JOURNAL OF BIOSCIENCE AND BIOENGINEElUNG 'Vo). 91. No.4, 396--402. 2001
Comparison of the Biological H
2S Removal Characteristics among
Four Inorganic Packing Materials
MITSUYO HIRAI, MANABU KAMAMOTO, MOHAMAD YANI. AND MAKOTO SHODA·
Chemical Resources lAboratory, Tokyo Institute o/Techn%gy. 4259 Nagatsutll-Cho. Midori-ku. Yokohama 226-8503. Japan
Received 14 September 20001 Accepted 31 January 2001
Fool' iDOfllUlif paddJJgmaterfais were evaiualed in tenns of their availability as pacld. materials of a packed tower deodorizatioll appuatu (bIoIiIter) lrom the 'riewpoiats of biologk:ai HJ8 remoYai dlanc:terisda ud
lOIRe ,lIysieal properties. AmOlll poJODII cenm1cs (A), caIduted cristobalHe (8), c:aIri .. led aad lor:aed obsidla (C), pu.wed ad ealdaaled soU (D), tile superiority of dIae
JNICIdnI
materials determlDed based 011 tile YlliIMS of DOobioiogic:al re.oyai per aoit weigllt 01' wait yolume of paddDI uu.teriaJ, complete relDoYlliatptICity of BJ8 per .llIt wddtt of packing materild per 0,. or wnlt "olame of paddDg material per
oy
u4 pl1!SSllJ't! tlrop of tile ptIded bed was III (he order of A :::::C> D:::::B, nicllis correlated willi! tile maximum waterCOD teD" porosity, and meaD pore diameter.
[Key wonk: biofilter, bydrogen sulfide, removal capacity. inorganic packing materials1 The applications of biological deodorizing methods
have been increasing (1-4) because of their cost effective-ness and simple maintenance (2, S) compared to chemi-cal and physichemi-cal methods. Biologichemi-cal deodorizations are divided into two types of system. gas-solid and gas-liC!-uid systems (6). Among the gas..solid systems. a packed tower deodorization system is efficient mainly because this can be constructed in a small construction area and can function sufficiently in urban a:eas. To reduce the scale of the apparatus, the selection of parldnc: m.:..terials is an important factor anJ. manj 、ゥヲヲセ・ョエ@ tyPeS of pack-ing materials suited for ュゥ」イッエZセ。ャ@ growth have been ac-tively researched. Some requirements for a good packing material are as follows: (i) high water-holding capacity, (ii) high porosity and large specific surface area, (iii) less compacting nature, (iv) low-pressure drop over a wiue range of water content, (v) small change in form in long periods of use, (vi) lightness, (vii) low cost, (viii) appro-priate adsorbing ability for malodorous gases and (ix) large buffering capacity for acidic end products. Re-quirements (ill). (iv). (v), (vi) and (vii) are mainly related to the construction and maintenance of the biological deodorization apparatus, and (i) and (il) are related to its biological activities. The acidity or basicity of gases may be one of the factors for selecting packing materi-als. As organic packing materials, soil, compost and peat were sbown as good packing materials (7-14) that meet requirements (i), (ii), (vii), (viii) and (ix). Inorganic packing materials, such as perlite (15). porous ceramics (16), activated carbon fiber (17,18) and porous lava (19), are used, because they meet requirements (ill), (iv) and (v). Because comparative study of different packing materials has been rarely conducte<t under the saDIe con-dition (20). the evaluation of many packing materials is difficult. In this study, biologi::al H2S removal charac-teristics of four inorganic packing materials were evaluat-ed under the same experimentaJ cOllditions of packing volume, Dow conditions and inoculation source. Addi-tionally, the H2S removal characteristics were discussed in terms of pllysicocbemica1 properties and microbi:ll
• Corresponding author. e-mail: [email protected] phone: +81-{a)4S-924-5274 fax: +81-(0)4.5-924-5276
396
distribution on the packing materials.
MATERIALS AND METHODS
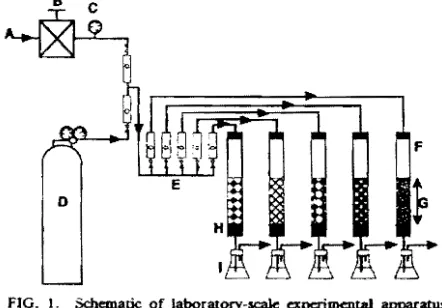
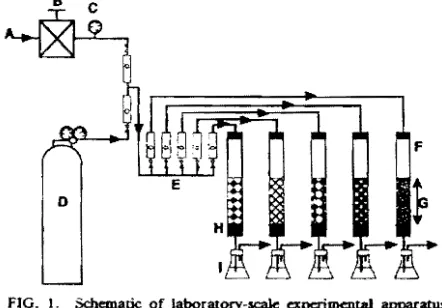
Flow system A gas flow system is snown in Fig. 1. Biofilter columns are made of glass and have a 50 mm inner diameter and 500 mm height. hセ@ gas from a gas cylinder was diluted with air from e compressor, then supplied to the biofilter downward after its flow was regulated with a flowmeter to the appropriate value.
Pacida& materials The chemical components of the inorganic packing materials used are shown in Table 1. True density (g·cm-l ). porosity (%). bulk density (g. cm-1), mean pore diameter (pm), pore distribution, pH, and maximum water content (%) were measured as fol-lows.
True density (PJ: distilled -.vater was placed into a lOO-ml Erlenmeyer flask. A mark was made at the water level inside and the system was weighed
b'1
gJ. Fifty ml of distilled water and xg of packing material dried at 100°C overnight were placed in the marked flask, then stirred with a magnetic stirrer and suctioned with anaspi-FIG. L Schematic of laboratory-scale apc:rimentaI apparatus. A., Air; B, pressure regulator; C, pressure gauge; D. gas cylinder Hhセ@
+Nl ); E, flow meter; F. glass column HsoセクsooュュhN@ packed
[image:1.599.318.539.507.661.2]VQL. 91, 2001 COMPARISON OF INORGANIC PACKING MATERIALS IN TERMS OF H2S REMOVAL 397 TABLE I. Chemical COInponcnts of the packing materials
used (wt.X) Packing material
A: P _ ceramics as.9 6.3 0.1 0.1 1.4 0.3 0.4 1.6 B: Calcinated aistoba1ile 80.3 9.8 3.1 0.4 0.2 2.9 0.3 I.S C: CakiDaled - ' 78.2 13.1 0.7 0.1 0.6 0.1 2.1 4.S
rormed obsidian
D; 0canWated ud SS.7 12.0 13.6 2.0 1.9 2.0 0.1 3.3
caIcinaIcd soil
rator for I h. DistiUed water was added at the marked level and the system was. weighed Hケセ@ g), and P. was calculated as follows.
P.=X/{X-(Y2-Yl)} (1)
Porosity (e): each packing material and distilled water were placed in a 100-00 volumetric cylinder, and their total volume (VI 00) was measured. Then, the volume of the packing material (Va) was calculated using the fol-lowing equation.
Va= V1-(Y2-YI)-50 (2)
Here, [weight of added waterl=[volume of added water]= [pore volume of the packing material].
Porosity was determined by the following e4uation. t=(yz -YI)/[(YCYI)+ Va] x 100 (3) As packing materials C and 0 Boated on the water sur-face, their true density and porosity were measured by the following method. Fifty 00 of distilled water and the pa-::kinc; material (XI g) were placed into a lOO-ml of Ellenmeyer ilask and sactioncd using an aspirator. The weighed packing material (x2 g) taken out from the flask and 20 mt of distilled water were placed into a graduated cylinder, and their total volume (VI ml) was measured. Apparent porosity was obtained using the following equ-ation.
Apparent volume= V1 - 20
True density and porosity were calculated from the apparent volume.
p.=xl/{(apparent volume)-(x,-x21} (4)
t= {(XI-x21/(apparent volume)} x 100 (5) Bulk density (PJ,): bulk density was calculated as fol-lows.
(6) Mean pore diameter (pm) a:ld pore distribution (pm)
TABLE 2. Packing conditions Packing Packed height
material (em)
A 18
B 18
C 18
D 18
Packed weight (g-dry)
Experiment on nonbiologica1
ュョッカ。ャッヲhセ@
86.0
233
39.1 139
Expcrimenton
biological
ュョッカ。ャッヲhセ@
33.1 194
38.6 188
were measured using the mercury pressure porosimeter method (porosimeter Type 220, Carlo Erba Strumen-tazione, Co. Ltd., Italy). The pH, maximum water con-tent and water recon-tentivity were measured by the same methods descn"bed jn a previous paper (20).
Noabiologieal bセ@ remoyal 011 paddag material A dry packing material (YI g) was soaked in water over-night, then packed in a glass column at 18 em height under the same conditions as those in the flow experiment shown in Fig.
t.
Packing material C with low density was floated on the water surface and suctioned using artaspirator to remove air inside of it. Then, it was placed back to atmospheric pressure to allow water absorption into the micropore. After no water was dripping from the bottom of the column with maximum water content, iOOppm hセ@ at about 60% relative humidity of atmos-phere was made to flow in the downstream direction at O.1l1min (SV=1l9h-I ). The load per unit volume of packing material to each column was set at the same value of 408 g-S . m -3 packing material· d -I. Loads per unit weight of packing material are different, because of their different paddng densities. Detailed packing condi-tions are shown in Table 2.
Biological hセ@ I'eQIOyai To seed the
microorgan-isms, each packing material was soaked in the sludge taken from a reservoir tank for UF film separation of a nondilution and high-load night soil treatment plant. Packing matericili inoculated with sludge were packed in the column at 18 em height and H2,S was supplied at 22°C. The load of the gas on each column increased gradually by changing the inlet concentration and/or space velocity. and the pH of each column was neutral-ized by NaHCOJ or HCJ (see legend in Fig. 4).
Microbial count Cell numbers were measured at the start and at the end of the exoeriment. About 10 g (wet weight) of each packing material was sampled and homogenized in 90 ml of sterilized water at 10,000 rpm for 10miD (Homogenizer EX-3, Nihon Sciki Ltd., Tokyo). Serially diluted suspension was streaked onto the follOwing solid media: Dutrient agar containing yeast
TABLE 3. Media used for Illicrobial count in hセ@ removal (g./-l)
Meat. el\:tract 3 Glucose J.O K2HPO. I.5.S KH,PO. 8 ·KH,PO. 2
Polypepton 15 "2HPO• I HaHzPO. 0.85 HILa 0.1 K,HPO. 2
Yeast extract 3 MgSO.·1HP O.S NH..Cl 2 CaCh·2H2O O.S NH.CI 0.4
Na1HPO.·12Hp 2 Kd O.S MgCh·6H::O 0.07 f・soNᄋWhセ@ 0.3 mァcィᄋVhセ@ 0.2
Had 3 FeSO.·7Hp 0.01
(NH..hSO.
0.1 Ha:sp,.3Hp 0.01 Fe50 •• 7H:rO 0.01HaNO, 2.5 DMSO" 1 NazS.O,·SH2O 8
Trace metal $01. (m1)" 0.2
Apr 15 Agar 15 Apr 15 Gdlansum S Apr IS
pH 7 pH 7 pH 7 pH 4 pH 7
• OMSO, dゥュ・エィIセ@ sultoxide.
セ@ Trace metal soiutiQp (g.I-I). (e1hyienedinitrilo)tetraacetic acid dUodium salt 50.
znSO.,
7HP 22. Caa2 5, MnCh·4H:rO S. PeSO.· 7Hz<> 5, [image:2.599.54.420.15.682.2] [image:2.599.310.543.71.158.2]398
.
HIRAI ET At.60
r - - - ,
50
セ@
セ@ 40
I:
II)
...
セ@ 30
u
"-iii 20
..
3: 10
o
o
48.5) I (2.33 - 0.17) '" - 8.57
""cr'
I
2 3
Time Cd)
4 5
FlO. 2. Tune course of water oootent unda-flow in packing material A.
extract (NY A) for heterotrophic bacteria, Czapex-Dox agar (COA) for fungi, dimethyl sulfoxide agar (DMSOA) for Hypomicrobium sp., thiosulfate agar (TSA) for less acidophilic sulfur-oxidizing bacteria and modified Waksman gellan gum (MWG) for acidophilic sulfur-oxidizir:g bacteria. The cell numbers were expres-sed in colony forming units (cfu). Detailed components of these media are summ:1r:iud in Table 3.
MISS measUreDleat of sludge used Sludge was filtered by suction using a cel1ulose illtrate fitter paper (0..2 pm pore size, 47 mm? Advantec Tayo. Tokyo). Filtered sludge was Jried using a filter paper at 100°C overnight and weighed. MLSS was determined as the mean value of three replicate.
H;tS gas The H:tS concentration in cylinders with Nl as a dilution gas was approximately 20,300 ppm (fa1cachiho Gas Ltd., Machida. Tokyo). hセ@ gas from the gas cylinder was diluted with air to appropriate con-centrations.
Gas alUllysis The hセ@ concentrat;on
was
measured using a gas chromatograph (Shimadzu GC-4BM) equipped with a flame photometric detector and a Teflon column (i.d., 3 mm; length, 6 m) packed witha
poJy-phenyl ether (S rings) on 60-80 mesh 10% Shimal.ite TPA. The column temperature, detector temperature and injection temperature were 70, 230 and 130°C, respec-tively, and N2 was used as a carrier gas.RESUL'IS AND DISCUSSION
Physical and cbem.ic:al propel1ies of packing materials The physical and chemical properties of each packing material are summarized in Table 4. Packing materials A and C have bigher porosity and maximum water con-tent, and larger mean pore diameter than packing materi-als Band C. Figure 2 shows tbe cbange in water content
J. B1osel, BIOENG., 125
E
Q.
.g 100
U!I
...
:x:
75
セ@
0
c 0
SO
セ@
III
...
...
I:II)
25 u I:
0 0
00 3 6 9 12 IS
Time (h)
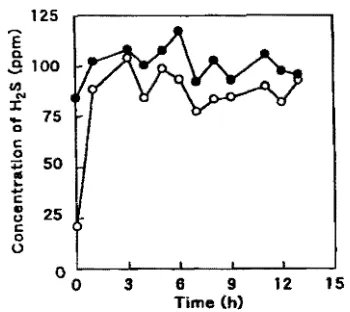
FIG. 3. Nonbiologkal mnoval of hセ@ 1D1dcr-80w in wetted packins material A. Symbols: _, inlet ccnemtl"afioa; o. outlet
c0n-centration,
from tbe saturated value at a space velocity of 119 b-I in
packing material A. From tbe decrease in water content on the first day as shown in Fig. 2, 40 mI of water manually supplied to the column daily was sufficient for maintaining the water content of packing materials at 70-80% of maximum water content. Packing materials B, C and D showed the same tendency as that observed
in packing material A (data not shown). Water retentiv-ity was calculated from the linear relationship between moisture content and time except for the initial drastic change and was expressed as cbange in moisture content (%) per day. The value for packing material C was the
smallest among all the packing materials used (fable 4). Noablological H;zS remoV1d Figure 3 shows the nonbiological H:tS removal in packing material A. The other packing materials used showed a pattern similar to that of packing material A. The nonbiologica1 removal capacity is the amount of net adsorbed gas on packing materials and amount of absorbed gas into free water on packing materials. Nonbiologica1 removal capacities per unit weight and volume of packing material shown in Table S were calculated from the difference between the
inlet and outlet concentrations. Packing material C has the largest capacity per unit weight, followed by packing material A.
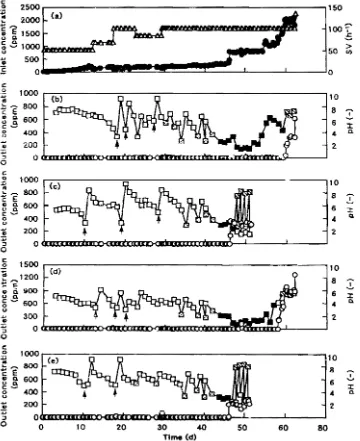
Biological H,5 removal MLSS of sludge used for inoculativn was セV@ g .[-1, and cell numbers in the sludge were 1.9 x lOS, 1.2 x lOS, 2.9x 107, 3.9x 107 and 1.3 X 107 (cfu·mI-1) as detected on NYA, TSA. mセwgN@ DMSOA and CDA media. respectively. Packing materials were inoculated into the sludge, and then H:zS was supplied to each packing material in a biofilter. Figure 4 shows the time course
of
hセ@ removal patternsin
each column. The inlet concentrations and/or space velocity (SV) grad-ually increased under the same volumetric load of hセ@TABLE 4. PbysicaJ and chemical properties of the packing materials used
Packing TJ"\1cdensity Porosity Bulk density Mean pore Maximum water Water
material (g.cm-3) (%') (J·an-3) Pore dia.lbution diameter pH coutel1t retentivity
(pm)
<">
(%'·d- ')A 2.31 79.6 0.47 I pill-leu than 100 pm 32.S 6.4 62.8 -8.57
B l.S9 42.6 0.91 mostly in 0.01 pm. 0.019 8.2 35.S -4.39
C 0,]] 89.0 0.12 0.1 flnHess than 100 pm 2.32 6.3 89.0 -3.26
[image:3.599.96.275.58.219.2] [image:3.599.337.512.65.221.2] [image:3.599.64.546.616.682.2]Vat. 91.2001 COMPARlSON OF INORGANIC PACKING MATERIALS IN TERMS OF H,s REMOVAL 399 TABLE 5. Amounts of H,s removed nonbiolo&ically by packing materials under p$ flow conditions, and complete H,s removal
capacity ami pressure drop in biofilter
Packing
material
A
B C
D
0.23 0.10 0.36 0.13
51 32.6 3SOO 6.1
64 2.1 1200 21.7
39 30.9 3400 U.S
50 3.0 1600 31.1
• Measured at the end of H,s removal.
c
2500
0
:p
<a) !III
2000
...
1
セ@
1500g
-91000 u....
•
500E
---
..
セ@II:
0 1000
:p
CIS
...
-::; E
800•
g:600 u c ...0 400
"
.., 200 41 :;:;
:::I 0
0
II:
0 1000
セ@
...
8001: 0) E
Q. 600
u Q.
11: .... 0 400
u
....
0) 200:;:; 0
:::I
0
セ@
1500セセMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMMML@
:;
... 1200
セ@ t::
III ii 900
U Q
セ@ .... 600
U
300
ッセュ。ュョセセセoomュョセセュZセセセ@
1000
(e)
セ@
800cャャZエZエエjセ@
600 400
•
•
200 0
0 10 20 30 40 50 60 80
TIme (d)
150
100
f'
r.
....
50>
CI)0
\0
8
'"'
t....
6 :I:
..
Q.2
10 B ..!., -. 6 :z: 4 Q. 2
[image:4.599.114.474.214.655.2]10 B ... 1
...
6
:c
..
Q2.
10 8
...
I
6
'"'
:c
..
Q.2.
..
40Q HIRAI ET AL. ""'
,
70 "!'iii 60 1:
..
...
...
50 E III"
..
c:: 40 :i 0 u.J 4111
SO
Q. >.
..
20"
I..
III 10.lO:
V,
I 0
..!!
0 10 20 30 40 50 60 70Time Cd)
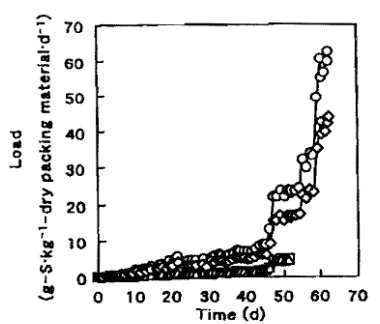
FIG. S. Time course of H;$ load. Symbob:
<>.
packing mat.eriaJ.A; A. pad.ing JD&!erial B; o. packing maurial Ci o. padins material D.
as shown in Fig. 4a. Loads of unit weight packing materials were different in each packing material as shown in Fig. 5 because the bulk density shown in Table 4 is <iifferent. Because rapid pH decrease of tbe drain water due to accumulated H:zSO .. oxidized from H:zS was observed when excellent removal was achieved. each column was washed with 100 mI of wate.-. Additional supply cf water and n。hcセ@ solutions to maintain pH at more than 2, which is shown in Fig. 4, was carried out based on the pH of the drain water. Although the pH decrease was observed at the time
0:
supplying 4 mIof 1% NaHCO, and 40ml of 0.1% NaHCO" bi('log:::al HzS removal continued even at high H;;S Joali. After several washings of the accumu:..ted sulfate in each
column. the load was increased to obtain the maximum removal capacity in an acclimated biofiJter, although H2S gas was detected in the outlet. During the final
period of extremely high H:zS load, a pH increase due to excessive NaHCO, supply was observed and 6% HO was added to adjust the pH. A rapid pH increase in the period indicates that no biological HzS removal ッ」セ@
curred. A plot of hセs@ load versus removal capacity in packing material A is shown in Fig. 6. The diagonal line shows the 100% removal capacity relative to the load. Deviated point from the line gave tbe complete removal capacity which is summarized in Table S. Nonbiological removals were temporary phenomena and all of their values were equivalent to biological removaJ values of less than 1 h. Therefore, they were neglected in calculat-ing the biological removal capacity. Evaluations of ー。」ャ」セ@
ing materials based on H:zS removal per unit weight of packing material and unit volume of packing material per day are in the order of A;;;:;C>Di$;B and A>C>D> B. As a whole, packing materials A and C were better than packing materials B and D in terms of their H:zS removal per unit weight. and packing material A was the
f
60"
50I. BIQSCI. BIOENG.,
... - - - - l i ! I
セ@
0o
10 20 30 40 50 60 Load(g-S-kg-l-dry paekin8 material'd-1)
FIO. 6. Plot of H"s.S load veuus removal capacity in packing materialA.
best among all in the packing materials used in HzS removal per unit volume, and subsequently pack.ing materials C
was
good. Pressure drops llt the end of HzS removal are shown in Table 5. Packing material C showed the ウュ。ャャセ@ value. The maximum water content is more closely related to the removal 」。セ」ゥエケ@ than water retentivity in Table 4. Therefore. frequent supp!y of water to meintain wate.- content close to the maximum value would improve the removal efficiency of packing material C.Microbial rount Results of microbial counts at the start and end of the flow experiments are summarized in Table (;. !ncreal:: in the cell number appeared on TSA, MWG DMSO and CDA. indicating growth of tィゥッ「。セ@
eillus sp. Hyphomicrobium sp. and fungi on biofilters.
In our previous reports, isolated microorganisms such
as Thiobaeiflus sp. セ、@ Hyphomicrobium sp. in a peat
biofilter acclimated by ウオャヲオイセエ。ゥョゥョァ@ odorous com-pounds were ascertained
as
the dominant species (8. 12, 21. 22). Therefore, similar species would also serve for H1S removal in the packing materials used. Participation of fungi in hセ@ removal was also confirmed (23)_ No decrease in microbial numbers counted on NY A demon-strates the contribution of heterotrophs in H2S removal which was shown in a previous paper (24).Ev"'ation of H:tS removal characteristics of foor packing materials The H1S removal characteristics of
the packing materials used shown in Table 4 are summa-rized as follows. Packing materials A and B have hlgh true densities. Packing materials C and A have high porosities. 68htness and high maximum water contents and are light. Packing material B has high pH. The mean pore diameters of A and C are 32.5 pm and 2.32 pm. respectively, and those of Band D are very small. Comparison of the hセ@ removal characteristics of ー。」ォセ@
ing materials A, B. C and D. in terms of biological
com-TABLE 6. Microorganisms acdimatcd oe pecting materials under hセ@ flow (cfu.
r
I セォゥョァ@ material)Pad:in& NYA TSA CDA MWG DMSOA
material Start Bod セ@ End Start Bod セAuGエ@ End Start End
A 1., X 1011 S.7X II)" RNVクiセ@ ,.,x lOll 1.6x 10'· '.Ox 10'1 2.1 x 10' 2.2>< 10" 1.9>< 10'· 1.4X 10"
B SAx 10'. 4.7 x lOll I.Sx
loa
2.7 x lOll 2.& x 10" 2.4 x 10" 3.2x 10' 2.0 x 10'1 2.6 x 10"' 105 x 10"C 1.2 x 10" '.4 x ]011 SNFxiセ@ 4.2 x 10" 1.4 x 101• 1.6 X 10" 6.7 x 10' 3.11 x 10" 1.1 x 10'. 2.2 x 10"
[image:5.599.326.531.64.239.2] [image:5.599.84.274.68.230.2]VOL. 91. 2001
•
COMPARISON OF INORGANIC PACKING MATERIALS IN TERMS OF H;i REMOVAL 401Packinc material A Packing material B
Packlnc materia' C Packing material 0
MonbioIocIcaI removal per unit weicht
100
Pre ... ure drop o
NonbloloeicaJ removal per unit
100
volume100
\'00
Complete removal "aPtW:1ty Complete removal capaelty per ul'llt volume per ""it weicht
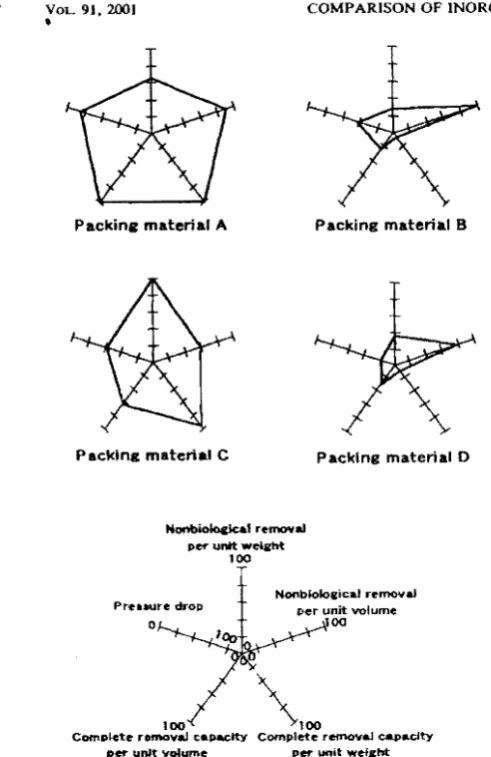
FIG. 7. Overall appraisal as a biological deodorization packing material from the results of H;i removal eJqJeriment on packing materials A, B. C and D. Axis is graduated in rdative ratio to the maximal value of all pa(:king materials.
plete removal per unit weight and unit volume, nonbio-logical removal per unit weight and volume and pressure drop, is shown in Fig. 7 as a radar graph. Each axis is graduated in. relative ratios to the maximal value of all packing materials, except for pressure drop which is
marked in opposite direction. The price of each packing material is in the order of A»D==B»C (detailed data not shown). Overall appraisal of the packing materials used from the results described above is A==C> D==B in excellence order. In the removal of acidic H2S gas,
pack-ing materials which have higher pH values are expected to be 1:uperior than tbose with acidic pH values, 「セオウ・@
of their physical and chemical adsorbiilg ability, However, in this experiment the pH of the packing materials did not correlate to their removal capacity.
The efficient and complete H:§ removal capacity
ofpack-ing materials A and C is correlated セッ@ their physical and chemical properties such as high porosity and mean pore diameter (Tab!e 4). Although there are no differences in cell numbers among the bioiilter packing materials at the end of experiment. the differences in physical properties such as porosity. mean pore diameter and maximum
water content contribute to the differences in mass trans-fer capacity of HzS. resulting in a diftrans-ferent complete hセ@
removal capacity of each packing material. In selecting inorganic packing materials for use in biofilters, these physical parameters are important factors for determin-ing the reaction rate between the microorganisms and H1S.
REFERENCES
]. SItMa,. M.l Metbocis for &be biological treatment of exhaust gases, p. 3]-46. lit Martin, A. M. (ed.), Biological degradation of wastes. EIse¥ier Science, London (1991).
2. OtiaIpaf. S. P. P.: Exhaust gas purification, p. 42$-452. In
Rehm, H. J. and Reed. O. (ed.), Biotechnol.. vol. 8. VCH. Weinheim (1986).
3. Glllap, D. L: BIOX hydrogen sulfide abatemc.ut process-appli-eation analysis, Trans. Geothenn. Resour. COUncl., 28, 11-17
(1996).
4. NacI, G.: Controlling H;) emission. Cbem.
Ene.,
104, 125-128 (1997).5. Elridge, A. F .... 4 BUtz, l.: Cost benefits of biological odour
control. Ind. Waste Maugt., 18-20 (1992).
6.
1CJuaa&a-.
T.: Fundamentals of biological deodorization, p.1-44. In Fukuyama, J. (ed.), Base and application of biological
deodorization. Odot Research and Engineering of Japan. Tokyo (1994). (10 Japanese)
7.
Zeisi&,
H.D., Hoimr, A., IUl4 K.rdtDlft-, S.: Anvendung von biologischen Filtem zur Reduzienmg von gauchs-intensiven Emissionen. Heft 2. Schriftreihe der l..andtechnik Weihenste-phan (Hrsg), fイ・ゥウゥョセ@ (1980).8. Fnrusawa, N., Togasld, I., Hirai, M., Sboda, M., and Kubota,
H.: Removal of hydrogen sulfide by a bio6lter with fibrous
peat. J. Ferment. Techno!., 61, 589-594 (1984).
9. Teruawll, M., iiinU, M., ud KBbota. H.: Soil deodorization systems. RioCycie., July 28-32 (1986).
10. ClIo, K. S., HInd, M., aad Shoda, M.l Degradation
characteris-tics of hydrogen sulfide, methanetiol, dimethyl sulfide and dimethyl disulfide by Thiobacillus thioparu5 DW44 isolated from peat biofilter. 1. Fennent. Bioeng., 71, 384-389 (1991). 1 J. ZhllDg, L., Hirai, M., .lId Sfloda, M.: Removal chara(:(eristics
of dimethyl sulfide, methanethiol and hydrogen sulfide by
Hyphomicrobium sp., 155 isolated from peat bi06lter. 1.
Fer-ment. Bioeng.. 12, SYRセSYV@ (1991).
12. 010, K. S., HInd, M., ud Sflada, M.: Enhanced removal efficiency of malodorous gases in a pilot-scale peat biofilter inoculated with 77.iobuci//us thiojXlt1l6 DW44. 1. Fennent. Bioeng .• 73, 46--50 (1992).
13. Dqorao-o..., l. R., Kow .. , S., aM Le 00irK, P.: Microbiologica1 Oxida1ivil .;;;.f !:.:1'::'vr;cu セキャGゥオオ」@ in it Oionner.
Can. 1. Microbial., 43, 264-271 (1997).
14. Va. Laagealtol'e, H., WByas, E., u.<I Sdiamp, N.: Elimination of hydrogen sulpbide from odorous air by a wood bark biofilt.:r. Water Res., 10, 1471-1416 (1986).
15. WIlDI, A. B., BnulioD, R.M.. R., a.d Lau, A. K.: Elfects of periods of starvation and fluctuating hydrogen sulfi<:!:: concen-tration on biofilter dynamics and performance. J. Hazard. Mater., 60, 287-303 (1998).
16. Sbinabe, 11:., Oketaal, S •• Odd, T., IlDd Matsam ... M.:
Chara(:(eristics of hydrogen sulfide removal by Thiobacil/us
thiooxidans KS I isolated from a carrier-packed biologic.al
deo-dorization mtem. J. Ferment. Bioeng., 18, 592-598 (1995). 17. Lee, S. K. ad Sltoda, M.: Biological deodorization using
acti-vated carbon fabric as a carrier of microorganisms. J. F.;:r-ment. Bioc:ng., A, 437-442 (1989).
18. Park, S. J.t BIrsi, M., ad Sttoda, M.: Treatment of exhaust
gases from a night soil treatmmt plant by a combined deodori-zation system of activated carbon fabric reactor and peat biofilter inoculated with Thiobacillus thlopar1lS DW44. J.
Fer-ment.
Bioeng •• 7', 42l-426 (]993). [image:6.599.46.292.50.429.2]No-/ ..
402 HIRAI ET AL.
•
bacillus thioporus. J. Biosci. BioeJl8., 91, 25-31 (2000).
1J). Kia, N. J., Hirai, M., ud SIwda. M.: Comparison of organic and inocgank c:anienI in nmt)'IJai of bydros;m IUlIide in bio-filter. Environ. TeclmoI.,
I'.
1233-1241 (1998).21. Wll4lla, A., SIIoda. M., Itabo ... H., ltohJatlU, T., ltatayama-F., Y., ... XtuaIIIIi, H.: セ@
or
H;iS oxidU:iD& bac-teria inhabitina II, peal biofilter. J. Fa:mcot. Tcclmol.., セN@ 161-167 (1986).22. ClIo, It. S., BinI, M •• ud SIIo4a, N.: Removal cl!araaerlstics
J. BlOSel. BIOENO.,
of hydrogen sulphide and methanethiol by Thiobacillus sp. isolated from peat biofiJler in biological deodorization. J. Fer-mcut. BioeD,., 71, 44--49 (1991).
23. ClIo, It. S., 1IIni, M., . . . " ' a . M.: [)epadatioa
or
hydro-,eo lulfide by XllIttltomonas 'P. strain DY44 isoJated from peat. Appl. Eoviroa. Microbiol.,5I, 1183-1189 (1992). 24. BinIl. Mo. "-toll, H.,
.JiM&.
L, 1ttoII,. ... I . . . SIIocIa,JOURNAL OF BIOSCIENCE AND BIOENGINEElUNG 'Vo). 91. No.4, 396--402. 2001
Comparison of the Biological H
2S Removal Characteristics among
Four Inorganic Packing Materials
MITSUYO HIRAI, MANABU KAMAMOTO, MOHAMAD YANI. AND MAKOTO SHODA·
Chemical Resources lAboratory, Tokyo Institute o/Techn%gy. 4259 Nagatsutll-Cho. Midori-ku. Yokohama 226-8503. Japan
Received 14 September 20001 Accepted 31 January 2001
Fool' iDOfllUlif paddJJgmaterfais were evaiualed in tenns of their availability as pacld. materials of a packed tower deodorizatioll appuatu (bIoIiIter) lrom the 'riewpoiats of biologk:ai HJ8 remoYai dlanc:terisda ud
lOIRe ,lIysieal properties. AmOlll poJODII cenm1cs (A), caIduted cristobalHe (8), c:aIri .. led aad lor:aed obsidla (C), pu.wed ad ealdaaled soU (D), tile superiority of dIae
JNICIdnI
materials determlDed based 011 tile YlliIMS of DOobioiogic:al re.oyai per aoit weigllt 01' wait yolume of paddDI uu.teriaJ, complete relDoYlliatptICity of BJ8 per .llIt wddtt of packing materild per 0,. or wnlt "olame of paddDg material per
oy
u4 pl1!SSllJ't! tlrop of tile ptIded bed was III (he order of A :::::C> D:::::B, nicllis correlated willi! tile maximum waterCOD teD" porosity, and meaD pore diameter.
[Key wonk: biofilter, bydrogen sulfide, removal capacity. inorganic packing materials1 The applications of biological deodorizing methods
have been increasing (1-4) because of their cost effective-ness and simple maintenance (2, S) compared to chemi-cal and physichemi-cal methods. Biologichemi-cal deodorizations are divided into two types of system. gas-solid and gas-liC!-uid systems (6). Among the gas..solid systems. a packed tower deodorization system is efficient mainly because this can be constructed in a small construction area and can function sufficiently in urban a:eas. To reduce the scale of the apparatus, the selection of parldnc: m.:..terials is an important factor anJ. manj 、ゥヲヲセ・ョエ@ tyPeS of pack-ing materials suited for ュゥ」イッエZセ。ャ@ growth have been ac-tively researched. Some requirements for a good packing material are as follows: (i) high water-holding capacity, (ii) high porosity and large specific surface area, (iii) less compacting nature, (iv) low-pressure drop over a wiue range of water content, (v) small change in form in long periods of use, (vi) lightness, (vii) low cost, (viii) appro-priate adsorbing ability for malodorous gases and (ix) large buffering capacity for acidic end products. Re-quirements (ill). (iv). (v), (vi) and (vii) are mainly related to the construction and maintenance of the biological deodorization apparatus, and (i) and (il) are related to its biological activities. The acidity or basicity of gases may be one of the factors for selecting packing materi-als. As organic packing materials, soil, compost and peat were sbown as good packing materials (7-14) that meet requirements (i), (ii), (vii), (viii) and (ix). Inorganic packing materials, such as perlite (15). porous ceramics (16), activated carbon fiber (17,18) and porous lava (19), are used, because they meet requirements (ill), (iv) and (v). Because comparative study of different packing materials has been rarely conducte<t under the saDIe con-dition (20). the evaluation of many packing materials is difficult. In this study, biologi::al H2S removal charac-teristics of four inorganic packing materials were evaluat-ed under the same experimentaJ cOllditions of packing volume, Dow conditions and inoculation source. Addi-tionally, the H2S removal characteristics were discussed in terms of pllysicocbemica1 properties and microbi:ll
• Corresponding author. e-mail: [email protected] phone: +81-{a)4S-924-5274 fax: +81-(0)4.5-924-5276
396
distribution on the packing materials.
MATERIALS AND METHODS
Flow system A gas flow system is snown in Fig. 1. Biofilter columns are made of glass and have a 50 mm inner diameter and 500 mm height. hセ@ gas from a gas cylinder was diluted with air from e compressor, then supplied to the biofilter downward after its flow was regulated with a flowmeter to the appropriate value.
Pacida& materials The chemical components of the inorganic packing materials used are shown in Table 1. True density (g·cm-l ). porosity (%). bulk density (g. cm-1), mean pore diameter (pm), pore distribution, pH, and maximum water content (%) were measured as fol-lows.
True density (PJ: distilled -.vater was placed into a lOO-ml Erlenmeyer flask. A mark was made at the water level inside and the system was weighed
b'1
gJ. Fifty ml of distilled water and xg of packing material dried at 100°C overnight were placed in the marked flask, then stirred with a magnetic stirrer and suctioned with anaspi-FIG. L Schematic of laboratory-scale apc:rimentaI apparatus. A., Air; B, pressure regulator; C, pressure gauge; D. gas cylinder Hhセ@
+Nl ); E, flow meter; F. glass column HsoセクsooュュhN@ packed
[image:8.599.318.539.507.661.2]