Research report
21 21
Ca
-independent activity of Ca
/ calmodulin-dependent protein
kinase II involved in stimulation of neurite outgrowth in
neuroblastoma cells
a a b ,
*
aYoshimi Sogawa , Yoshiyuki Yoshimura , Akira Otaka
, Takashi Yamauchi
a
Department of Biochemistry, Faculty of Pharmaceutical Sciences, The University of Tokushima, Shomachi 1, Tokushima 770-8505, Japan b
Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto 606-8501, Japan Accepted 8 August 2000
Abstract
21 21
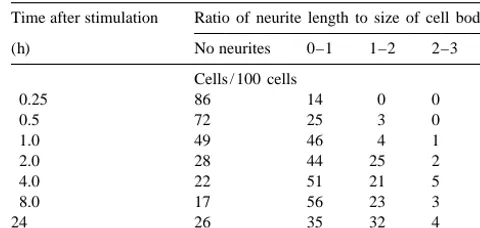
We investigated the involvement of Ca -independent activity of Ca / calmodulin-dependent protein kinase II (CaM kinase II) in stimulation of neurite outgrowth. When neuroblastoma Neruo2a (Nb2a) cells expressing theaisoform of CaM kinase II (Nb2a /acells) were stimulated by plating, they changed shape from round to flattened, and began to form neurites within 15 min. Numbers of cells bearing neurites increased from 15 min to about 2 h. Neurite length increased markedly from 30 min to 2 h after stimulation.
21
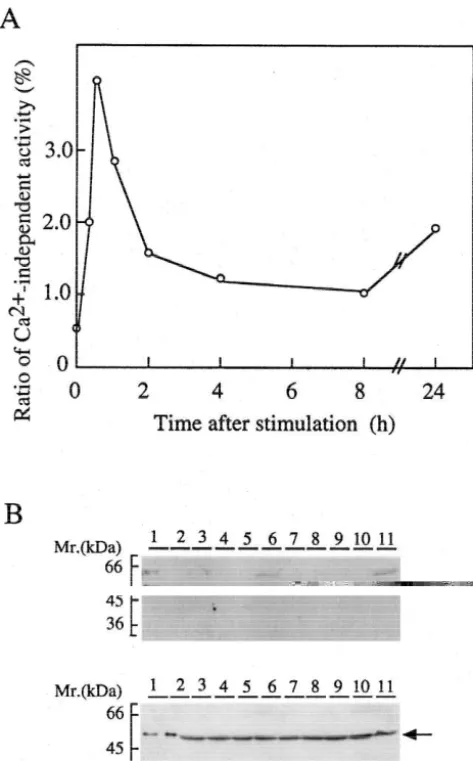
Ca -independent activity of CaM kinase II increased immediately after stimulation, peaked at about 30 min, and then gradually
21
decreased. Autophosphorylation of Thr-286 followed the same time course as the increase in Ca -independent activity. The
21
autophosphorylation and appearance of Ca -independent activity preceded the formation of neurites. The effect of mutation of the autophosphorylation site in the kinase whose Thr-286 was replaced with Ala (aT286A kinase) or Asp (aT286D kinase) was examined.
21 21
aT286A kinase was not converted to a Ca -independent form, andaT286D kinase had Ca -independent activity significantly as an autophosphorylated kinase. Cells expressingaT286A kinase did not form neurites, and were indistinguishable from control Nb2a cells. Cells expressingaT286D kinase had much longer neurites than Nb2a /acells expressing the wild type kinase, although the initiation of
21
neurite outgrowth was very late. These results indicated that Ca -independent activity of the kinase autophosphorylated at Thr-286 involves for neurite outgrowth. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regulation
Topic: Process outgrowth, growth cones, and sprouting
21 21
Keywords: Ca / calmodulin-dependent protein kinase II; Autophosphorylation; Neurite outgrowth; Ca -independent activity; Neuroblastoma
1. Introduction brain, and in particular in cortical and hippocampal
neurons. Many of its substrates are involved in neuronal
21
Ca / calmodulin-dependent protein kinase II (CaM signaling. Neuronal CaM kinase II plays important roles in kinase II) is a multifunctional mediator of the activity the control of nerve functions in response to intracellular
21
dependent calcium increase in excitable cells Ca , including the synthesis and secretion of neuro-[3,13,21,43,44]. It is expressed at very high levels in the transmitters, a receptor function, structural modification of the cytoskeleton, axonal transport, long term potentiation
21 (LTP) and learning and memory [6,25]. It has been
Abbreviations: CaM kinase II, Ca / calmodulin-dependent protein
reported that transgenic mice lacking theaisoform of this kinase II; DMEM, Dulbecco’s modified Eagle’s medium; EGTA,
ethyl-ene glycol bis (b-aminoethylether)-N,N,N9,N9-tetra acetic acid; FBS, fetal kinase are defective in long-term potentiation and spatial
21
bovine serum; GFP, green fluorescence protein; Nb2a, Neuro 2a; PAGE, learning [2,36]. Brief Ca signals activate CaM kinase II, polyacrylamide gel electrophoresis; PMSF, phenylmethylsulfonyl fluo- and stimulate an autophosphorylation of Thr-286 which ride; SDS, sodium dodecyl sulfate
allows the kinase to maintain its activated state after the *Corresponding author. Tel.: 181-88-633-7250; fax: 181-88-633- 21
Ca concentration has returned to basal levels [11,18,32]. 9514.
E-mail address: [email protected] (T. Yamauchi). Autophosphorylation of CaM kinase II occurs in situ. For 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
example, depolarization or electrical stimulation increased cence reagent were purchased from DuPont New England
21
Ca -independent activity in PC 12 cells [28], pituitary Nuclear Research, G418 (geneticin) from Sigma, horserad-cells [10] and hippocampal neurons [12]. However, since ish peroxidase-coupled anti-mouse goat IgG and anti-rabbit
TM6
the autophosphorylation of the kinase occurs relatively goat IgG from Bio-Rad, FuGENE transfection reagent quickly, in just a few minutes, little is known about how from Bohringer Mannheim, pEGFP-C1 vectors from
21
the Ca -independent activity of the kinase influences CLONTECH. The CaM kinase II substrate peptide
au-physiological events. tocamtide 2 (KKALRRQETVDAL) [18] was synthesized
CaM kinase II is a multigene family, in which each of by Sawady Tech, Tokyo. Anti-phospho-CaM kinase II four isoforms (a, b, g, and d) is encoded by a separate antibody was provided by Dr. Yoko Yamagata, National gene [4,24,39,40]. The a and b isoforms are expressed Institute for Physiological Sciences, Okazaki, Japan, and almost exclusively in the nervous system [6]. Brain CaM was also prepared by our hand as described [42]. Phospho-kinase II consists of a distinct homo-oligomer of either a peptide containing an autophosphorylation site was syn-or b polypeptides [20]. The concentration of a and b thesized with a ABI peptide synthesizer. CaM kinase II isoforms varies markedly in different brain regions with was purified from rat forebrain as described previously postnatal age [38]. The role of each isoform in the [44]. Monoclonal antibody specific to the a isoform of regulation of nerve function has been investigated in CaM kinase II was as described [19].
neuroblastoma cells expressing these isoforms [15,31]. CaM kinase II promotes neurite outgrowth, and the b
isoform induces a greater change in morphology than thea 2.2. Cell culture isoform [31].
21
Neurite outgrowth is triggered by the influx of Ca in Mouse neuroblastoma Neuro 2a cells (Nb2a) and human response to various stimuli [1]. The neuronal growth cone, embryonal kidney cells (HEK 293T) were cultured in a specialized structure at the distal end of a developing Dulbecco’s modified Eagle’s medium (DMEM) sup-neurite, is responsible for neurite outgrowth and path plemented with 10% fetal bovine serum (FBS), penicillin, finding in the developing nervous system [29]. The motili- and streptomycin at 378C in a 7% CO2 humidified ty of neurites and growth cones is important to synaptic incubator, and the media changed every 2 to 3 days as plasticity. Alterations in cell shape result from directed needed. Cells transfected with neo alone,aCaM kinase II
21
changes in cytoskeletal dynamics regulated by Ca and its mutant cDNAs were maintained in the presence of signaling. CaM kinase II is one of the major mediators of 0.7 mg / ml G418. Assessments of neurite outgrowth were
21
Ca signaling in the central nervous system [6]. It carried out as described previously [15]. Cells with one regulates polymerization-depolymerization of microtubules process greater than one cell diameter were considered and microtubule-microfilament interaction through phos- neurite bearing. Neurite length for a given cells was phorylation of microtubule-associated proteins [14,45,46]. quantified as the average radial distance from the neurite Furthermore, it plays important roles in synaptic plasticity tips to the soma.
[6,25]. We have shown that CaM kinase II is involved in controlling neuronal morphogenesis using neuroblastoma
cells expressing the kinase [15,31], suggesting that these 2.3. Isolation of Nb2a cells stably expressinga isoform cells could be used as a model system for studying the of CaM kinase II and its mutants
motility of neurites. On the other hand, calcineurin is a
21
Ca / calmodulin-dependent protein phosphatase that has The wild type and mutant cDNAs of the a isoform of been reported to regulate neurite outgrowth [9,23,27]. CaM kinase II were inserted into the EcoRI site of the Moreover, growth cones retain the ability to modulate in pEF321 and pcDNA3 expression vectors in the sense
21 21
response to Ca elevation [34], suggesting that Ca direction under the control of human elongation factor 1 signaling plays an important role in the regulation of and CMV promoters, respectively. pEF321- or pcDNA3-a,
neurite outgrowth. or a-mutant DNAs (aT286A and aT286D DNAs) and
21
To elucidate the mechanism of Ca signaling through pEF321-neo DNA (pEF321-neo) were introduced into the action of CaM kinase II on the control of neurite Nb2a cells by the calcium phosphate gel method [15], or
TM6
outgrowth, we investigated the relation between neurite by treatment with FuGENE transfection reagent
ac-21
outgrowth and Ca -independent activity induced by cording to the manufacturer’s instructions. Mutant cDNAs autophosphorylation of the kinase in neuroblastoma cells ofaT286A andaT286D were prepared from theaisoform expressing the aisoform and its mutants. of CaM kinase II cDNA by site directed mutagenesis as described previously [32]. After 2 to 4 weeks of growth in selection medium containing G-418, several individual
2. Materials and methods G-418-resistant clones were isolated. The cells were
further cloned from a single cell by a limited dilution 2.1. Materials
method. Expression of the wild and mutant kinases was
32
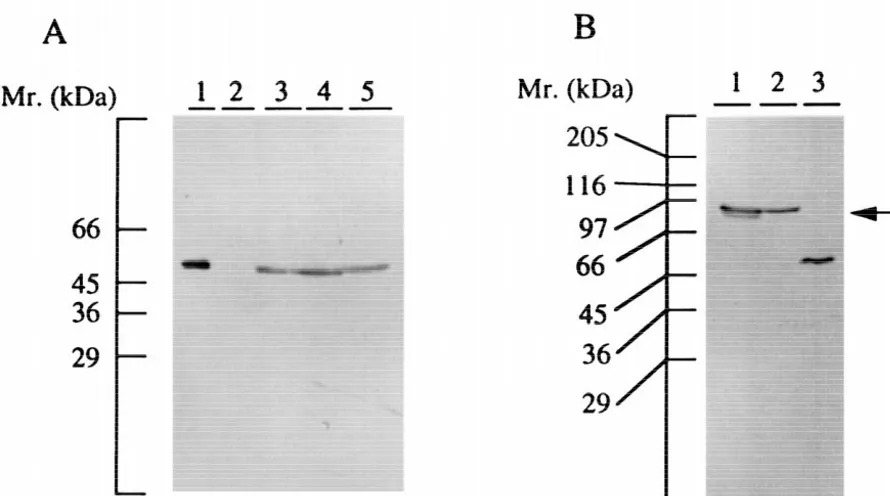
Fig. 1. Detection of CaM kinase II and its mutants in Nb2a cells stably expressing the enzymes and GFP-tagged kinases by immunoblotting. (A) Cell extracts (50mg of protein) of Nb2a cells stably expressing theaisoform of CaM kinase II and its mutants were subjected to immunoblotting. Lane 1, purified CaM kinase II (30 ng); lanes 2–5, Nb2a control cells, Nb2a /a cells, Nb2a /aT286A cells, and Nb2a /aT286D cells, respectively. Molecular markers are shown on the left. (B) pEGFP-C1 /aand pEGFP-C1 /aT286D DNAs were transfected to HEK 293T cells, and cultured for 24 h, and then cells were extracted as described under Materials and methods. Cell extracts (50mg of protein) were subjected to immunoblotting. Lane 1, transfected with pEGFP-C1 /aDNA; lane 2, transfected with pEGFP-C1 /aT286D DNA; lane 3, purified CaM kinase II (30 ng). An arrow indicates the GFP-tagged kinase. Molecular markers are shown on the left.
2.4. Expression of GFP-CaM kinase II 2.5. Preparation of cell extracts
The sequence corresponding to the a isoform of rat Cells were cultured in tissue culture dishes, washed once
21 21
brain CaM kinase II was ligated with EcoRI and HindIII with Ca -Mg -free DMEM, harvested, and then soni-sites at the 59and 39noncoding regions, respectively, and cated in 200 ml of extraction buffer / 10 cm dish. The cloned into pEGFP-C1 expression vector to yield plasmid extraction buffer consisted of 40 mM Tris–HCl, pH 7.6, 1 pEGFP-C1 /ain frame. GFP was tagged to the N-terminus mM dithiothreitol, 1 mM EGTA, 10% glycerol, 1 mM
5
of CaM kinase II. Nb2a cells were seeded at 2310 / 35 phenylmethylsulfonyl fluoride (PMSF), 0.1 mM okadaic mm dish and cultured for 24 h. The plasmid DNA (2mg) acid, 0.1 mM calyculin A, and 10 mg / ml each of the
TM6
was introduced to the cells with 3 ml of FuGENE antibiotic protease inhibitors, antipain, leupeptin, and transfection reagent. The number of cells, and amount of pepstatin A. After centrifugation at 18,5003g for 30 min,
TM6
FuGENE transfection reagent and plasmid DNA were the soluble and particulate fractions were separated. changed in proportion to the size of the culture dish. After
an appropriate period of incubation, the medium was 2.6. Immunoblot analysis changed to DMEM without phenol red, and the expression
of GFP-fusion protein was monitored by fluorescent Immunoblot analysis of transfected cells was carried out
microscopy. as described previously [19]. The soluble fraction of
incu-Table 1
bated with horseradish peroxidase-coupled antibody (di- a
Neurite length of Nb2a /acells luted 1:2000). Immunoreactive bands were detected using
Time after stimulation Ratio of neurite length to size of cell body enhanced chemiluminescence reagents.
For reprobing, membranes were washed with PBS2 (h) No neurites 0–1 1–2 2–3 3
0.05% Tween 20, and then incubated with 62.5 mM Cells / 100 cells
Tris–HCl, pH 6.8, containing 2% SDS and 100 mM 0.25 86 14 0 0 0
2-mercaptoethanol for 30 min at 508C. After the mem- 0.5 72 25 3 0 0
1.0 49 46 4 1 0
branes had been washed with PBS-0.05% Tween 20 and
2.0 28 44 25 2 1
reused, the detection reaction was started from the
block-4.0 22 51 21 5 1
ing step as described above. 8.0 17 56 23 3 1
Protein was measured with bovine serum albumin as a 24 26 35 32 4 3
standard as described [5]. SDS–PAGE was performed by a 5
Nb2a /a cells were seeded at 2310 / well of 24-well plates, and the method of Laemmli [22]. photographed at 15 min, 30 min, 60 min, 2 h, 4 h, 8 h, and 24 h after stimulation. Neurite length for a given cell was quantified as the average 2.7. Assay of CaM kinase II radial distance from the neurite tips to the soma. The values are
representative of data obtained from four independent experiments. CaM kinase II activity was assayed by phosphorylation
of autocamtide 2 at 308C for 1 min as described previously
[38]. The standard reaction mixture contained 50 mM increased in number and composed about 27% of the total
32 5
Hepes buffer, pH 8.0, 50mM [g- P]ATP (4–6310 cpm), at 2 h (Fig. 2 and Table 1). Neurite-bearing cells were not 8 mM Mg(CH COO) , 103 2 mM autocamtide 2, 2 mM increased significantly after 2 h. Neurite length increased calmodulin, 0.2 mM CaCl , 0.1 mM EGTA, and a suitable2 markedly from 30 min to 2 h after stimulation, indicating amount of enzyme in a total volume of 50 ml. The that the formation of neurites was initiated soon after
21
Ca -independent activity was measured under the same stimulation. Control Nb2a cells did not form neurites, conditions except that 1 mM EGTA was added in place of consistent with previous results [31]. Neurite outgrowth of
21
Ca . Nb2a /a cells was observed when cells were stimulated by
21
ionomycin, a Ca -ionophore (data not shown). But, the 2.8. Autophosphorylation of CaM kinase II morphological change could only be investigated for a short time, since this reagent had strong cell toxicity. The Autophosphorylation of CaM kinase II was carried out initiation of neurites was faster than that of differentiation at 08C. The reaction mixture contained 50 mM Hepes of neuroblastoma cells by cAMP [30] or that of pheochro-buffer, pH 8.0, 100mM ATP, 8 mM Mg (CH COO) , 0.23 2 mocytoma PC12 cells by nerve growth factor [16], sug-mM CaCl , 0.1 sug-mM EGTA, 22 mM calmodulin, l mM gesting that the action of CaM kinase II was related to the dithiothreitol, and suitable amounts of CaM kinase II in a morphological change of the growth cone.
total volume of 30ml. Control reactions were carried out in
21
the absence of Ca and calmodulin. After incubation for 3.2. Expression of GFP-CaM kinase II 10 min, aliquots of the reactions were removed and
assayed for enzymatic activity. To investigate the involvement of CaM kinase II in
neurite outgrowth directly, the distribution of CaM kinase II in neurites was examined using GFP-fusion protein.
3. Results When pEGFP-C1 /aplasmid DNA was introduced in HEK
293T cells, expression of enzyme protein was detected by
3.1. Neurite outgrowth immunoblotting (Fig. 1B). The molecular mass of the a
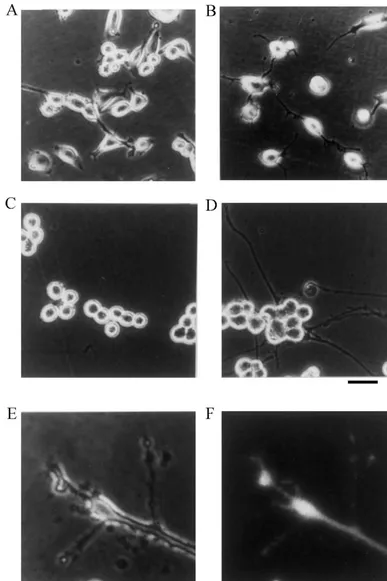
isoform of CaM kinase II and GFP is 50 and 27 kDa, We previously demonstrated that CaM kinase II pro- respectively. GFP-CaM kinase II was found to be 77 kDa, moted neurite outgrowth in neuroblastoma cells, and these consistent with the mass of respective proteins. Specific cells formed neurites within 2 h of stimulation [31]. To enzyme activity was 9.40 and 5.71 nmol / min / mg protein clarify the involvement of CaM kinase II in neurite in soluble and particulate fractions, respectively, indicating outgrowth, morphological change was investigated in the that the activity of the GFP-tagged kinase was comparable
21 period just after stimulation. Cells were stimulated by to that of the native kinase. The activity was Ca and
21
5
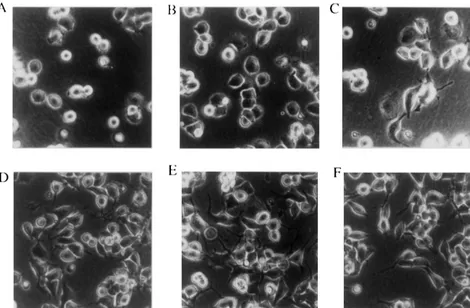
Fig. 2. Morphological change of Nb2a /acells. Nb2a /acells were seeded at 2310 / well of 24-well plates, and photographed at 15 min, 30 min, 60 min, 2 h, 4 h, and 8 h after stimulation by plating (A–F, respectively). The center of each well was chosen as the site for analysis. A phase contrast photomicrograph is shown. Bar, 40mm.
transiently expressed in Nb2a cells (Fig. 3). These cells 30 min, and then gradually decreased. The enzyme activity had neurites and the GFP-tagged kinase was found to be was significantly high at 4 to 8 h as compared with the
21
distributed in the neurites as well as in the cell body. Cells basal level. However, after 24 h, the Ca -independent
21
transfected with control pEGFP-C1 plasmid DNA did not activity was only slightly increased. The Ca -independent change cell morphology and did not have neurites. The activity accounted for about 4% of total activity at 30 min.
21 efficiency of the transfection was about 30%. These results This may be physiologically significant, since the Ca -indicated that CaM kinase II localized in neurites and independent activity of the extract composed at maximum promoted neurite outgrowth even in cells transiently about 50% of total activity when the kinase was auto-expressing the kinase. The kinase was clearly detectable in phosphorylated in vitro, and the kinase in neurites may be neurites of Nb2a /a cells when pEGFP-C1 /a DNA was present at relatively low concentrations as judged from the introduced into Nb2a /acells (Fig. 3E and F). The kinase distribution of GFP-CaM kinase II.
was detected throughout the neurite. These results suggest Immunoblotting using phospho-kinase specific antibody that the kinase may phosphorylate some proteins involved demonstrated phosphorylation of Thr-286 at 15 min, and
in neurite outgrowth. with a maximum at 30 min and then a decrease (Fig. 4B).
Phospho-kinase was detected at 2 and 4 h after stimulation.
21
3.3. Detection of Ca -independent activity and After 24 h, phospho-kinase activity significantly increased autophosphorylation of CaM kinase II again. The a isoform of CaM kinase II was expressed at almost the same level in these cell extracts (Fig. 4B, lower
21
To clarify the relation of Ca / calmodulin-independent panel). The change of autophosphorylated kinase was
21
activity with neurite outgrowth, autophosphorylated kinase compatible with that of Ca -independent activity. These
21 was investigated in Nb2a /a cells by immunoblotting and results indicated that autophosphorylation and Ca -in-activity assay (Fig. 4). When cells were seeded on new dependent activity preceded the initiation of neurite
out-21 21
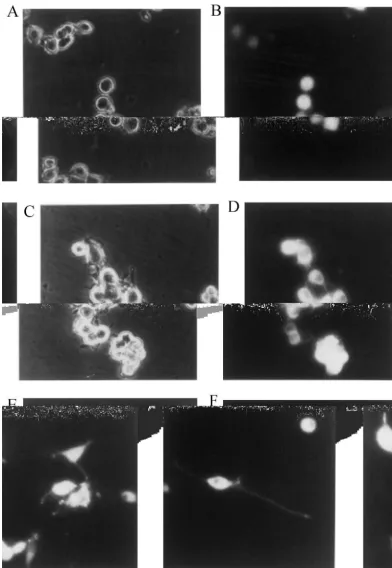
Fig. 3. Expression of GFP-CaM kinase II in Nb2a or Nb2a /acells. Nb2a or Nb2a /acells were transfected with pEGFP-C1 /aDNA as described under Materials and methods. Cells were cultured for 24 h and then photographed. (A and B) control pEGFP-C1 DNA transfected to Nb2a cells; (C and D) pEGFP-C1 /aDNA transfected to Nb2a cells; E and F, pEGFP-C1 /aDNA transfected to Nb2a /acells; A and C, phase contrast photomicrographs; (B, D, E and F) fluorescent photomicrographs. E and F show typical samples bearing short and long neurites, respectively.
3.4. Effect of mutation of Thr-286 in CaM kinase II on growth was investigated in cells expressing aT286A and neurite outgrowth aT286D kinases (Nb2a /aT286A and Nb2a /aT286D cells,
21 respectively). aT286A kinase did not generate Ca
-in-21
significantly high as compared with that of wild type (less
21 than 1%) and aT286A kinase (about 1%). The Ca -independent activity of aT286D and aT286A kinases did not increase significantly on autophosphorylation, whereas that of the wild type increased about 50%. These experi-ments were done in three independent clones, and similar results were obtained in each clone (data not shown).
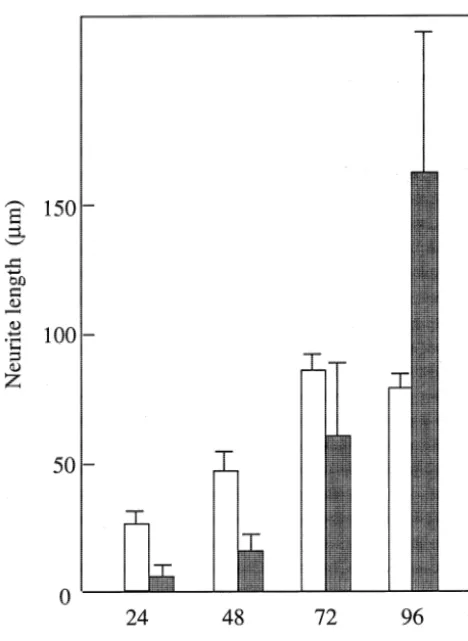
The morphological change caused by the stimulation was investigated as described above. Nb2a /aT286A cells did not form neurites and were indistinguishable from control Nb2a cells at all time points (data not shown), consistent with a previous report [15]. On the other hand, Nb2a /aT286D cells had neurites, although they formed very late. The adhesion of Nb2a /aT286D cells to culture dishes was weak as compared with that of Nb2a /a cells. The cells did not proliferate for 24 h, but then increased with a doubling time of about 24–30 h, which was almost the same as that of Nb2a /aand Nb2a /aT286A cells (data not shown). Nb2a /aT286D cells did not change shape and were round for a period of 2 days. After 48 h, the cells were flattened and neurite outgrowth initiated, with a rapid increase in the number of processes. About 20% of cells bore short neurites at that time. From 48 to 96 h, the neurites lengthened considerably, and relatively large differences in length were observed between the cells (Figs. 5 and 6), suggesting that the initiation of neurite outgrowth was rate limiting step of these process. The expression of the kinase in the growth cone was clearly demonstrated using the GFP-tagged kinase (Fig. 6E and F). On the other hand, Nb2a /a cells had neurites at an early period. Neurites gradually increased in number from 24 to 72 h, and did not change after 72 h. These results indicated that initiation of neurite outgrowth might require 21
Fig. 4. Appearance of Ca -independent activity and autophosphorylated a threshold level of CaM kinase II activity, and that 6
21 kinase. Nb2a /a cells (5310 ) were stimulating by plating on 10-cm
Ca -independent activity of the kinase induced by auto-dishes and extracted at the times indicated. (A) Time course of
appear-21 phosphorylation of Thr-286 involved in neurite outgrowth
ance of Ca -independent activity. An aliquot was withdrawn, and total
21 of neuroblastoma cells.
and Ca -independent activities of the kinase were determined. The enzyme activity was normalized to that of control cells without
stimula-21
tion, and expressed as a percent age of Ca -independent activity to total activity. Data are representative of at least 5 experiments done in duplicate. (B) Time course of the change of phospho-kinase detected by
4. Discussion
immunoblotting. Cell extracts (50 mg of protein) were subjected to SDS–PAGE, and then immunoblotting as described under Materials and
methods. Upper panel, detected with anti-phospho-kinase antibody. Cell shape is an inherent property and closely related to Lower panel, after detection of phospho-kinase, membranes were re- cell function. Neuronal cells make specific connections probed using anti-CaM kinase II antibody specific to the a isoform as with one another. Despite the differences among nerve described under Materials and methods..
cells, the basic mechanisms of these processes may have many similarities. Changes in cell shape and motility are induced to make neuronal connections and related to the activity. The expression of these kinases was confirmed by
function of cytoskeletal proteins. The functions of these immunoblotting (Fig. 1A). The CaM kinase II activity in
proteins are regulated by phosphorylation-dephosphoryla-the soluble fraction of Nb2a /a, Nb2a /aT286A and Nb2a /
21
tion, and Ca signaling plays an important role in these aT286D cells was 6.960.4, 6.860.4, and 3.060.1 nmol /
21 processes. CaM kinase II is a major mediator of Ca min / mg, respectively. Cells expressing aT286D kinase
signaling in the central nervous system. It regulates had weak activity and cells with strong enzyme activity
polymerization-depolymerization of microtubules and in-were not obtained under the experimental conditions. This
termediate filaments, and microtubule-microfilament inter-may be due to the fact that the cell cycle arrests at a level
actions [14,45–47]. In the present study, we demonstrated over the threshold of constitutively active kinase [33]. The
21 21
in-5
teins important for dendritic outgrowth are transported to dendrites and growth cones, several mRNAs, including those for theaisoform of CaM kinase II [7], microtubule-associated protein 2 (MAP2) [8], and activity-regulated cytoskeleton associated protein (Arc) [26], are identified in dendrites of hippocampal neurons, suggesting that local translation of the mRNA within dendrites could also generate proteins necessary for dendritic function. CaM kinase II was found to be expressed in neurites and growth cones of Nb2a /a cells. These cells are thought to be a useful model for studying dendritic growth cones, although the mRNA of CaM kinase II was not investigated in neurites. The morphological changes to neurites in neuro-blastoma cells may be related to the motility changing synaptic connections of brain neuronal cells.
21
Ca -independent activity of the kinase was found to be induced by the stimulation of neuroblastoma cells and involved in promoting neurite outgrowth. Mutation of Thr-286 to Asp resulted in a kinase with some of the properties of the phosphorylated enzyme. When cDNA of the mutant kinase was transfected to neuroblastoma cells and stable transformants (Nb2a /aT286D cells) were iso-lated, the CaM kinase II activity of these cells was found to be very weak. Since it has been reported that cells expressing constitutively active CaM kinase II were ar-rested in G2 of the cell cycle [33], the cells expressing high levels of aT286D kinase may be arrested in that phase. However, isolated Nb2a /aT286D cells were viable Fig. 6. Neurite length of Nb2a /aand Nb2a /aT286D cells. Nb2a /aand
5 and proliferated with a doubling time of about 24–30 h
Nb2a /aT286D cells were seeded at 0.5–2310 / well of 24-well plates,
and photographed. Average neurite length of at least 25 long neurites was after the adaptation, although they did not proliferate for determined from photomicrographs. Open bar, Nb2a /acells; filled bar, 24–30 h after seeding. Nb2a /aT286D cells formed long Nb2a /aT286D cells. Data are representative of at least 3 experiments.
neurites, although the process started very late. These cells might be weakly but constantly stimulated in culture, and aT286D kinase might be spontaneously activated at a level volved in the neurite outgrowth of neuroblastoma cells above threshold for the initiation of neurite outgrowth.
expressing CaM kinase II. Neurite outgrowth might be promoted by the constitutively
21
When Nb2a /a cells expressing the a isoform of CaM active kinase. Therefore, though the Ca -independent
21
kinase II were stimulated, Ca -independent activity of the activity necessary for neurite outgrowth may be rather kinase was rapidly induced, and maximized at about 30 weak, the initiation of outgrowth may require a relatively min. It then gradually declined, but remained significantly high level of CaM kinase II activity.
high for at least 4 h. Neurite outgrowth was initiated after On the other hand, the Rho family proteins, newly
21
activation of Ca -independent activity. Although induc- described members of the Ras superfamily of small
21
tion of Ca -independent activity is observed in PC 12 GTPases, are known to participate in regulation of cell cells, pituitary cells and hippocampal neurons, the auto- morphology in neuroblastoma cells including growth cone phosphorylation of the kinase lasts only a relatively short morphology [17]. These proteins control the assembly and time [10,12,28]. CaM kinase II was found to be distributed organization of the actin cytoskeleton. It has been also in growth cones and neurites as well as throughout the cell shown that a conventional myosin is required for growth body using the GFP-tagged kinase. It has been reported cone motility of neuroblastoma cells using antisense
21
that the influx of Ca triggers neurite outgrowth after oligodeoxyribonucleotides targeting myosin IIB transcript
21 stimulation in neuroblastoma cells [1]. The localization of [41]. However, little is known about the effect of Ca CaM kinase II in neurites and growth cones supported the signaling through CaM kinase II and signaling through the result that the kinase promoted neurite outgrowth [31]. Rho family and myosin on neurite outgrowth or retraction CaM kinase II translocates to the postsynaptic density in in neuroblastoma cells, and further studies are required to
21
eluci-dependent and cyclic-AMP-eluci-dependent kinases, J. Neurochem. 45 date the molecular mechanism of morphological change of
(1985) 900–905.
neuronal cells. 21
[15] Y. Goshima, S. Ohsako, T. Yamauchi, Overexpression of Ca / calmodulin-dependent protein kinase II in Neuro2a and NG108-15 neuroblastoma cell lines promotes neurite outgrowth and growth cone motility, J. Neurosci. 13 (1993) 559–567.
Acknowledgements
[16] L.A. Greene, A.S. Tischler, Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve We are deeply indebted to Dr. Y. Yamagata for gracious- growth factor, Proc. Natl. Acad. Sci. USA 73 (1976) 2424–2428.
[17] A. Hall, Rho GTPases and the actin cytoskeleton, Science 279 ly supplying phospho-kinase specific antibody. This work
(1998) 509–514. was supported in a part by a grant-in-aid for Scientific
[18] P.I. Hanson, M.S. Kapiloff, L.L. Lou, M.G. Rosenfeld, H.
Schul-Research from the Ministry of Education, Science, and 21
man, Expression of a multifunctional Ca / calmodulin-dependent Culture of Japan. protein kinase and mutational analysis of its autoregulation, Neuron
3 (1989) 59–70.
[19] T. Ichikawa, S. Sekihara, S. Ohsaho, Y. Hirata, T. Yamauchi, 21
Ca / calmodulin-dependent protein kinase II in the rat cerebellum;
References An immunohistochemical study with monoclonal antibodies specific
to eitheraorbsubunit, J. Chem. Neuroanat. 5 (1992) 383–390. [20] T. Kanaseki, Y. Ikeuchi, H. Sugiura, T. Yamauchi, Structural features [1] L. Anglister, I.C. Farber, A. Shahar, A. Grinvald, Localization of
21
of Ca / calmodulin-dependent protein kinase II revealed by elec-voltage-sensitive calcium channels along developing neurites: Their
tron microscopy, J. Cell Biol. 115 (1991) 1049–1060. possible role in regulating neurite elongation, Dev. Biol. 94 (1982)
[21] M.B. Kennedy, P. Greengard, Two calcium / calmodulin-dependent 351–365.
protein kinases, which are highly concentrated in brain, phosphor-[2] M.E. Bach, R.D. Hawkins, M. Osman, E.R. Kandel, M. Mayford,
ylate protein I at distinct sites, Proc. Natl. Acad. Sci. USA 78 (1981) Impairment of spatial but not contextual memory in CaM KII
1293–1297. mutant mice with a selective loss of hippocampal LTP in the range
[22] U.K. Laemmli, Cleavage of structural proteins during the assembly of theufrequency, Cell 81 (1995) 905–915.
of the head of bacteriophage T4, Nature 227 (1970) 680–685. [3] M.K. Bennett, N.E. Erondu, M.B. Kennedy, Purification and
charac-[23] N.J. Lautermilch, N.C. Spitzer, Regulation of calcineurin by growth terization of a calmodulin-dependent protein kinase that is highly
cone calcium waves controls neurite extension, J. Neurosci. 20 concentrated in brain, J. Biol. Chem. 258 (1983) 12735–12744.
(2000) 315–325. [4] M.K. Bennett, M.B. Kennedy, Deduced primary structure of theb
21
[24] C.R. Lin, M.S. Kapiloff, S. Durgerian, K. Tatemoto, A.F. Russo, P. subunit of brain type II Ca / calmodulin-dependent protein kinase
Hanson, H. Schulman, M.G. Rosenfeld, Molecular cloning of a determined by molecular cloning, Proc. Natl. Acad. Sci. USA 84
brain-specific calcium / calmodulin-dependent protein kinase, Proc. (1987) 1794–1798.
Natl. Acad. Sci. USA 84 (1987) 5962–5966. [5] A. Bensadoun, D. Weinstein, Assay of proteins in the presence of
[25] J. Lisman, The CaM kinase II hypothesis for the storage of synaptic interfering materials, Anal. Biochem. 70 (1976) 241–250.
memory, Trends Neurosci. 17 (1994) 406–412. [6] A.P. Braun, H. Schulman, The multifunctional calcium /
calmodulin-[26] G.L. Lyford, K. Yamagata, W.E. Kaufmann, C.A. Barnes, N.G. dependent protein kinase: From form to function, Annu. Rev.
Copeland, D.J. Gilbert, N.A. Jenkis, A.A. Lanahan, P.F. Worley, Physiol. 57 (1995) 417–445.
Arc, a growth factor and activity regulated gene, encodes a novel [7] K.E. Burgin, M.N. Waxham, S. Rickling, S.A. Westgate, W.C.
21
cytoskeleton-associated protein that is enriched in neuronal de-Mobley, P.T. Kelly, In situ hybridization histochemistry of Ca /
ndrites, Neuron 14 (1995) 433–445. calmodulin-dependent protein kinase II in developing rat brain, J.
[27] W.E. Lyons, E.B. George, T.M. Dawson, J.P. Steiner, S.H. Snyder, Neurosci. 10 (1990) 1788–1798.
Immunosuppressant FK506 promotes neurite outgrowth in cultures [8] A. Caceres, G.A. Banker, O. Steward, L. Binder, M. Payne, MAP2
of PC12 cells and sensory ganglia, Proc. Natl. Acad. Sci. USA 91 is localized to the dendrites of hippocampal neurons in culture, Dev.
(1994) 3191–3195. Brain. Res. 13 (1984) 314–318.
21
[28] M. MacNicol, A.B. Jefferson, H. Schulman, Ca / calmodulin [9] H.Y. Chang, K. Takei, A.M. Sydor, T. Born, F. Rusnak, D.G. Jay,
kinase is activated by the phosphatidylinositol signaling pathway Asymmetric retraction of growth cone filopodia following focal
21
and becomes Ca -independent in PC12 cells, J. Biol. Chem. 265 inactivation of calcineurin, Nature 376 (1995) 686–690.
(1990) 18055–18058. [10] Z.J. Cui, F.S. Gorelick, P.S. Dannies, Calcium /
calmodulin-depen-[29] B.K. Mueller, Growth cone guidance: First steps towards a deeper dent protein kinase II activation in rat pituitary cells in the presence
understanding, Annu. Rev. Neurosci. 22 (1999) 351–388. of thyrotropin-releasing hormone and dopamine, Endocrinology 134
[30] M. Nirenberg, S. Wilson, H. Higashida, A. Rotter, K. Krueger, N. (1994) 2245–2250.
Busis, R. Ray, J.G. Kenimer, M. Adler, Modulation of synaptic [11] Y.-L Fong, W.L. Taylor, A.R. Means, T.R. Soderling, Studies of the
21
formation by cyclic adenosine monophosphate, Science 222 (1983) regulatory mechanism of Ca / calmodulin-dependent protein kinase
794–799. II. Mutation of threonine 286 to alanine and aspartate, J. Biol.
Chem. 264 (1989) 16759–16763. [31] T. Nomura, K. Kumatoriya, Y. Yoshimura, T. Yamauchi, Over-21
expression of a and b isoforms of Ca / calmodulin-dependent [12] K. Fukunaga, L. Stoppini, E. Miyamoto, D. Muller, Long-term
21
protein kinase II in neuroblastoma cells — H7 promotes neurite potentiation is associated with an increased activity of Ca /
cal-outgrowth, Brain Res. 766 (1997) 129–141. modulin-dependent protein kinase II, J. Biol. Chem. 268 (1993)
7863–7867. [32] S. Ohsako, H. Nakazawa, S. Sekihara, A. Ikai, T. Yamauchi, Role of 21 threonine-286 as autophosphorylation site for appearance of Ca -[13] J.R. Goldenring, B. Gonzalez, J.S. McGuire Jr, R.J. Delorenzo,
21
independent activity of Ca / calmodulin-dependent protein kinase Purification and characterization of a calmodulin-dependent kinase
IIasubunit, J. Biochem. 109 (1991) 137–143. from rat brain cytosol able to phosphorylate tubulin and
micro-tubule-associated proteins, J. Biol. Chem. 258 (1983) 12632–12640. [33] M.D. Planas-Silva, A.R. Means, Expression of a constitutive form of calcium / calmodulin dependent protein kinase II leads to arrest of [14] J.R. Goldenring, M.L. Vallano, R.J. DeLorenzo, Phosphorylation of
calmodulin-[34] V. Rehder, S. Cheng, Autonomous regulation of growth cone autophosphorylated state of Ca / calmodulin-dependent protein filopodia, J. Neuobil. 34 (1998) 179–192. kinase II by acute neuronal excitation in vivo, J. Neurochem. 71 [35] K. Shen, T. Meyer, Dynamic control of CaM KII translocation and (1998) 427–439.
localization in hippocampal neurons by NMDA receptor stimulation, [43] T. Yamauchi, H. Fujisawa, Evidence for three distinct forms of Science 284 (1999) 162–166. calmodulin-dependent protein kinases from rat brain, FEBS Lett. [36] A.J. Silva, R. Paylor, J.M. Wehner, S. Tonegawa, Impaired spatial 116 (1980) 141–144.
learning in a-calcium-calmodulin kinase II mutant mice, Science [44] T. Yamauchi, H. Fujisawa, Purification and characterization of the 257 (1992) 206–211. brain calmodulin-dependent protein kinase (kinase II), which is [37] S. Strack, R.J. Colbran, Autophosphorylation-dependent targeting of involved in the activation of tryptophan 5-monooxygenase, Eur. J.
calcium / calmodulin-dependent protein kinase II by the NR2B Biochem. 132 (1983) 15–21.
subunit of the N-methyl-D-aspartate receptor, J. Biol. Chem. 273 [45] T. Yamauchi, H. Fujisawa, Disassembly of microtubules by the (1998) 20689–20692. action of calmodulin-dependent protein kinase (kinase II) which [38] H. Sugiura, T. Yamauchi, Developmental changes in the levels of occurs only in the brain tissues, Biochem. Biophys. Res. Commun.
21
Ca / calmodulin-dependent protein kinase IIa andb proteins in 110 (1983) 287–291.
soluble and particulate fractions of the rat brain, Brain Res. 593 [46] T. Yamauchi, H. Fujisawa, Regulation of the interaction of actin (1992) 97–104. filaments with microtubule-associated protein 2 by calmodulin-[39] T. Tobimatsu, I. Kameshita, H. Fujisawa, Molecular cloning of the dependent protein kinase II, Biochim. Biophys. Acta 968 (1988)
cDNA encoding the third polypeptide (g) of brain calmodulin- 77–85.
dependent protein kinase II, J. Biol. Chem. 263 (1988) 16082– [47] T. Yano, T. Tokui, Y. Nishi, K. Nishizawa, M. Shibata, K. Kikuchi,
16086. S. Tuiki, T. Yamauchi, M. Inagaki, Phosphorylation of keratin
[40] T. Tobimatsu, H. Fujisawa, Tissue-specific expression of four types intermediate filaments by protein kinase C, by calmodulin-depen-of rat calmodulin-dependent protein kinase II mRNAs, J. Biol. dent protein kinase and by cAMP-dependent protein kinase, Eur. J. Chem. 264 (1989) 17907–17912. Biochem. 197 (1991) 281–290.
[41] S.R. Wylie, P.-J. Wu, H. Patel, P.D. Chantler, A conventional myosin [48] Y. Yoshimura, T. Yamauchi, Phosphorylation-dependent reversible 21