsgrown
onTSA (control)
and prechalienged atreduced a\\,. obtained
with
NaCl,
to
hypochlorite
solutionsat 25"C
is
shorvnin
Figure 5. Experiments showed thai theNaCl
dou,nshockby
serialdilutions
in
PSSdid
not influ-ence the results (data not shown).At
a chlorineconcenira-tion
of
300 ppm (4.2mM),
a 3-log reduction was observedfor
controi ceils aiter 60
min of
exposure.Cells
that wereshott-
short-non
bng-vi&le
long-nonviable viaue
viable FIGURE4.
VicLbilitl'ol
Salmoneila Ertreritidis strain 1418 clal'lenged at a,,.0.95 oi ZS"C, determinetl b1, direct microscopl' coultl'
ing after stttining v,itlt Live/Deatl BacLight viability kir (n-=
tt'
-Cells longertlnn
6 p,nt v'ere considered as elongatedcells'
';:*..-;lg:.++ffi
.o o\
a
q)E
C 0)
c.)
o-E
:)
TL
O Z
o
o
J
.4#
,ffi
-'':j ,-l
D
g6
daYsPHYSIOLOGICAL BEHAViOR OF SALMONELLA ENTERITIDIS AT REDUCED 4", 2685
6
.5
E
io
I
*-o c
'c
?,
0
J. Food Prot., Vol. 69, tr-o l l
0 20 .{o 6,0
80Timc (rrinute s)
[image:10.595.33.279.37.169.2]020106080
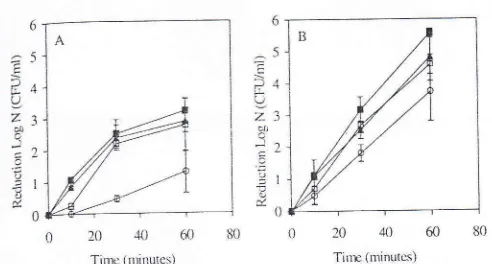
Tirrc (minutus)FIGURE 5. ToLerance trt sodium b'pot'hlorite ry' Salmonella
Erz-rcritidis
srrain
1448 t1t(A)
300 ppm (4'2 mM) and (B) 400 ppnt(5.6 ntM)
(n
:
i).a,
Control.eil't;
' cells prechallenged cLt an'0.97;
C,
cells prechallengedtil
a,' 0 95;E
cells prechal'lenged at a,.. 0.91.prechallenged to a'" 0.95 were the most tolerant to the treat-ment,
foilowed by
the cells challenged to aw 0.94 and 0'97'At
400 ppm(5.6
rrM),
a higher-killing
efficiency
wastbund
with
the same trend.After
60rrin
of
exposure, nlore than a5-log
reduction was observedfbr
thecontrol
cells, whereascells
prechallengedto
a,"0.95 were
reduced byapproximatell'
3
log
units. Thecells that
wereprechallen-ged
to
a,. 0.97
and0.94
decreasedby
approxinlately 4'5log
units.Ol'erall,
the cells challengedto
reduceda*
dem-onstrated better toleranceto
hypochlorite
than the controlce11s.
DTSCUSSION
In
this
study,the
responsesof
eight rvild-ti'pe
strainsof Solnonella Enteritidis
to recluced aw environments wereanalyzed. Challenge
at
a*,0.95
and0.94
resultedin
cell elongationof all
tested SalntonellaEnteritidis
strains'Mattick
et
a|.(19)
demonstratedthat
filamentoussal-monellas contained
regularly
spaced nucleoids,which
in-dicated that cells were probablyblocked
in
septation'lt
is conceivablethat the cells
elongation resulted fron.r inacti-vationor
inhibition
of
cell division
proteins,which
in
turn blocks the septation during thecell division
(16,23)' Ithas
been reported
that FtsZ
is by far
the
best-conserved celldivision protein:
it
is
also presentin
most
speciesof
bac-teria(16,2J).
Next
to
FtsZ,
when ar.ry oneof
thecell
di-vision
proteins
in
Escfterichia
col.i,including
FtsA,
FtsI,FtsK, FtsL.
FtsN. FtsQ, FtsW, andZipA, is
nonfunctionalor
absent, cells -growrvithout
dividing'
which
leadsto
theformation
of
filaments (-3, 6).Because
not
all
cells
rvere elongated, as \\tasparticu-larly
observed at a,n, 0.95. the responseof
SaLntonella E'n'teriti<lis strains
to
a,., t'eduction occursat the
level
of
theindividual cell.
Booth(2)
suggested that responseto
stressIargely takes place at the single
cell
level
and can lead toheterogeneity
in
a bacterial population.This
heterogeneity is a recognized propertyof
bacterial populations and allowsadaptation
to
adivelsitl' of
niches'Any
protein thatis
re-quired
for
survi"'al is capableof
contributing to thehetero-geneity (21.
Cellular
parameters. essentialto
survival
under stressconditions are the
integrity
of
theceil
membrane,nlainte-6
E
?-4 U
Z3
xr:)
a
=)
! I0
nance
of
the
folding
of
proteins, andthe
integrity
of
theDNA
(2, 5). Discrirnination
betweenintact
and permeablecells
by
fluorescent stains has been usedin
many
studieson
viability
of
bacteria (6)-Examining
thecell
viability
bymeans
of
fluorescent techniqueshighlighted
theheteroge-neity
of
Salntonel.LaEnteritidis
populationsin
response tochallenge
to
lorv
a*.
becauseboth viable
and
nonviableshort and elongated cells were observed' The filamentation
of
the cells resultedin
higher signals on FSC and SSC byflorv cytometry.
FSClight is
laserlight
diffracted
aroundthe cells
andis
relatedto cell
surfacearea'
SSClight
isrellected and refracted laser
light;
is
relatedto
the internalcompiexity
or
granularityof
aceIl
(6)'
A
large populationof
cells(i.e., i0,000
events) was measuredby
flow
cytom-etry,
which otfered substantialinformation
on themorpho-logical heterogeneity
of
this particular bacterial population'albwing
sorting and
subsequent characterizationof
fila-mentous cells
in
luture experimeltts.Studies
have
demonstratedthe
fact
that
SaLmonellacells
adaptedto
certain
stressconditions show
cross-pro-tection
againstother
stresses(17, 21)'
In
this
study,
we investigated the elTectof
sodium hypochlorite on cellspre-challenged
to
low
a'uobtained
with an ionic
humectant'When
mixe<iwith
water,sodium hypochlorite
dissociatesand forms
hypochlorousacid (HOCI),
an active
form
otcl.rlorine. HOC1
is
aneffective
disinfectantpartly
becausemost microorganisms
do not
posses specific enzymesfor
detoxificationof HOCI' like
they dofor
other oxidants such as reactiveoxygen
species ( 14)- Tl'risstudy
indicated thatthe cells
prechallengedto lou'
a',, show better
toleranceagair.rst sodium hypochlorite than the control cells
with
ion-ic
humectant. Cross-protection to hypochlorite may becon-f'erred by the expression
of
the stress sigma factor rpoS andthe
subiequent synthesisof
stress-relatedproteins
in
thecells exposed
to
low a*
(8r.
Moreover, theaddition
of
theionic
humectantNaCl may
induce
the
accumulation
of
compatible solutes such as betaine that may confer
protec-tion
a-sainst the detrirnental eft-ectsof
sodium hypochloriteby
maintainingcellular protein conformation
and enzymeactivities and
supportingcell
membraneintegrity
(7'
10't 5).
The
survival of
Saltttonella E'nteritidis at reduced a*-asiow
as0.94-increases the
risk of
cross-contamination because thesetolerant
cells can
come
into
contact with
foodstuffs
placedon
these surfaces'In this
study,we
ob-served that the filanrentationof
thecells
resultedin
anin-crease
of
the optical
densityin
broth without
apparentin-crease
in colony-forrning
units (data not shown), indicating tl.rat filamentous cellsfbrm
single colonies on plates'How-evel
r.l'iren these elongatedceils
were l'ecovered underfa-vorable conditions,
the
filamentscould
split up
and fbl'm rlumerous singlecells' The
possible presenceof
elongatedcellsonsurfacesshouldbeconsideredapotentialinf.ectiorr
risk
because these filaments are viablefor
several days andcan
rapidly split up
under favorable conditions
in
food-stuffs,
resulting
in
a large numberof
viable cells'
Further-more, the existenceof
a population tolerant to hypochlorite after chailenge tolow
aw poses an importantrisk for
pubiic2686 KIIIBOOM
E'I-AI-plants or on household surfaces lnore etficiently. Therefore, increased attention should be paid
to
the cleaning anddis-infection
procedures usedon
surfacesin
theseenviron-ments.
ACKNOWLEDGMENTS
We thank Kaouther Ben-An-ror lor her valuable comments on flow cytometry assessments. This project was partly funded by the Health, Saf'ety and Sustainability program of The Netherlan<is Organization for
Health Research and Dcvelopment.
REFERENCES
1.
Bender, D. I-1, J, U Gould. J. D. Johnson, and A.ll
Paiin. 1985.Determination of inorganic nonnretallic cousiitueuts: chlorine
(resid-ual). /n A. E. Greenberg. R. R. Trussell. and L. S. Clesceri (ed.),
Standard methods fbr the exanrination of rvatel and wastewater. l6th
ed. American Public Health Associatjon, Washington, D.C.
2-
Booth, I. R 2002. Stress and tlrc single ccll: intrapopulation diversityis a mechanism to ensure survival upon exposure to stress. r?1. J. Food MicrobioL T8:19 30.
3.
Chen, J. C.. and .1. B ecku,ith. 200 I . FtsQ. FtsL and FtsI requ ire FtsK,but not Fts1.\, fbr co locaiization u,ith FtsZ during Eschtrithia coli cell division. Mol. Microl:iol. 42:395-413.
4-
Comit6 Europ6en de Normaiisation. European Committee lorStan-dardization (CEN). 1997- L,uropean Nornr 127(;. Chernical disinfec-tants and antiseptics quantitatiYe suspensjon test for tl)e evaluation
of bactericidal activity of chenlical disinfcctants and antiseptics used
in food. industrial. domestic. and institutional areas test nlcthod and requirentents (L,hase 2. itep 1). British Standartl lnstitutc,
I-on-don.
5.
Csonka. L. N. 1989. I']hlsiolo-tical and gcnetic responses of bacteriato osn.rotic stress. Microbictl. Rri'.53:121 147.
6.
Darvey. H. M.. and D. B. Kell. 1996. Flow cytometr'1,and ccll soningof helerogeneous microbial populations: the intportance of
single-cell analyscs. Microbiol. Rev. 60:641-649.
1.
Diamant. S.. D. Rosenthal. A. Azenr. N. Eliahu. A. P Ben-Zvi, and P GoloubinotT. 2003. Dicarboxi,lic amino acids and glycine-betaineregulate chaperone-mediated protei n-disag-uregation under stress. Mol. Microbiol. :19:.101 410.
8.
Dodd, C. E., and T. G. Aldsrvorth. 2002. The imponance of RpoSin the survivai of bacteria through food processing. Int. J- Food
Microbiol. 74: i 89 194.
9"
Dufienne. J.. \\r. Ritmeester E. Deifgou-van Asch. E van Leusden.and R. de Jonge. 2001. Quantification of the contarnination
ofchick-en and chickofchick-en products in The Netherlands with Sultnonella and CanptltLbacre r. J. Food P rot. 64:538--5.{ l.
10.
Gutierrez. C-. T. Abee, andI.
R. Booth. 199-5. Physiology of the osmotic stress response in microorganisms. lilt. J. Food Microbiol. 28:233-214.J. Food prot., Vol.
69, No,.ii
Humphrey, T. J.. E. Slater, K. Ir{cAlpire, R. J. Rowbury, "nd R";;
Gilbert. 1995. Salntortella-Enteritidis phage type-4 isolates moretoL- 1
eranr of hear. acid, or hydloge",p::":19: also survive tong., oo
il1]
faces. Appl. Environ. Microbiol. 61:3161-3164. t1.
12
13
11
l5
16.
11
21.
22.
l8
t9
20
Kirby, R. N,I.. and R. Davies. I990. Survival of dehydrated
Salmonella Typhirnurium LT2 at high+emperatures. ./. Appl.
iol. 68:241 746.
Backr
lr..f
Kusumaningrurn, H. D., G. Ribotdi,
W
C. Hazeleger, and R R_ , .,.:jBeumer. 2003. Survival of foodborne pathogens on stainless
surfd
,r,and cross-contaminarion to fbods. lnt. J. Food Microbiol.25:22i ,:...: j 10.
Leyer. C. J., and E. A. Johnson. 1997. Acid adaptation sensidzrr
;
Salnnnellatl'phimuriumtohypoChlorouSacid-Appl.Environ.L|i"
crobiol.6f:461-461-
r,Lloyrt, A. W., J. A. Baker, C. Smith, C. J. Olliff, and K. J.
Rur i'
1992. A cornparison of glycine, sarcosine, N,N-dimethylglycine, glv-cinebetajne and N-modified betaines as liposome cryoprotecrant;
J-Pharm. Pharnacot.
44'.50'/-51l.
'","\4argolin. W. 2000. Themes and variations in prokaryotic cell
di\i.
. t: sion. FEMS Microbiol. Rer,.2,1:531 548.Mattick, K. L., E Jorgensen, J. D. Legan, M. B. Cole, J. porter, H.
M. Lappin-Scott. and T. J. Humphrey. 2000. Survival and filamrn.
tation of -Snlnronrl/a Enteritidis PT4 and Salnrcnella enterica serotu
Typhimuriurn DT104 at low water acrtyity. Appl. Environ. Micro-bi,,1.6r, 1211 l)11).
Mattick. K. L., E Jorgensen, J. D. Lcgan. H. M. Lappin-Scorr. and
T
J- Hurnphrey. 2000. Habituati<tn of Salntonella spp. at reduccd tt,,water activity and its et'tect on heat tolerance. AppL. Environ.
Mi-crobiol.66:4921
4925.
.::Mattick. K. L., 1-. E. Phillips, Ii Jorgensen, H. N4. Lappin-Scon. and
T. J. I{urnphrey. 2003. Fiiament formation by Sahzonella spp.
in-
'oculated into liquid tbod matrices at rcfiigeration temperatures, afld srou,th patterns when u,arnred. J. Food Prot.66:215-219. O'Byrne. C. P.. and I. R. Booth. 2002. Osmoregulation and its im-po(ance to food-borne nricroorganisn.is. Int. J. l-ood Microbiol.'71: 10-l I Io.
Olsen. S. J., L. C. l\4acKiron, J. S. Goulding, N. H. Bean, and
L
Slutsker. 2002. Surveillance for tbodborne disease outbreaks-Unit-ed States. 1993-1997. MMWR surveillance summaries 49 (SS0l).
Available
at:
http://u'ww.cdc.gov/nrmwr/preview/mmwrhtml/ ss490lal.htm. Accessed 25 July 2006.Potts,M.1994.Desiccaticlnto1eranCeofprokaryotes.MicrobioL
Rer,. 58:755-805.
Rothfield, L., S. Justice, and J. Garcia-Lara.1999. Bacterial cell di-vision. Arrnu- Rev. Genel. 33:423-448.
Saby, S.. P Leroy. and J. C. Block. 1999. E,scherichiacoli resistancc to chlorine antl glutathione synthesis in response to oxygenation
and
lstarvatioil. Appl. Envirort. Microbiol.. 65:5600 5603.
Schmidt, K., and C. Tir-atlo (ed.). 200i. WHO surveillance prG
gramme for control of foodborne infections and intoxications in Eu-rope, 7th report, 1993-1998. Federal Institute for Health Protection
of Consumers and Veterinary Medicine, Berlin, Gernrany.
23.
z1
25
-t.t:&