www.elsevier.com / locate / bres
Interactive report
Hypothermia as an adjunctive treatment for severe bacterial
1meningitis
a ,
*
a a b cJose E. Irazuzta
, Robert Pretzlaff , Mark Rowin , Kevin Milam , Frank P. Zemlan ,
a
Basilia Zingarelli
aDivision of Critical Care, Children’s Hospital Medical Center, Cincinnati, OH, USA b
Marshall University, Huntington, WV, USA c
University of Cincinnati College of Medicine, Cincinnati, OH, USA
Accepted 30 August 2000
Abstract
Brain injury due to bacterial meningitis results in a high mortality rate and significant neurologic sequelae in survivors. The objective of this study was to determine if the application of moderate hypothermia shortly after the administration of antibiotics would attenuate the inflammatory response and increase in intracranial pressure that occurs in meningitis. For this study we used a rabbit model of severe Group B streptococcal meningitis. The first component of this study evaluated the effects of hypothermia on blood–brain barrier function and markers of inflammation in meningitic animals. The second part of the study evaluated the effects of hypothermia on intracranial pressure, cerebral perfusion pressure and brain edema. This study demonstrates that the use of hypothermia preserves CSF / serum glucose ratio, decreases CSF protein and nitric oxide and attenuates myeloperoxidase activity in brain tissue. In the second part of this study we show a decrease in intracranial pressure, an improvement in cerebral perfusion pressure and a decrease in cerebral edema in hypothermic meningitic animals. We conclude that in the treatment of severe bacterial meningitis, the application of moderate hypothermia initiated shortly after antibiotic therapy improves short-term physiologic measures associated with brain injury. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Infectious diseases
Keywords: Meningitis; Hypothermia; Intracranial pressure; Cerebral perfusion pressure; Inflammation; Cleaved Tau protein
1. Introduction maintaining an adequate cerebral perfusion pressure and
avoiding hyperventilation [43,49]. This approach to the Current management of brain injury emphasizes the treatment of brain injury is developed largely from work maintenance of adequate physiologic substrate and attenua- on traumatic brain injury [48]. The study of the application tion of the inflammatory response in an attempt to prevent of these principles in other forms of brain injury (i.e. secondary brain injury. This approach applies the princi- infectious) is less well established.
ples of improved cerebral perfusion and oxygen delivery in Providing adequate oxygen delivery and cerebral perfu-all patients with techniques to decrease oxygen consump- sion does not reverse the primary insult and the injured tion in the most severely affected [30]. Current recom- brain responds with a complex inflammatory cascade that mendations include lowering intracranial pressure (ICP), is responsible for much of the secondary damage observed. The infiltration of neutrophils [6,17,60] the production of inflammatory mediators [9,36,44] and the liberation ex-citatory amino acids [12,59,29] are common findings in
1
Published on the World Wide Web on 13 Semptember 2000. brain injury. Additionally, meningitic brain injury results *Corresponding author. Division of Critical Care, One Children’s
in areas of ischemia and necrosis secondary to vasculitis
Plaza, Dayton, OH 45404-1815, USA. Tel.: 11-937-463-5168; fax:
and areas of cerebritis secondary to neutrophil infiltration
11-937-463-5082.
E-mail address: [email protected] (J.E. Irazuzta). [32,57].
Cerebral edema with an increase in ICP and sudden Care and Use of Laboratory Animals published by the herniation is the often fatal outcome of bacterial meningitis National Institutes of Health (National Academy Press — [26,31,46]. The raised ICP common to patients with revised 1996) and was performed with the approval of the meningitis often occurs within 12 h of admission to the Wright State University Laboratory Animal Care and Use hospital [27,46,47]. This time coincides with the increase Committee. Male white New Zealand rabbits (2.5–3 kg) in the inflammatory response generated by antibiotic were housed under conventional conditions. All surgical therapy. This increase in the inflammatory response ex- procedures and the administration of bacteria were per-acerbates blood–brain barrier dysfunction and cerebral formed under anesthesia. Anesthesia consisted of an edema [53]. Recognizing the sequence of these events intramuscular administration of ketamine (30 mg / kg) and offers a window of opportunity for studying interventions xylazine (3 mg / kg) followed by a continuous administra-designed to attenuate the inflammatory response and treat tion of Isoflurane (1.5%) and nitrous oxide (50%).
or prevent secondary brain injury. Group B Streptococcus — type III (GBS) were cultured
To be successful interventions designed to attenuate the overnight in Todd–Hewitt broth and grown to logarithmic inflammatory response, brain edema and increased ICP phase. The morning of the experiment the bacteria were described in meningitis should modulate multiple points of washed and re-suspended in sterile saline. Meningitis was the cascade of events that leads to increased brain damage. induced by inoculation into the cisterna magna of the The neuroprotective role of hypothermia has been known animals with 0.4 ml of the GBS suspension after removal for some time and hypothermia has been demonstrated to of 0.4 ml of cerebrospinal fluid (CSF). Sham animals have a beneficial effect at multiple points of brain injury received an inoculum containing sterile saline.
progression [40,50]. Moderate hypothermia reduces
cyto-kine production, nitric oxide generation, leukocyte infiltra- 2.2. Randomization tion and the release of excitatory amino acids [18,22,63]. A
decrease in cerebral metabolism and preservation of energy Prior to randomization and sixteen hours after GBS stores has been documented, as well as, a decrease in inoculation the severity of meningitis was determined by cerebral edema [8,45,62]. In our laboratory we have two observers assigning a clinical severity score. Severity demonstrated that the application of moderate hypothermia of illness was scored as follows: 05Normal activity, 15
decreases excitatory amino acid release and neuronal stress Reduced ambulation, 25inability to stand for .5 s, 35
in meningitis [29]. Moderate hypothermia has also been evidence of paralysis, 45actively seizing for more than 5 utilized in traumatic brain injury to control increased min or comatose. Animals were paired by severity of intracranial pressure that has failed conventional therapy clinical symptoms and randomized to hypothermic (32–
[55]. 348C) or normothermic (37–398C) conditions. Animals
We postulate that the application of moderate hypo- were enrolled until there was a minimum of six survivors thermia shortly after the administration of the antibiotics in each treatment group. Four control (Sham) animals will attenuate the inflammatory response and increase in underwent the entire procedure but did not receive the ICP that occurs in meningitis. Instituting hypothermia bacterial inoculum and were handled as animals in the following antibiotic therapy has two characteristics that normothermic group.
make its study appealing. Hypothermia following
anti-biotic therapy simulates a realistic clinical situation. The 2.3. Induction of hypothermia second advantage is that because inflammation increases
dramatically following antibiotic administration the use of Following randomization and instrumentation animals in hypothermia as a therapeutic intervention will be coordi- the hypothermic group were rendered hypothermic by nated with the time during which inflammation is in- covering their torso with a plastic bag containing ice. creased. This study was performed in two parts. The first Stable rectal temperature conditions (hypothermic, 32–
1
] was to evaluate the effects of moderate hypothermia on the 348C; normothermic, 37–398C) were obtained within 1 h2
integrity of the blood–brain barrier and inflammatory of initiating the antibiotic therapy. In a pilot study rectal markers of brain injury. The second was to evaluate the and brain temperatures were equivalent in this model (data effects of hypothermia on intracranial pressure and the not shown). Instrumentation involved placement of a maintenance of an adequate cerebral perfusion pressure in tracheotomy, a rectal probe, as well as, arterial and venous animals with severe bacterial meningitis. catheters. Ventilatory support was initiated and titrated to maintain a pH of.7.28, a PaCO of between 30 and 402
Torr and a PaO2.100 Torr. Following instrumentation
2. Material and methods animals were given a 20 ml / kg bolus of Hespan (Excel ,
Irvine, CA) and a continuous saline infusion at 20 ml / h
2.1. Animal preparation was started and continued for the duration of the study.
Blood gasses were obtained every 2 h. If the PaCO was2
adminis-tered at a dose of 0.5 mEq / kg or 1 mEq / kg for a pH,7.2 termined after enzymatic reduction of nitrate to nitrite or pH,7.1 respectively. The temperature was monitored using nitrate reductase (670 mU / ml) with NADPH (160 with a rectal probe Physitemp 700 1 HT (Physitemp mM) at ambient temperature for 3 h. Following incubation Instrument Inc., Clifton, NJ) The temperature was main- an equal volume of the Griess reagent (1% sulfanilamide tained in both groups with warming blankets Baxter K- and 0.1% naphthylethylenediamide in 5% phosphoric acid) MOD 100 (Baxter Health Corporation, Deerfield, IL). was added and nitrite concentrations were measured
spectrophotometrically at 550 nm. 2.4. Protocol — the effects of hypothermia on
inflammation 2.7. Measurement of myeloperoxidase activity
In this component of the study animals received 0.4 ml Myeloperoxidase activity (MPO) in brain tissue was
5
of GBS (10 cfu / ml). Sixteen hours after inoculation the determined as previously described with minor modifica-animals received ceftriaxone (Roche Laboratories) (50 mg / tion [6]. The mid and anterior portions of the left hemi-kg IV), were instrumented and maintained under tempera- sphere were homogenized in a solution containing 50 mM ture control conditions for 10 h. A dopamine infusion was Tris–HCl (pH 7.4) at a ratio of 4 ml / g of wet weight. An initiated and titrated to maintain a mean arterial blood aliquot of 1 ml of the homogenate was added to 20 ml of 5 pressure of greater than 40 mmHg in all animals. The mM phosphate buffer (pH 6) and centrifuged twice at 48C. hypothermic animals were re-warmed prior to euthanasia. The supernatant was discarded, the pellet washed in At the conclusion of the study anesthesia was briefly phosphate buffer and re-suspended in 0.5% hexa-decyl-lightened to observe the presence of corneal reflexes and trimethyl-ammonium bromide. Three cycles of freezing / spontaneous respirations. The study animals then under- thawing in liquid nitrogen followed by sonication were went a second cisternal tap for CSF collection and blood performed before incubation of the sample for 20 min at samples were obtained by cardiac puncture. The animals 48C. An aliquot of the supernatant was mixed with a were euthanized by an intracardiac administration of a solution of tetramethyl benzidine 3.9 mg / ml in di-methyl
saturated solution of KCl formamide and 0.004% of H O . The rate of change in2 2
Following euthanasia an autopsy was immediately per- absorbance was measured spectrophotometrically at 405 formed. The cranial vault was opened and its contents nm. Myeloperoxidase activity was defined as the quantity extracted en masse. Following removal the right brain was of enzyme degrading 1 mmol of hydrogen peroxide per isolated, stripped of the meninges, dissected on filter paper minute at 258C and expressed as units per gram of protein. and frozen. In three animals from each group coronal The protein content of the homogenized brain was mea-sections of the left brain were obtained fixed in formalde- sured spectrophotometrically by the Bradford method (Bio
hyde, embedded in paraffin and cut for the preparation of Rad Sigma Diagnostics ). microscopic slides. The slides were stained with
hemato-xylin and eosine according to standard techniques. 2.8. Protocol — the effects of hypothermia on cerebral perfusion and edema
2.5. Measurements of blood–CSF barrier function and
5
Infection We increased the bacterial load to 5310 cfu / ml and the
dose of ceftriaxone to 100 mg / kg in this component of the Blood and CSF specimens were centrifuged, the super- study. Following the administration of antibiotics and natant collected and rapidly frozen for further analysis. instrumentation animals in the hypothermic group were Alteration of blood–CSF barrier function was evaluated by cooled as previously described. Animals were in-measurement of protein and glucose content in CSF and strumented as previously described with the addition of an
serum samples. Protein content was measured spectro- intracranial pressure monitor (Camino , San Diego, CA) photometrically utilizing the Bradford method (Bio Rad being placed by standard technique. After the surgical
Sigma Diagnostics ). Glucose was measured spectro- procedures were performed nitrous oxide was discontinued photometrically utilizing a commercially available assay and the isoflurane was decreased to 0.5%. An infusion of
(Glucose HK, Sigma Diagnostics ). diazepam (0.7 mg / kg / h) and fentanyl (35 mg / kg / h) was Bacterial presence was evaluated by culturing 10ml of administered during the remainder of the study. Animals both CSF and blood on 5% blood agar plate at 378C, were maintained hypothermic for 6 h.
followed by colony counts 24 h later.
2.9. Hemodynamic management 2.6. Measurement of NO production
Maneuvers to maintain mean arterial pressure above 45 Nitric oxide was determined by measuring nitrate and mmHg were as follows. If the mean arterial pressure fell a
improve. If volume resuscitation was inadequate a continu- were determined by unpaired t-test with the Bonferroni ous dopamine infusion was initiated and titrated to main- correction for multiple comparisons. Regression analysis tain an arterial blood pressure greater then 45 mmHg. was utilized when the association between continuous During the last hours of the protocol the normal saline variables was sought. Determination of changes in heart infusion was adjusted so that all animals received the same rate, blood pressure, intracranial pressure and cerebral water load. Before euthanasia, the hypothermic animals perfusion pressure between experimental groups utilized were re-warmed and the anesthesia was briefly lightened to repeated measures factorial ANOVA. Mann–Whitney U observe the presence of corneal and paw reflexes as well as test was utilized for no-parametric data. Results were spontaneous respiration. Bacterial presence was evaluated considered significant at P,0.05
in this group also by culturing 10 ml of both CSF and blood on 5% blood agar plate at 378C, followed by colony
count 24 h later. 3. Results
Cerebral edema was determined by comparison of the
brain wet to dry ratio. Briefly, the brain was removed, 3.1. Hypothermia attenuates markers of inflammation placed on filter paper and dried in a vacuum oven at
1058C. Drying continued until a stable dry brain weight In the initial experiment 33 animals were used. Eight was obtained. Brain water content is expressed as water animals died during the instrumentation process and were
per 100 g dry weight. excluded from analysis. The experiment was carried out on
the surviving rabbits: eleven infected animals were in the 2.10. Measurements cleaved Tau protein (cTau) hypothermic group, ten infected animals were in the normothermic group and four animals were used as the A post-hoc analysis of available CSF samples was control (Sham) group. All animals receiving the intrathecal performed to detect the presence of cTau in CSF. Seven injection of GBS animals developed clinical signs of pre-meningitic samples and nine from the end of the study meningitis characterized by meningismus, lethargy, or period were available (2 Sham, 4 normothermic, 3 hypo- inability to ambulate within 16 h. The average clinical thermic) were available. Measurement of CSF cTau em- severity score at entry to the study was 1.460.2 in the ploys a previously characterized ELISA assay [65]. This normothermic animals and 1.560.2 in the hypothermic ELISA employs three Mabs (7A5, 8A12, and 12B2) that animals ( p5NS). All sham animals were behaving nor-recognize forms of cleaved MAP-tau present in human mally upon entry into the study.
CSF. Immulon 2 plates are coated with affinity-purified
Mab 12B2 (100ml / well, 5 mg / ml) for 1 h and then coated 3.2. The effect of hypothermia on glucose ratio, CSF overnight with 5% nonfat dry milk and 0.5% gelatin in protein and NO production
Tris buffered saline (TBS). Plates are washed with 0.1%
Tween in TBS (TBST), and serial dilutions of rabbit CSF Analysis of the CSF of normothermic animals demon-added in triplicate, incubated for 1 h, and then washed with strated the characteristic increase in CSF protein and a TBST. One hundredml / well of HRP-conjugated 7A5 and decrease in the CSF / serum glucose ratio that is the 8A12 diluted 1:2000 is added for 1 h, plates washed with hallmark of bacterial meningitis (Table 1) CSF protein in TBST and 100 ml / well of a 3 mg / ml solution of biotin- the hypothermic animals was significantly decreased when tyramine in 50 mM Tris–HCl, 0.001% H O2 2 (pH 8.0) compared to normothermic animals, but remained elevated added for 15 min. Color is developed with Vector ABC-AP when compared to Shams (hypothermic 3965 mg / dl vs. using nitrophenylphosphate as substrate and is read at 405 normothermic 5666 mg / dl; P,0.05). Evaluation of the nm. Affinity-purified human cTau is employed as the CSF / serum glucose ratio revealed a decrease in the standard. Negative controls include exclusion of 12B2, glucose ratio in normothermic meningitic animals with deletion of sample, or deletion biotinylated 7A5 and 12B2
from the assay. Experimental CSF cTau samples
demon-Table 1
strating O.D. values less than the sensitivity of the assay a
Inflammatory changes in GBS meningitis with and without hypothermia
(standard curve value demonstrating a coefficient of
vari-Variable Sham Normothermia Hypothermia
ation.10%) are assigned a value of zero. The sensitivity
[ [
Glucose ratio (%) 5567 3964* 5667*
of this ELISA is 30 pg per well and demonstrates an
[ [
CSF protein (mg / dl) 662 5666* 3965*
intra-assay variation of less than 7% and an inter-assay [ [
CSF nitric oxide (mmol / l) 2662 4665* 3165*
variation of less than 10%. [ [
Myeloperoxidase (U / gram) 7.260.9 19.362.9* 12.761.4* a
Values are equal to the mean (6S.E.M.). CSF; cerebral spinal fluid.
2.11. Data analysis
Glucose ratio is equal to the serum glucose / CSF glucose*100. *5P,
[
0.05 when compared to Sham treatment. 5P,0.05 when comparing
All data are presented as mean6standard error for each normothermic animals to hypothermic animals. Significance determined
preservation of the normal glucose ratio in the hypothermic group. (hypothermic 5667% vs. normothermic 3964%; P,0.05).
The presence of nitric oxide was determined as a marker of CNS inflammation. Nitric oxide was determined by analysis of serum and CSF nitrate / nitrite levels as previ-ously described. Serum nitrate / nitrite values were similar between sham and infected animals. (Sham 249618mmol / l vs. normothermic 289681 mmol / l vs. hypothermic 213628, p5NS). Differences in serum nitrate / nitrite levels in the hypothermic and normothermic groups did not reach statistical significance. However, meningitis induces an increase in nitric oxide levels in the CSF of infected animals (Shams 2662 mmol / l vs. normothermic 4665
mmol / l; P,0.05). Meningitic animals in the hypothermic group had CSF nitrate / nitrite levels that were significantly lower than their normothermic counterparts (hypothermic 3165 mmol / l vs. normothermic 4665 mmol / l, P,0.05) (Table 1). There was a correlation between the increase in
2
CSF nitric oxide and decreased glucose ratio (r 50.259, P,0.05), between nitric oxide levels and increased CSF
2
protein (r 50.241, P,0.05) and between CSF nitric oxide
2
and myeloperoxidase activity (r 50.254, P,0.05).
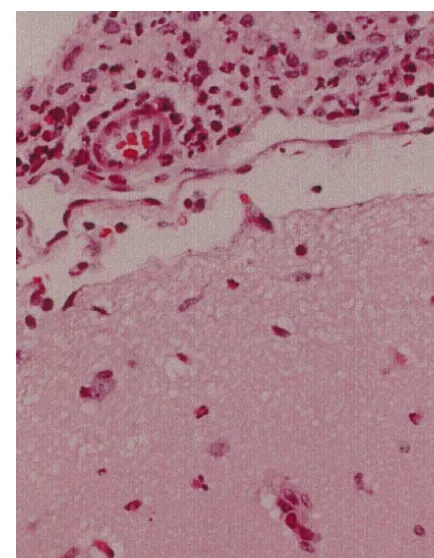
Fig. 1. Paraffin embedded microscopic section (3100) stained with
3.3. Neutrophil infiltration
hematoxylin-eosin of a rabbit brain infected with Group B Streptococcus. There is a thick infiltrate of polymorphonuclear cells present in the
Histologic examination of the brain of animals with subarachnoid space. Several polymorphonuclear cells are seen infiltrating meningitis using hematoxylin and eosin staining revealed the cortical surface. These lesions were distributed in a heterogeneous
extensive neutrophil exudate in the subarachnoid space pattern in both the hypothermic and normothermic groups making comparative analysis difficult.
with a patchy pattern of neutrophils infiltrating the cortical surface. Fig. 1 depicts a representative profile of
inflamma-tion in infected animals. These lesions were distributed in a correlation between bacterial presence and any of the heterogeneous pattern in both normothermic and hypo- above measured parameters.
thermic animals. Because of the heterogeneous pattern we could not find an acceptable method for quantifying
differences between the two groups. 3.5. Hypothermia attenuates increased intracranial
Evaluation of myeloperoxidase in brain tissue of ex- pressure perimental animals was determined as a marker of
neutro-phil infiltration. Analysis demonstrated an increase in Following an intrathecal injection of GBS all animals tissue MPO activity in the brain of infected animals developed clinical signs of meningitis characterized by compared to shams (Table 1). When the brain tissue was meningismus, lethargy or an inability to ambulate. Eleven evaluated, we noted a significant reduction in tissue MPO animals died from meningitis prior to randomization and in the hypothermic group when compared to normothermic were excluded. Following instrumentation and randomiza-animals (hypothermic 12.761.4 U / gram vs. normothermic tion, 9 animals in the normothermic group and 8 animals in
19.362.9 U / gram; P,0.05). the hypothermic group were entered into the study. The
average clinical severity score at entry to the study was
3.4. Bacterial clearance 2.260.3 in the normothermic animals and 2.860.4 in the
of the possibility of contamination resulting from place-ment of the ICP monitor.
3.6. ICP and CPP
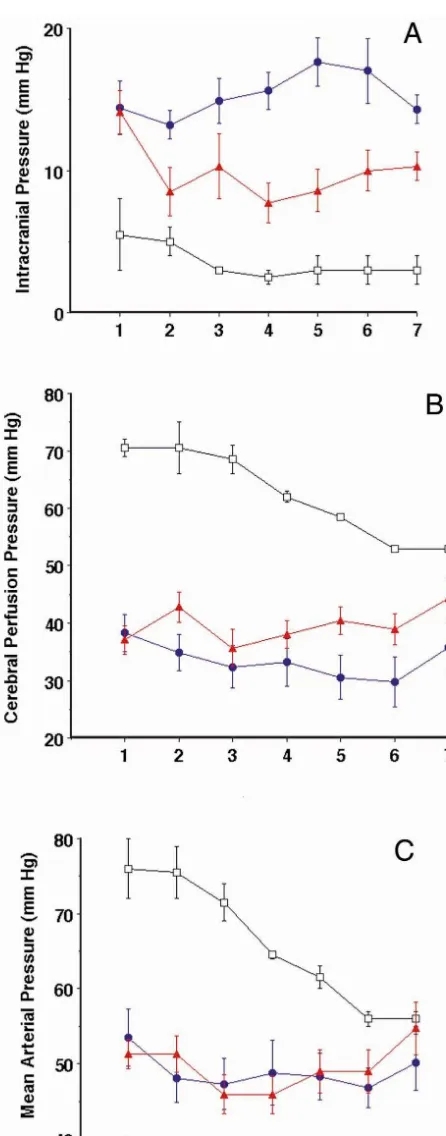
All infected animals demonstrated an increase in ICP and decreased blood pressure when compared with the Sham group. Prior to cooling, animals in the hypothermic and normothermic groups demonstrated no difference in ICP, CPP, mean arterial pressure or heart rate. As can be seen in Fig. 2a there is a significant reduction of the ICP in the hypothermic group (P,0.05). This reduction remains throughout the experiment though this decrease in ICP does not fall to the level of the non-meningitic (Sham) animals. Cerebral perfusion pressure is affected while mean arterial pressure was maintained without a significant difference between the two groups by titrating the dopa-mine infusion. (Fig. 2b,c) Heart rate in the normothermic animals was greater than either the hypothermic or sham treated animals (P,0.05).
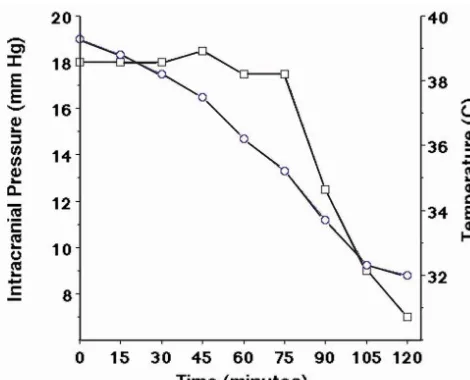
During this part of the study it was noted that hypo-thermic animals exhibited a rapid decrease in ICP in the first 2 h after initiating external cooling. Because this largely occurred during instrumentation two additional animals were instrumented prior to external cooling. These animals were cooled after instrumentation about 18 h after inducing meningitis to explore the relationship between the induction of hypothermia and intracranial pressure. As can be seen in Fig. 3, the ICP remains elevated until the core temperature decreases below 368C. Below this temperature ICP readily decreases with this decrease being seen at about 90 min after initiating cooling.
3.7. Cerebral edema
To evaluate if the decreased ICP observed in hypo-thermic animals had a relationship with the development of cerebral edema we measured the brain water content in each group. Hypothermia induced a small but statistically significant difference in brain water content between the two groups. The average brain water content of normother-mic meningitic animals was 489613 gH20 / 100 g dry weight and that of hypothermic meningitic animals was 44968 gH20 / 100 g dry weight (P,0.05). The brain water content of the Sham animals was intermediate to these values and was 461647 gH20 / 100 g dry weight and the difference was not significant when compared to either the hypothermic or normothermic groups.
Fig. 2. (a)–(c) depict the response to hypothermia in meningitic animals for intracranial pressure, cerebral perfusion pressure, and mean arterial
3.8. Cleaved Tau protein in CSF
pressure respectively. Three groups of animals are represented (1) Sham treatment (no meningitis, normothermia), (2) Normothermic treatment
(meningitis, normothermia) and (3) Hypothermic (meningitis, hypother- cTau was undetectable in the CSF in the pre-meningitic mia). Each time point represents 90 min of elapsed time. Data is samples and after the experiment in the Sham operated presented as the mean6S.E.M. Repeated measures factorial ANOVA was
animals. In contrast cTau was elevated in all animals after
used to determine differences between hypothermic and normothermic
meningitis (normothermic, 28.7614 hypothermic 29.965
animals. (*5P,0.05) d5Normothermic treatment, m5Hypothermic
meningitis [7,19,20]. Breakdown in the blood–CSF barrier, leukocyte accumulation, an increase in nitric oxide and secondary areas of ischemia and necrosis are well char-acterized during meningitis [32,35,57]. Our previous work has demonstrated an attenuation of excitotoxic amino acid release and decreased neuronal stress with the application of moderate hypothermia in bacterial meningitis [29].
The goal of this study was to determine if the salutary effects of hypothermia could be extended to ameliorate the secondary brain injury associated with meningitis. Our study demonstrated that the application of moderate hypo-thermia in a model of severe bacterial meningitis preserves markers of blood–brain barrier function, decreases CSF nitric oxide, and decreases myeloperoxidase activity in brain tissue. We have further demonstrated that hypo-thermia decreases intracranial pressure without negatively effecting blood pressure thereby allowing for maintenance
Fig. 3. Demonstrates the relationship between body temperature and ICP
of an improved cerebral perfusion pressure. The efficacy of
during the first two hours of external cooling. Each time point represents
ensuring an adequate perfusion pressure has recently been
the average value of two meningitic animals. s5temperature (8C),
demonstrated in adults with bacterial meningitis [42]. Our
h5intracranial pressure (mmHg).
study has also documented a small but statistically signifi-cant decrease in brain water in the hypothermic animals
4. Discussion suggesting a decrease in cerebral edema even in this
short-term study. The increase in the CSF nitrate / nitrate in Evidence supporting the use of hypothermia to attenuate meningitis with no significant change in serum nitrate / brain injury following trauma dates back greater than 40 nitrate has been reported as evidence of in-situ NO years [50]. Hypothermia improves neurologic outcome in production and has been correlated with increased morbidi-animal models and human clinical trials of traumatic brain ty [1,11,13,33,34,36]. In line with these findings, we found injury [14,40]. Acceptance of hypothermia as a treatment that CSF nitrate / nitrate levels are increased in meningitis modality has been limited by the acknowledgment that and this increase is attenuated by hypothermia.
hypothermia is known to cause myocardial depression, Neutrophil infiltration contributes to tissue damage by arrhythmias, coagulopathy and immune dysfunction. The releasing radicals and oxidant molecules [37,61]. A recent adverse effects of hypothermia are obviated by the use of study showed that hypothermia decreases the influx of moderate hypothermia and a slower rather than rapid rate leukocytes into the CSF of animals with meningitis [2]. In of cooling [39]. Moderate hypothermia has been generally this study we sought to evaluate neutrophil infiltration by defined as a temperature greater than 308C, with most histology and determination of myeloperoxidase activity. studies targeting a temperature of 32–348C. Because of the heterogenous distribution of neutrophils an Moderate hypothermia applied after an insult plays a acceptable method of quantifying them was not found. role in protecting neurons in ischemic and traumatic brain Myeloperoxidase activity has been used as a marker of damage by modifying multiple points of the cascade of neutrophil presence and has been evaluated in different events that leads to secondary injury [18,22]. Hypothermia types of brain injuries [7,25,28]. Although the MPO gene preserves cellular energy stores reducing the cerebral is expressed in the myeloid lineage, MPO protein remains metabolic rate for oxygen associated with electrophysio- stored in large quantities in neutrophils in the azurophilic logical activity and homeostatic functions required to granules [23]. We have documented a decrease in MPO maintain cellular integrity [8,45,16]. It is reported that activity in the hypothermic group. We interpret this differ-cerebral metabolic rate decreases approximately 7% for ence to be secondary to a decrease in neutrophil accumula-each degree Celsius reduction in temperature [8]. Further tion. Supporting this interpretation, we recently character-cerebral protective effects include the preservation of the ized integrin expression on CSF neutrophils in meningitis blood–brain barrier function, a decreased neutrophil infil- and have observed that the activation of b1-integrins on trate and a reduction in the release of excitatory amino extravasated neutrophils is decreased by hypothermia acids and cerebral edema [15,56,63]. These beneficial [51,52]. b1-integrins are adhesion molecules involved in effects appear to be secondary to a suppression of the the binding of neutrophils to the extracellular matrix [10]. inflammatory cascade and not due to a delay in the injury The decrease in integrin activation may be in part
respon-process [41]. sible for the decrease in neutrophil infiltration.
infection. However, due to our short observation period, tality due to meningitis is usually secondary to brain we cannot rule out the possibility that hypothermia may herniation within the initial 12 h following admission and adversely affect bacterial clearance from the subarachnoid initiation of medical treatment [27,31,46,47].
space. In this regard, it is crucial to note that delayed We conclude that the initiation of moderate hypothermia sterilization of the CSF in meningitis has been associated at the time of antibiotic therapy in an animal model of with an adverse outcome [38]. Hypothermia also has been bacterial meningitis modulates the inflammatory response. found to interfere with the up-regulation interleukin-1b Hypothermia produces an attenuation of blood–brain after traumatic brain injury [21,24]. Interleukin-1b medi- barrier disruption, nitric oxide activation and decreases ates the increase in nerve growth factor, an endogenous myeloperoxidase activity. Improvement in the increase in neuroprotective factor. Thus, raising the possibility that by intracranial pressure with preservation of cerebral perfu-suppressing the inflammatory cascade may also hinder the sion pressure accompanies treatment with moderate
hypo-reparative process [21]. thermia. Further studies to investigate whether these
Tau proteins are structural microtubule binding proteins effects are transitory and whether hypothermia ultimately primarily localized in the axonal compartment of neurons. affects morbidity and mortality should be entertained. Functionally, Tau binds to axonal microtubules resulting in
the formation of axonal microtubule bundles. Loss of
axonal microtubules resulting from direct or indirect injury Acknowledgements to central nervous system axons is a common feature of
several types of brain injury [64,66]. This loss of axonal This work was supported by a grant from The Chil-microtubules following injury releases intracellular micro- dren’s Medical Center Research Foundation, Dayton OH. tubule binding proteins, such as Tau, into the extracellular The author’s would also like to thank Roche Laboratories space, where they are transported to the CSF.CSF levels of for the gift of the ceftriaxone used in this study.
a cTau reflect axonal damage after traumatic head injury [65]. We found that cTau protein is elevated in CSF during meningitis. There is no previous data that allows any
References
speculation on the relationship between the time or
severi-ty of meningitis and the appearance of cTau. The post-hoc G
[1] F.R. Amaee, S.D. Comis, M.P. Osborne, N -methyl-L-arginine
nature of this part of the study only allow us to conclude
protects the guinea pig cochlea from the cytotoxic effects of
that cTau is elevated in meningitis and further studies are pneumolysin, Acta Otolaryngol. 115 (1995) 386–391.
necessary to see if it could be a utilized as a biological [2] K. Angstwurm, S. Reub, D. Freyer, G. Arnold, U. Dirnagl, R. Schumann, J. Weber, Induced hypothermia in experimental
pneumo-marker of neurological injury in meningitis. Another
coccal meningitis, J. Cereb. Blood. Flow. Metab. 20 (2000) 834–
limitation is that the monoclonal antibodies were
de-838.
veloped against human protein. Based on our results we
[3] M. Arditi, L. Ables, R. Yogev, H. Cerebrospinal fluid endotoxin
feel that sufficient cross reactivity exists to support our levels in children with, Influenzae meningitis before and after
conclusion. administration of intravenous ceftriaxone, J. Infect. Dis. 6 (1989)
1005–1011.
In an attempt to make this study more clinically relevant
[4] M. Arditi, E.O.J. Mason, J.S. Bradley, T.Q. Tan, W.J. Barson, G.E.
the application of hypothermia occurred following the
Schutze, E.R. Wald, L.B. Givner, K.S. Kim, R. Yogev, S.L. Kaplan,
administration of antibiotics in a live-bacterial model.
Three-year multicenter surveillance of pneumococcal meningitis in
Because the application of hypothermia is likely to be children: clinical characteristics, and outcome related to penicillin reserved for only the most severely affected patients our susceptibility and dexamethasone use, Pediatrics 5 (1998) 1087–
1097.
model used a substantial bacterial inoculum. Further,
[5] L.J. Baraff, S.I. Lee, D.L. Schriger, Outcomes of bacterial
meningi-antibiotic therapy was used to stimulate an increase in the
tis in children: a meta-analysis, Pediatr. Infect. Dis. J. 12 (1993)
inflammatory response and the application of hypothermia
389–394.
was used to coincide with the time that the inflammatory [6] F.C. Barone, L.M. Hillegass, W.J. Price, R.F. White, E.V. Lee, G.Z. response was greatest. The results of this study suggest a Feuerstein, H.M. Sarau, R.K. Clark, D.E. Griswold, Polymorphonu-clear leukocyte infiltration into cerebral focal ischemic tissue:
beneficial short-term effect of the application of
hypo-myeloperoxidase activity assay and histologic verification, J.
Neuro-thermia in severe bacterial meningitis.
sci. Res. 29 (1991) 336–345.
Mortality from meningitis remains between 7 and 20%
[7] F.C. Barone, L.M. Hillegass, M.N. Tzimas, D.B. Schmidt, J.J.
despite advances in antibiotic therapy [4,54]. Additionally, Foley, R.F. White, W.J. Price, G.Z. Feuerstein, R.K. Clark, D.E. significant neurologic sequelae occur in greater than 20% Griswold, Time-related changes in myeloperoxidase activity and
of survivors [5]. At the time a patient arrives for medical leukotriene B4 receptor binding reflect leukocyte influx in cerebral focal stroke, Mol. Chem. Neuropathol. 24 (1995) 13–30.
attention, the conventional treatment of using antibiotics
[8] E.A.J. Bering, Effect of body temperature change on cerebral
induces a massive release of bacterial products with a
oxygen consumption of the intact monkey, Am. J. Physiol. 200
subsequent exacerbation of inflammation and resultant (1961) 417–419.
secondary brain damage [3,58]. This rapid exacerbation of [9] S.M. Black, M.A. Bedolli, S. Martinez, J.D. Bristow, D.M. Ferriero,
to regions of selective vulnerability to hypoxia-ischaemia in the [29] J. Irazuzta, J. Olson, M.P. Kiefaber, H. Wong, Hypothermia at-developing rat brain, Neurobiol. Dis. 2 (1995) 145–155. tenuates excitatory neurotransmitter release in meningitis, Brain Res. [10] J. Bohnsack, S. Akiyama, C.H. Damsky, W. Knape, G. Zimmerman, 847 (1999) 143–148.
Human neutrophil adherence to laminin in vitro, J. Exp. Med. 171 [30] B. Kelly, J. Luce, Current concepts in cerebral protection, Chest 103
(1990) 1221–1237. (1993) 1246–1254.
[11] K. Boje, Inhibition of nitric oxide synthase attenuates blood–brain [31] T. Kilpi, M. Anttila, M.J.T. Kallio, H. Peltola, Length of pre-barrier disruption during experimental meningitis, Brain Res. 720 diagnostic history related to the course and sequelae of childhood (1996) 75–83. bacterial meningitis, Pediatr. Infect. Dis. J. 12 (1993) 184–188. [12] A.V. Buryakova, M.S. Cand, I.A. Sytinsky, Amino acid composition [32] Y.S. Kim, R.A. Sheldon, B.R. Elliott, Q. Liu, D.M. Ferriero, M.G.
of cerebrospinal fluid in acute neuroinfections in children, Arch. Tauber, Brain injury in experimental neonatal meningitis due to Neurol. 32 (1975) 28–31. group B streptococci, J. Neuropathol. Exp. Neurol. 54 (1995) [13] B. Buster, A. Weintrob, B. Townsend, W. Scheld, Potential role of 531–539.
nitric oxide in the pathophysiology of experimental bacterial menin- [33] Y.S. Kim, M.G. Tauber, Neurotoxicity of glia activated by gram-gitis in rats, Infection and Immunity 63 (1995) 3835–3839. positive bacterial products depends on nitric oxide, Infect. Immun. [14] R. Busto, W.D. Dietrich, Y.T. Globus, I. Valdes, P. Scheinberg, M.D. 64 (1996) 3148–3153.
Ginsberg, Small differences in intraischemic brain temperature
[34] U. Koedel, A. Bernatowicz, R. Paul, K. Frei, A. Fontana, H.W. critically determine the extent of ischemic neuronal injury, J. Cereb.
Pfister, Experimental pneumococcal meningitis: cerebrovascular Blood Flow Metab. 7 (1987) 729–738.
alterations, brain edema, and meningeal inflammation are linked to [15] R. Busto, M.T.-T. Globus, D. Dietrich, E. Martinez, I. Valdes, M.D.
the production of nitric oxide, Annals Neurol. 37 (1995) 313–323. Ginsberg, Effect of mild hypothermia on ischemia-induced release
[35] U. Koedel, H.W. Pfister, Oxidative stress in bacterial meningitis, of neurotransmitters and free fatty acids in rat brain, Stroke 20
Brain Pathol. 9 (1999) 57–67. (1989) 904–910.
[36] R.F. Kornelisse, K. Hoekman, J.J. Visser, W.C.J. Hop, J.G.M. [16] L. Canevary, A. Console, E.A. Tendi, J.B. Clarck, T.A. Bates, Effect
Huijmans, P.J.C. van der Straaten, A.J. van der Jeijden, R.N. Sukhai, of postischaemic hypothermia on the mitochondrial damage induced
H.J. Neijens, The role of nitric oxide in bacterial meningitis in by ischaemia and reperfusion in the gerbil, Brain Res. 817 (1999)
children, J. Infec. Dis. 174 (1996) 120–126. 341–345.
[37] F. Lahrtz, L. Piali, K.S. Spanaus, J. Seebach, A. Fontana, [17] R.S.B. Clark, T.M. Carlos, J.K. Schiding, M. Bree, L.A. Fireman,
Chemokines and chemotaxis of leukocytes in infectious meningitis, S.T. DeKosky, P.M. Kochanek, Antibodies against Mac-1 attenuate
J. Neuroimmunology 85 (1998) 33–43. neutrophil accumulation after traumatic brain injury in rats, J.
[38] M.H. Lebel, G.H. McCracken Jr, Delayed cerebrospinal fluid Neurotrauma 13 (1996) 333–341.
sterilization and adverse outcome of bacterial meningitis in infants [18] G.L. Clifton, J.Y. Jiang, B.G. Lyeth, L.W. Jenkins, R.J. Hamm, R.L.
and children, Pediatrics 83 (1989) 161–167. Hayes, Marked protection by moderate hypothermia after
ex-[39] D. Marion, Therapeutic moderate hypothermia for severe traumatic perimental traumatic brain injury, J. Cereb. Blood Flow Metab. 11
brain injury, J. Intensive Care Med. 12 (1997) 239–248. (1991) 114–121.
[40] D.W. Marion, L.E. Penrod, S.F. Kelsey, W.D. Obrist, P.M. Kochanek, [19] C.S. Cobbs, A. Fenoy, D.S. Bredt, L.J. Noble, Expression of nitric
A.M. Palmer, S.R. Wisniewski, S.T. DeKosky, Treatment of oxide synthase in the cerebral microvasculature after traumatic brain
traumatic brain injury with moderate hypothermia, NEJM 336 injury in rats, Brain Res. 751 (1997) 336–338.
(1997) 540–546. [20] B.J. Darakchiev, M. Itkis, T. Agajanova, A. Itkis, R.J. Hariri,
[41] H. Minamisawa, C.H. Nordstrom, M.L. Smith, B.K. Siesjo, The Changes of MPO activity and brain water accumulation in traumatic
influence of mild body and brain hypothermia on ischemic brain brain injury experiments, Acta Neurochir. 70 (1997) 98–101.
damage, J. Cereb. Blood Flow Metab. 10 (1990) 365–374. [21] S.T. DeKosky, S.D. Styren, M.E. O’Malley, J.R. Goss, P. Kochanek,
[42] K. Moller, F. Larsen, J. Qvist, J. Wandall, G. Knudsen, I. Gjorup, P. D. Marion, C.H. Evans, P.D. Robbins, Interleukin-1 receptor
antago-Skinhoj, Dependency of cerebral blood flow on mean arterial nist suppresses neurotrophin response in injured rat brain, Ann.
pressure in patients with acute bacterial meningitis, Crit. Care Med. Neurol. 39 (1996) 123–127.
28 (2000) 1027–1032. [22] W.D. Dietrich, O. Alonso, R. Busto, M.Y.T. Globus, M.D. Ginsberg,
[43] J. Muizelaar, A. Marmarou, J. Ward, H. Kontos, S. Choi, D. Becker, Post-traumatic brain hypothermia reduces histopathological damage
H. Gruemer, H. Young, Adverse effects of prolonged hyperventila-following concussive brain injury in the rat, Acta Neuropathol. 87
tion in patients with severe head injury: a randomized clinical trial, (1994) 250–258.
J. Neurosurg. 75 (1991) 7310–7739. [23] P. Fouret, R.M. duBois, J.K. Bernaudin, H. Takahashi, V.J. Ferrans,
[44] M.M. Mustafa, O. Ramilo, K.D. Olsen, P.S. Franklin, E.J. Hansen, R.G. Crystal, Expression of the neutrophiles elastase gene during
B. Beutler, G.H. McCracken Jr, Tumor necrosis factor in mediating human bone marrow cell differentiation, J. Exp. Med. 169 (1989)
experimental haemophilus influenzae type B meningitis, J. Clin. 833–845.
Invest. 84 (1989) 1253–1259. [24] J.R. Goss, S.D. Styren, P.D. Miller, P.M. Kochanek, A.M. Palmer,
[45] E.M. Nemoto, R. Klementavicius, J.A. Melick, H. Yonas, Suppres-D.W. Marion, S.T. DeKosky, Hypothermia attenuates the normal
sion of cerebral metabolic rate for oxygen (CMRO2) by mild increase in interleukin 1B RNA and nerve growth factor following
hypothermia compared to thiopental, J. Neurosurg. Anesthesiol. 8 traumatic brain injury in the rat, J. Neurotrauma 12 (1995) 159–167.
(1996) 52–59. [25] J.F. Hansbrough, T. Wikstrom, M. Braide, M. Tenehaus, O.H.
[46] G. Rennick, F. Shann, J. de Campo, Cerebral herniation during Rennekampee, V. Kiessig, L.M. Bjursten, Neutrophil activation and
bacterial meningitis in children, Br. Med. J. 306 (1993) 953–955. tissue neutrophil sequestration in a rat model of thermal injury, J.
[47] R.H. Rischbieth, Pneumococcal meningitis — A killing disease, Surg. Res. 61 (1996) 17–22.
Med. J. Aust. 47 (1960) 578–581. [26] L. Horowitz, D. Kaufman, Y. Kong, An antibody to leukocyte
[48] M. Rosner, S. Daughton, Cerebral perfusion pressure management integrins attenuates coronary vascular injury due to ischemia and
in head injury, J. Trauma 30 (1990) 933–939. reperfusion in dogs, Am. J. Physiol. 272 (1997) H614–H618.
[27] S.J. Horwitz, B. Boxerbaum, J. O’Bell, Cerebral herniation in [49] M. Rosner, S. Rosner, A. Johnson, Cerebral perfusion pressure: bacterial meningitis in childhood, Ann. Neurol. 7 (1980) 524–528. management protocol and clinical results, J. Neurosurg. 83 (1995) [28] J. Irazuzta, M. Mirkin, B. Zingarelli, Mercaptoethylguanidine at- 949–962.
tenuates inflammation in bacterial meningitis in rabbits, Life Sci. 67 [50] H. Rosomoff, A study of experimental brain injury during
[51] M.E. Rowin, V. Xue, J. Irazuzta, Integrin expression on neutrophils meningitis in rabbits treated with monoclonal antibodies against in a rabbit model of Group B Streptococcal meningitis, Inflamma- adhesion promoting receptors of leukocytes, J. Exp. Med. 170
tion 23 (2000) 23–23. (1989) 959–968.
[52] M.E. Rowin, V.W. Xue, J. Irazuzta, Hypothermia attenuates b1 [61] N.B. Vedder, R.K. Winn, C.L. Rice, Y. Chi, K.E. Arfors, J.M. Harlan, integrin expression on neutrophils in an animal model of meningitis, A monoclonal antibody to the adherence-promoting leukocyte Pediatric Res. 45 (1999) 173. glycoprotein CD-13 reduces organ injury and improves survival [53] X. Saez-Llorens, O. Ramilo, M.M. Mustafa, J. Mertsola, G.H. from hemorrhagic shock and resuscitation in rabbits, J. Clin. Invest.
McCracken Jr., Molecular pathophysiology of bacterial meningitis: 81 (1988) 939–944.
current concepts and therapeutic implications, J. Pediatr. 5 (1990) [62] F. Welsh, R. Sims, V. Harris, Mild hypothermia prevents ischemic
671–684. injury in gerbil hippocampus, J. Cereb. Blood Flow Metab. 10
[54] A. Schuchat, K. Robinson, J.D. Wenger, L.H. Harrison, M. Farley, (1990) 557–563. A.L. Reingold, L. Lefkowitz, B.A. Perkins, Bacterial meningitis in
[63] M.J. Whalen, T.M. Carlos, R.S.B. Clark, D.W. Marion, M.S.T. the United States in 1995, NEJM 337 (1997) 970–976.
DeKosky, S. Heineman, J.K. Schiding, F. Memarzadeh, C.E. Dixon, [55] T. Shiozaki, H. Sugimoto, M. Taneda, H. Yoshida, A. Iwai, T.
P.M. Kochanek, The relationship between brain temperature and Yoshioka, T. Sugimoto, Effect of mild hypothermia on
uncontroll-neutrophil accumulation after traumatic brain injury in rats, Acta able intracranial hypertension after severe head injury, J. Neurosurg.
Neurochir. S70 (1997) 260–261. 79 (1993) 363–368.
[64] F.P. Zemlan, T. Campbell, G.E. Dean, Cerebrospinal fluid levels of [56] S.L. Smith, E.D. Hall, Mild pre- and post-traumatic hypothermia
tau protein in Alzheimer’s disease and CNS injury, Neurosci. Abstr. attenuates blood–brain barrier damage following controlled cortical
23 (1997) 2220. impact injury in the rat, J. Neurotrauma 13 (1996) 1–9.
[65] F.P. Zemlan, W.S. Rosenberg, G.E. Dean, P. Luebbe, T.A. Campbell, [57] M.N. Swartz, Bacterial meningitis, NEJM 14 (1984) 912–914.
Quantification of axonal damage in traumatic brain injury: Affinity [58] M.G. Tauber, A.M. Shibl, C.J. Hackbarth, J.W. Larrick, M.A. Sande,
purification and characterization of cerebrospinal fluid tau proteins, Antibiotic therapy, endotoxin concentration in cerebrospinal fluid,
J. Neurochem. 72 (1999) 741–750. and brain edema in experimental Escherichia Coli meningitis in
[66] F.P. Zemlan, W.S. Rosenberg, Characterization of antibodies recog-rabbits, J. Infect. Dis. 3 (1987) 456–462.
nizing cerebrospinal fluid structural proteins, in press (2000). [59] S. Tucci, C. Pinto, J. Goyo, P. Rada, L. Hernandez, Measurement of
glutamine and glutamate by capillary electrophoresis and laser [67] B. Zingarelli, M. O’Connor, H. Wong, A.L. Salzman, C. Szabo, induced fluorescence detection in cerebrospinal fluid of meningitis Peroxynitrite mediated DNA strand breakage activates poly-ADP sick children, Clin. Biochem. 31 (1998) 143–150. ribosyl synthetase and causes cellular energy depletion in macro-[60] E.I. Tuomanen, K. Saukkonen, S. Sande, C. Cioffe, S.D. Wright, phages stimulated with bacterial lipopolysaccharide, J. Immunol.