Atherosclerotic arterial remodeling and the localization of
macrophages and matrix metalloproteases 1, 2 and 9 in the human
coronary artery
Gerard Pasterkamp
a,b,* , Arjan H. Schoneveld
a,b, Dirk Jan Hijnen
a,b,
Dominique P.V. de Kleijn
a,b, Hans Teepen
c, A.C. van der Wal
d, Cornelius Borst
a,baDepartment of Cardiology,Room G02-523,Heart Lung Institute,Utrecht Uni6ersity Hospital,Heidelberglaan100, 3584CX Utrecht,The Netherlands
bThe Interuni6ersity Cardiology Institute of the Netherlands,Utrecht,The Netherlands cDepartment of Pathology,Elisabeth Hospital,Tilburg,The Netherlands dCardio
6ascular Pathology,Academic Medical Center,Amsterdam,The Netherlands
Received 11 January 1999; received in revised form 6 August 1999; accepted 8 September 1999
Abstract
Atherosclerotic luminal narrowing is determined by plaque mass and the mode of geometrical remodeling. Recently, we reported that the type of atherosclerotic remodeling is associated with the presence of histological markers for plaque vulnerability. Inflammation and matrix degrading proteases (MMPs) may play a role in both plaque vulnerability and in expansive arterial remodeling. The aim of the present study was to investigate the association between the remodeling mode and the localization of macrophages and MMPs in coronary atherosclerotic segments. From 36 atherosclerotic coronary arteries, 45 and 51 segments were selected with a vessel area that was\10% smaller and larger compared with the adjacent segments, respectively. No significant difference in staining for macrophages was observed between segments with expansive and constrictive remodeling. More MMP-2 and MMP-9 staining was observed in plaques of expansively remodeled segments compared with constrictively remodeled segments. In general, MMP-staining was less evident in the adventitial layer compared with the plaque. Zymography revealed more active MMP-2 in expansively remodeled segments compared with constrictively remodeled segments (3409319 vs. 1999181 (adjusted counts/mm2), respectively,P=0.019). Zymography did not show differences in inactive MMP-2 or MMP-9 among groups. It might be postulated that MMPs within the plaque play a causal role not only in plaque vulnerability but also in de novo atherosclerotic remodeling. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Atherosclerosis; Remodeling; Matrix metalloproteinases; Artery; Plaque rupture
www.elsevier.com/locate/atherosclerosis
1. Introduction
Atherosclerotic luminal narrowing is determined by plaque mass and the mode of geometrical remodeling. Constrictive remodeling accelerates and expansive re-modeling prevents luminal narrowing by plaque forma-tion [1 – 6]. In the acute phase luminal narrowing may be enhanced by plaque rupture and subsequent throm-bus formation [7 – 10]. Recently, we reported that in
femoral artery segments the type of atherosclerotic remodeling is associated with the presence of histologi-cal markers for plaque vulnerability: more inflamma-tory cells but less collagen and smooth muscle cells were observed in the caps and shoulders of cross-sec-tions that showed expansive enlargement compared with the more stable constrictively remodeled cross-sec-tions [11]. The concept of this remodeling paradox, that expansive enlargement prevents luminal narrowing on one hand but may be associated with vulnerable plaques on the other hand, is supported by recently reported clinical ultrasound studies in which the mode of remodeling was found to be associated with the patients’ clinical syndrome [12,13].
* Corresponding author. Tel.: +31-30-2507155; fax: + 31-30-2522693.
E-mail address:[email protected] (G. Pasterkamp)
Vascular and inflammatory cells can modulate the structure and composition of the extracellular matrix by producing enzymes involved in its degradation. In vulnerable plaques matrix metalloproteinases (MMPs), that are secreted by macrophages, digest the matrix components within the fibrous cap. In cross-sections of atherosclerotic arteries revealing extreme expansive re-modeling, like aneurysms, increased MMP activity within the arterial wall and a higher density of macrophages may be observed within the arterial wall [14]. Mainly the gelatinases MMP-2 and MMP-9 are highly expressed and more active in enlarged aneurys-matic arteries.
It remains to be investigated whether the increased prevalence of inflammatory cells in expansively remod-eled segments compared with constrictively remodremod-eled segments is also evident for the atherosclerotic coronary artery. In addition, it is unknown if such higher preva-lence of macrophages in enlarged segments is also associated with an enhanced release of matrix metallo-proteases that are thought to play a pivotal role in both matrix degradation and plaque destabilization [15 – 18] and atherosclerotic remodeling [14,19].The aim of the present study was to investigate the association between the mode of remodeling and the presence of macrophages and MMP-1, MMP-2 and MMP-9 in coronary atherosclerotic cross-sections. In addition, zy-mography was performed to study the gelatinase activ-ity in atherosclerotic cross-sections that show expansive and constrictive remodeling.
2. Methods
Fourteen hearts were obtained within 24 h post-mortem (eight men and six women, 7198 years) from patients who did not die of cardiovascular disease. The right coronary artery (RCA, n=13), left anterior de-scending coronary artery (LAD, n=12) and left cir-cumflex (LCX, n=11) were dissected from the epicardium over a length of 5 – 10 cm from their origin. Six arteries were not used for analysis due to severe calcifications or extensive cutting artifact. The dissected coronary arteries were frozen in liquid nitrogen and stored at −80°C.
2.1. Morphometric analysis
The frozen coronary arteries were cut into segments of 2.5 mm that were subsequently numbered from proximal to distal. From each segment the side of the proximal cutting face was marked with east-Indian ink. Odd numbered segments were stained by Lawson elastin stain. Even numbered segments were stored for additional immunohistological staining. Morphometric measurements were performed on both the proximal
and sital site from each odd-numbered segment. If selected, the adjacent cutting face of the even-numbered segment was used for additional staining. For example, if the distal part of segment 1 was selected, then the proximal part of segment 2 was used for additional staining [20].
The microscopic images of the cross-sections stained with Lawson elastic tissue stain were recorded on VHS videotape with a 3CCD video camera for further image analysis. A ruler was used for distance calibration. From each cross-section we measured the following parameters: lumen area, area encompassed by the inter-nal elastic lamina (IEL-area) (mm2
), IEL-area circum-ference (mm), area encompassed by the external elastic lamina (EEL-area) (mm2) and EEL-area circumference
(mm).
Plaque area (mm2) was calculated by subtracting the
lumen area from the IEL-area. The media area was calculated by subtracting the EEL-area from the IEL-area. The area encompassed by the EEL-area was recalculated as if the vessel was circular (corrected EEL-area). Subsequently, the corrected IEL-area and corrected lumen area were calculated assuming a circu-lar EEL-area. The corrected IEL-area and lumen area were calculated by subtracting the media area from the corrected EEL-area and the plaque area from the cor-rected IEL-area, respectively. Throughout the text, the lumen area and IEL-area will be referred as being the corrected lumen areas and corrected IEL-areas.
2.2. Selection and immunohistochemical staining
Prior to histological staining a selection was made based on arterial geometry. All measurements obtained from the arterial segments were plotted as demon-strated in Fig. 1. Two groups of cross-sections were selected for staining that met the following criteria: A, IEL-area \10% larger compared with adjacent proxi-mally and distally located cross-sections (within 5 mm) (expansive remodeling); B, IEL-area \10% smaller compared with adjacent proximally and distally located cross-sections (within 5 mm) (constrictive remodeling). No major side branches should originate between the cross-section under investigation and the adjacent cross-sections.
In short, 8mm frozen sections were cut and stored at −80°C until use. The sections were dried at room temperature for 1 h and fixed for 10 min in fresh aceton, containing 0.03% H2O2 to block endogenous
peroxidase. The sections were dried, rinsed in PBS and incubated with normal horse serum (10% in PBS) for 30
min. Additional incubation with the correct primary monoclonal antibody was performed overnight at 4°C in PBS containing 0.1% BSA. After the overnight incu-bation the sections were rinsed in PBS (three times for 5 min) and incubated with 2.5 mg/ml biotynilated horse anti-mouse polyclonal antibody (Vector) in PBS
con-Fig. 1. Cross-sectional changes along a post mortem obtained coronary artery segment. The arterial segment is represented as if all lesions were completely concentric. The interrupted line represents the luminal border, the solid line the internal elastic lamina. Arrows indicate the selected cross-sections which revealed an IEL-area 10% larger or smaller than the adjacent cross-sections representative for local expansive and constrictive remodeling and shrinkage, respectively.
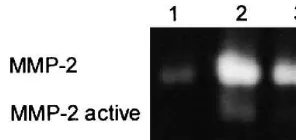
Fig. 3. Zymogram of three atherosclerotic coronary artery segments. Equal amounts of protein were used. The arterial segment in lane 1 shows less MMP-2 compared with the segments in lanes 2 and 3. Active MMP-2 is most prevalent in lane 2.
o/n incubation was followed by staining the gel with Coommassie blue stain and additional destaining of the gel so that clear white bands could be seen against a blue background. The white bands were quantified using a densitometric method on a Geldoc 1000 system (Bio-Rad) using the Molecular Analist software. The white bands for (active)MMP-2 (Fig. 3) and MMP-9 were identified on the basis of their molecular size and by taking into account the recombinant MMP-2 and MMPs-9 proteins (activated and non-activated) on zy-mography gel.
2.4. Analysis
The sections immunostained on the presence of macrophages, MMP-1, MMP-2 and MMP-9 were ana-lyzed quantitatively by light microscopy using Sis-anal-ysis 2.1 software. The sections were carefully studied and color thresholds were set and adjusted until the computerized detection met the visual interpretation. The degree of staining was expressed in mm2. For all
stains, the plaque, media and adventitia were analyzed separately. For macrophages and MMP-1 the cap/ shoulder of the plaque and the core of the plaque were analyzed separately. In addition, the type of staining (co-localization with macrophages, diffuse staining) was noted.
For each immuno staining, cross sections were cate-gorized into three groups based on the values obtained after computerized quantitative analysis: A, absent/ mi-nor staining (0 – 1000mm2; B, moderate staining (1000 –
10 000 mm2); and C, extensive staining (\10 000 mm2).
2.5. Statistical analysis
All values are presented as mean9S.D. AP-value of B0.05 was considered significant. Student’s t-test was used to compare morphometric measurements and zy-mographic determined activity among groups. Ax2-test was used to compare the area of immuno-stain among groups.
3. Results
From 36 coronary arteries, 45 and 51 segments were selected with a vessel area that were found to be \10% smaller and larger compared with the adjacent seg-ments, respectively (two to four segments/artery). Mor-phometric measurements of the cross-sections that demonstrated expansive or constrictive remodeling are depicted in Table 1. Lumen area, plaque area, IEL-area and EEL-area were all larger in the expansively remod-eled cross-sections compared with the cross-sections classified in the constrictive remodeling group.
taining 1% BSA and 1% normal human serum for 1 h. Next, the sections were rinsed in PBS (three times for 5 min) and incubated for one hour with 2 mg/ml strep-tavidine-horse radish peroxidase (Southern Biotechnol-ogy Associates).
To visualize the horse radish peroxidase, the sections were treated for 7 min with a sodium acetate buffer (pH 5.0) containing amino-ethyl-carbazole (AEC) substrate or with a Tris – HCl buffer (pH 7.5) containing DAB/ imidazole substrate. In addition, the sections were counterstained with heamatoxylin, to visualize all nuclei in the sections. For each staining, extra stainings with an omission of the primary antibody that served as negative controls were taken into account. When using the monoclonal antibodies for detection of MMP-2 and CD68, additional stainings were performed using an isotype matched control antibody (in the same concen-tration) and served as negative controls.
2.3. Zymography
For a subgroup of cross-sections stained for MMP-2 and MMP-9 of which adjacent tissue was available, zymography was performed (n=35 for segments with constrictive remodeling and n=37 for segments with expansive remodeling). The zymography procedure was performed using the next 10 – 15 adjacent 10 mm sec-tions. The sections were dissolved in 100 ml PBS con-taining 0.1% Tween and 1% SDS. Non dissolved tissue remnants were removed by centrifuging the samples for 5 min at 13 000 rpm. In addition, the amount of protein in each sample was determined by using a standard Lowry protein assay kit (Bio-Rad) and measuring at 655 nm with a automated ELISA-plate reader (Bio-Rad).
After equalizing the protein levels for all samples (by dilution with ddH2O) to 0.5 mg/ml, 30 ml of each
Table 1
Measurements of geometric variables obtained in cross-sections that showed constrictive remodeling and expansive remodeling compared with the adjacent cross-sections within the arterial segmenta
Expansion Constriction
Number of cross-sections 45 51 5.893.4* Lumen area (mm2) 3.592.6
13.795.9* 8.094.5
IEL-area (mm2)
7.995.0* Plaque area (mm2) 4.593.3
17.197.3* 10.495.4
EEL-area (mm2)
aValues are mean9S.D.
*PB0.05.
Table 2
Computerized measured values for macrophage, MMP-1, MMP-2 and MMP-9 staininga
Expansion Constriction
Macrophages, plaque (mm2) 56835 87974
38118/49856 Luminal/medial border 31609/25226
57948 45352
Macrophages, adventitia (mm2)
MMP-1, plaque (mm2) 52275 59432
MMP-1, adventitia (mm2) 10578 11343
MMP-2, plaque (mm2) 91985 148093
54605 MMP-2, adventitia (mm2) 55010
MMP-9, plaque (mm2) 149787 256861
20328 MMP-9, adventitia (mm2) 15800
aThe degree of staining was not normally distributed because of a
substantial number of cross-sections with extreme staining for the different assays. The measured values may differ among macrophages and the different MMPs because of different threshold values for the different stains.
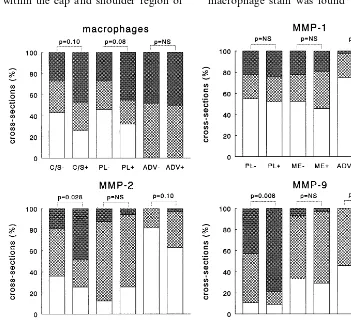
The area of staining for macrophages in the plaque tended to be more prevalent in expansively remodeled cross-sections compared with the cross-sections in the constrictive remodeling group. This difference, how-ever, was not significant (P=0.08) (Fig. 4). The com-puterized measured areas of immunostaining on the presence of macrophages were 56.835 versus 87.974mm2
for the constricted and expansively remodeled cross-sec-tions, respectively. These values for macrophage stain-ing within the total plaque were representative for the observed values within the cap and shoulder region of
the plaque as well as at the medial border of the plaque (Table 2). Overall, the media lacked macrophage stain-ing in the cross-sections under study. The degree of macrophage stain did not differ for the adventitia among groups. In the adventitia the extent of macrophage stain was found to be moderate in 42/96
Fig. 4. Percentage of cross-sections with minor (0 – 1000mm2), moderate (1000 – 10 000mm2) and heavy (\10 000mm2) staining for macrophages,
cross-sections and heavy in 45/96 cross-sections (Fig. 4).
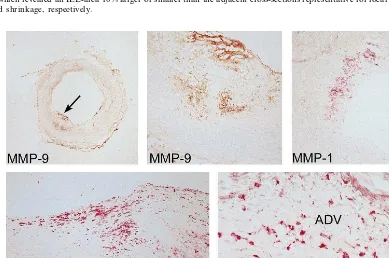
No difference in MMP-1 staining was observed be-tween the cross-sections that showed expansive and constrictive remodeling in the different layers of the arterial wall (total plaque, cap and shoulder of the plaque and adventitia) (Fig. 4). In contrast to the macrophages, the extent of staining for MMP-1 was more prevalent in the plaque than in the adventitia (Table 2).Both MMP-2 and MMP-9 immunostaining were more prevalent in plaques of the expansively remodeled segments versus constrictively remodeled cross-sections (Fig. 4). This enhanced staining in the expansively enlarged segments was evident in the cap and shoulder region as well as at the medial border of the plaque. MMP-9 mostly co-localized with present macrophages. MMP-2 showed more diffuse staining throughout the atherosclerotic plaque not specifically co-localizing with macrophages. The differences in staining among groups for MMP-2 and MMP-9 were not observed in either the media or the adventitia of the atherosclerotic cross-sections. MMP-2 and MMP-9 staining in the adventitia was much less prevalent when compared with the plaque (Table 2).
The plaque area was larger in the segments with expansive remodeling, compared with segments that showed constrictive remodeling (Table 1). The plaque area may, therefore, be considered a confounder for the interpretation of the differences in staining among groups. Zymography, in which equal amount of proteins had been loaded, revealed more active MMP-2 in the segments with expansive compared with segments with constrictive remodeling (3409319 vs. 1999180 (adjusted pixel counts/mm2), respectively (P=0.018))
whereas the inactive MMP-2 did not differ (30309 1290 vs. 308091339, respectively). MMP-9 did not differ for expansively and constrictively remodeled cross-sections (288491988 vs. 259791674, respec-tively).
4. Discussion
The extracellular matrix plays a role in maintaining structural integrity of the arterial wall. The matrix remodels during the course of development, growth and wound healing. Comparable shifts in the synthesis/ degradation balance occur in the pathogenesis of many diseases [21,22]. MMPs are natural matrix degrading enzymes that are present in active as well as in inactive forms within the arterial wall. The role of MMPs in plaque destabilization is well recognized: MMPs are more prevalent within high stress regions like the shoul-ders of the plaque and may contribute to the break-down of the collagen and thinning of the fibrous cap [18].
A role for MMPs in arterial wall remodeling is supported by several studies. Abbruzzese et al. [23] recently showed that blocking MMPs may inhibit flow dependent enlargement of the artery. In addition, inhi-bition of MMP-activity also reduces constrictive re-modeling after balloon angioplasty [24]. Enhanced MMP-activity has been described extensively in in-tracranial and abdominal aneurysms, suggesting an eti-ological role within this pathologic enlargement of the artery [14,19,25,26].
Recently, we demonstrated in the femoral artery that the type of remodeling is related to histological markers for plaque vulnerability: expansive remodeling is associ-ated with the presence of more inflammatory cells and less collagen in the cap and shoulder of the plaque [11]. In the present study we investigated the prevalence of macrophage staining in expansively and constrictively remodeled segments, in both the plaque and the adven-titia of the atherosclerotic coronary artery. In addition, staining for the different matrix degrading MMPs were evaluated.
The present study revealed that macrophages tend to be more prevalent in the plaques of enlarged segments compared with shrunken segments. Differences among groups did not reach significance, however. The preva-lence of macrophages in the adventitial layer was high but did not differ among groups. Immunohistochemical stainings for MMP-2 and MMP-9, but not MMP-1, were more prevalent in the plaques with expansive remodeling compared with segments with constrictive remodeling. In addition, active MMP-2 was increased in expansively remodeled segments compared with con-strictively remodeled segments.
4.1. Remodeling and plaque 6ulnerability in the coronary artery
4.2. Remodeling and MMPs
The present study revealed that MMP-stainings were more prevalent at the luminal border as well as the medial border of the plaque. Whether inflamma-tion within the atherosclerotic plaque with subsequent release of matrix degrading enzymes is a causal factor for expansive remodeling, remains speculative. Aneu-rysms are considered a pathologic subtype of expan-sive remodeling. These pathologic forms of enlargement have consistently been associated with enhanced inflammation and protease activity within the arterial wall, which makes a causal relation more plausible [14,19,25,26,30]. Mainly 2 and MMP-9 have been studied as proteases that play an impor-tant role in matrix degradation with subsequent enlargement of the artery in aneurysm formation. The present study supports the hypothesis that next to aneurysm formation, MMPs are involved in other forms of atherosclerotic enlargement as well.
Adventitial macrophage staining was extensive in most cross-sections, irrespective of the vessel size. In contrast with the macrophages in the plaque, the macrophages in the adventitia did not contain intra-cellular fat and were moderately co-localized with MMP-1, MMP-2 or MMP-9. Thus, if MMP staining represents active matrix degradation with subsequent arterial remodeling, then our observations suggest that this remodeling process is probably not initiated from the adventitia but more likely from the atherosclerotic plaque. Knox et al. [26] previously showed that in aortic aneurysms, proteolytic activity is particularly prevalent on the luminal and neointimal part of the specimens, which supports our observations.
We did observe a discrepancy in staining for MMP-1 versus MMP-2 and MMP-9 among constrictive and expansive arterial segments. MMP-2 and MMP-9 staining differed among expansively remodeled and constrictively remodeled cross-sections, whereas MMP-1 staining did not. It may well be that this difference in MMP-1 staining among enlarged and shrunken segments is reflected in MMP-1 activity.
The plaque area was larger when the segments showed expansive enlargement. Thus, the increased immunostaining for MMP-2 and MMP-9 may be due to the increased total plaque mass, rather than the expansive mode of remodeling. However, zymography was performed with equal amounts of protein and revealed higher levels of active MMP-2 in expansively, compared with constrictively, remodeled segments.
4.3. Limitations
In immunohistochemical staining, the presence of MMPs do not establish their catalytic capacity, as the
zymogen may lack activity. In addition, TIMPs may block activated MMPs. Thus, the stain for the differ-ent MMPs may not reflect their capability to digest the different matrix components necessary to induce arterial remodeling. Both remodeling and plaque infl-ammation are local phenomena (Fig. 1). Since adja-cent segments were already used for morphometric measurements and immunostaining for localization purposes it was not possible to perform zymographic studies of all investigated segments. Due to the dis-tance between the sites used for morphometry and zymography, the measured MMP-activity may not be representative for the cross-section in which the vessel size had been determined.
This is a cross-sectional study. Thus, it is difficult to establish causal relations between remodeling, plaque vulnerability and inflammation within the arte-rial wall. Previously, others have shown that MMPs do play a predominant role in plaque vulnerability [16,17]. Recent interventional studies showed that acti-vated MMPs are obligatory in flow related remodel-ing and post angioplasty remodelremodel-ing [23,24]. No animal models have been described in which both re-modeling modes, expansive and constrictive remodel-ing, occur. Thus far, studies in which alterations in de novo atherosclerotic remodeling is investigated in re-sponse to MMP inhibition are lacking. Based on the present results it might be postulated that, next to aneurysm formation, MMPs play a role in moderate forms of de novo atherosclerotic expansive remodel-ing.
The cross-sectional study design also limits the choice of reference segments. ‘Constrictive’ and ‘ex-pansive’ should therefore be considered as relative rather than absolute terms. A difference in vessel area between the site of interest and adjacent reference sites is an arbitrary but widely used measure for sig-nificant remodeling in cross-sectional studies [31 – 33]. In data analysis, the degree of staining was treated as a categorical variable. The cut off values for the different groups were arbitrarily chosen, which may have influenced the outcome.
5. Conclusions
Expansive remodeling in atherosclerotic coronary artery segments is associated with more MMP-2 and MMP-9 in the plaque compared with constrictive re-modeling. This association between remodeling mode and MMPs was evident for the plaque but not for the adventitia. Corrected for the amount of protein, more active MMP-2 was observed in plaques of expansively remodeled segments compared with constrictively re-modeled segments. It might be postulated that MMPs in the plaque play a causal role not only in plaque vulnerability but also in de novo atherosclerotic remodeling.
References
[1] Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolet-tis G. Compensatory enlargement of human atherosclerotic coronary arteries. New Engl J Med 1987;316:1371 – 5.
[2] Pasterkamp G, Wensing PJW, Post MJ, Hillen B, Mali WPTM, Borst C. Paradoxical arterial wall shrinkage con-tributes to luminal narrowing of human atherosclerotic femoral arteries. Circulation 1995;91:1444 – 9.
[3] Nishioka T, Luo H, Eigler NL, Berglund H, Kim C-J, Siegel RJ. Contribution of inadequate compensatory enlargement to development of human coronary artery stenosis: an in vivo intravascular ultrasound study. J Am Coll Cardiol 1996;27:1571 – 6.
[4] Mintz GS, Kent KM, Pichard AD, Satler LF, Popma JJ, Leon MB. Contribution of inadequate arterial remodeling to the de-velopment of focal coronary artery stenoses: an intravascular ultrasound study. Circulation 1997;95:1791 – 8.
[5] Pasterkamp G, Schoneveld AH, van Wolferen WA, et al. The impact of atherosclerotic arterial remodeling on percentage lu-minal stenosis varies widely within the arterial system: a post mortem study. Arterioscler Thromb Vasc Biol 1997;17:3057 – 63.
[6] Smits PC, Bos L, van Ufford MAQ, Eefting FD, Pasterkamp G, Borst C. Shrinkage of human coronary arteries is an impor-tant determinant of atherosclerotic luminal stenosis: an in vivo intravascular ultrasound study. Heart 1998;79:143 – 7.
[7] Davies MJ, Thomas AC. Plaque fissuring — the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J 1985;53:363 – 73.
[8] Fuster V, Badimon L, Badimon J, Chesebro JH. The patho-genesis of coronary artery disease and the acute coronary syn-dromes. New Engl J Med 1992;326:242 – 50.
[9] Fuster V, Badimon L, Badimon J, Chesebro JH. The patho-genesis of coronary artery disease and the acute coronary syn-dromes. New Engl J Med 1992;326:310 – 8.
[10] Falk E, Shah PK, Fuster V. Coronary plaque disruption. Cir-culation 1995;92:657 – 71.
[11] Pasterkamp G, Schoneveld AH, van der Wal AC, et al. The relation of arterial geometry with luminal narrowing and plaque vulnerability: the remodeling paradox. J Am Coll Car-diol 1998;32:655 – 62.
[12] Nishioka T, Luo H, Nagai T, et al. Impact of coronary artery remodeling on clinical manifestations of patients with de novo coronary artery lesions. J Am Coll Cardiol 1997;29(Suppl.):125A.
[13] Smits PC, Pasterkamp G, Eefting FD, Stella PR, de Jaegere PPT, Borst C. Arterial remodeling mode of the culprit lesion is related to clinical presentation in patients selected for PTCA. Eur Heart J 1998;19(Suppl.):587.
[14] Freestone T, Turner RJ, Coady A, Highman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscl Thromb Vasc Biol 1995;15(8):1145 – 51.
[15] Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atheroscelrotic plaques. Arteioscl Thromb Vasc Biol 1998;18:1707 – 15.
[16] Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expres-sion of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94(6):2493 – 503.
[17] Shah PK, Falk E, Badimon JJ, et al. Human monocyte derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix de-grading metalloproteinases and implications for plaque rupture. Circulation 1995;92(6):1565 – 9.
[18] Lee RT, Schoen FJ, Loree HM, Lark MW, Libby P. Circum-ferential stress and matrix metalloproteinase 1 in human coro-nary atherosclerosis. Implications for plaque rupture. Arterioscl Thromb Vasc Biol 1996;16(8):1070 – 3.
[19] Patel MI, Melrose J, Ghosh P, Appleberg M. Increased syn-thesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg 1996;24(1):82 – 92.
[20] Pasterkamp G, Schoneveld AH, van der Wal AC, et al. Infl-ammation of the atherosclerotic cap and shoulder of the plaque is a common and locally observed feature in unrup-tured plaques of femoral and coronary arteries. Arterioscler Thromb Vasc Biol 1999;19:54 – 8.
[21] Perez Tamayo R. Pathology of collagen degradation. A review. Am J Pathol 1978;92:509 – 66.
[22] Tryggvarson K, Hoyhtya M, Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochem Biophys Acta 1987;907:191 – 217.
[23] Abbruzzese TA, Guzman RJ, Martin RL, Yee C, Zarins CK, Dalman RL. Matrix metalloproteinase inhibition limits arterial enlargement in a rodent arteriovenous fistula model. Surgery 1998;124:328 – 34.
[24] de Smet BJGL, Robertus JL, Rebel JMJ, et al. Metallo-proteinase inhibition reduces constrictive arterial remodeling following balloon angioplasty: a study in the atherosclerotic Yucatan micropig. J Am Coll Cardiol 1999;33:88A.
[25] Bruno G, Todor R, Lewis I, Chyatte D. Vascular extracellular matrix remodeling in cerebral aneurysms. J Neurosurg 1998;89:431 – 40.
[26] Knox JB, Sukhova GK, Whittemore AD, Libby P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 1997;95:205 – 12.
[27] van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Cir-culation 1994;89:36 – 44.
[28] Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes: im-plications for plaque rupture. Circulation 1994;90:775 – 8. [29] Galis ZS, Muszynski M, Sukhova GK, Simon Morrisey E,
metallo-proteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann New York Acad Sci 1995;748:501 – 7.
[30] Freestone T, Turner RJ, Higman DJ, Lever MJ, Powell JT. Influence of hypercholesterolemia and adventitial inflammation on the development of aortic aneurysms in rabbits. Arterioscl Thromb Vasc Biol 1997;17:10 – 7.
[31] Timmis SBH, Burns WJ, Hermiller JB, Parker MA, Meyers SN, Davidson CJ. Influence of coronary atherosclerotic remodeling
on the mechanism of balloon angioplasty. Am Heart J 1997;134:1099 – 106.
[32] Tauth J, Pinnow E, Sullebarger JT, et al. Predictors of coronary arterial remodeling patterns in patients with myocardial is-chemia. Am J Cardiol 1997;80:1352 – 5.
[33] Pasterkamp G, Borst C, Gussenhoven EJ, et al. Remodeling of de novo atherosclerotic lesions in femoral arteries: impact on the mechanism of balloon angioplasty. J Am Coll Cardiol 1995;26:422 – 8.
.