www.elsevier.com/locate/ibmb
Characterization and developmental regulation of tyramine-
β
-hydroxylase in the CNS of the moth, Manduca sexta
Herman K. Lehman
a,*, Cristina M. Murgiuc
b, John G. Hildebrand
baDepartment of Biological Sciences, Hamilton College, Clinton, NY 13323, USA
bArizona Research Laboratories, Division of Neurobiology, University of Arizona, Tucson, AZ 85721-0077, USA
Abstract
Octopamine (OA) is present in insect nervous tissue, but little is known about its biosynthesis. In the CNS of Manduca sexta, OA levels increase markedly during postembryonic adult development. To study this increase, we developed an assay for
tyramine-β-hydroxylase, the putatively rate-limiting enzyme for OA biosynthesis. Tyramine-β-hydroxylase activity in extracts of M. sexta CNS tissue: (1) was time- and protein-dependent, and with protein concentrations up to 2µg/µl, was linear for 20 min; (2) had a pH optimum of 7.0 for conversion of tyramine to OA; (3) required ascorbate, copper, and catalase; and (4) had an apparent KM,
tyramineof 0.22±0.04 mM. These characteristics resemble those of the mammalian enzyme dopamine-β-hydroxylase, suggesting that
these two enzymes are functionally related. During adult development, tyramine-β-hydroxylase activity increased 11-fold in the brain and 9-fold in the abdominal ganglia, paralleling increases in OA levels in those CNS structures during metamorphosis. The apparent kinetic constants of tyramine-β-hydroxylase suggested that the amount of this enzyme present in the tissues increases. The increase in OA levels during adult development thus appears to be due to an increase in the level of enzyme available for OA synthesis and may reflect an increase in the number of octopaminergic neurons. 2000 Published by Elsevier Science Ltd. All rights reserved.
Keywords: Insect; Biogenic amines; Tyramine; Neurotransmitter synthesis; Tyramine-β-hydroxylase; Dopamine-β-hydroxylase
1. Introduction
Neuromodulation of CNS function plays fundamental roles in the behavior of insects and other animals. Octo-pamine (OA) is one of the most abundant neuromodula-tors in the arthropod nervous system, and its high levels reflect a wide distribution and broad spectrum of actions (Bodnaryk, 1980; Na¨ssel and Laxmyr, 1983; Evans, 1985; Davenport and Wright, 1986; Sloley and Okikaza, 1988; Nagao and Tanimura, 1988; Linn and Roelofs, 1993). In insects, OA has been shown to be involved in various peripheral functions, including control of the heart and visceral muscle contractions, control of
phero-Abbreviations: DβH, dopamine-β-hydroxylase; DUM, dorsal unpaired median; ETH, ecdysis-triggering hormone; OA, octopamine; PNMT, phenylethanolamine-N-methyl transferase; SAM, S-adenosyl-l -meth-ionine; TβH, tyrosine-β-hydroxylase; TES, N-tris[hydroxymethyl]me-thyl-2-aminoethanesulfonic acid; VUM, ventral unpaired midline.
* Corresponding author. Tel.:+1-315-859-4298; fax:+ 1-315-859-4807.
E-mail address: [email protected] (H.K. Lehman)
0965-1748/00/$ - see front matter2000 Published by Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 1 1 - 4
mone biosynthesis and perception, modulation of flight-muscle activity, and control of the release of neurohor-mones (reviewed by: David and Coulon, 1985; Evans, 1985; Orchard et al., 1993; Homberg, 1994). The actions of OA within the CNS are similarly diverse, and this amine has been implicated in such functions as olfactory learning and dishabituation (Dudai et al., 1987; Bacon et al., 1995). The levels of OA in the CNS are not constant, however, but increase during metamorphosis (Bodnaryk, 1980). In holometabolous insects, this increase in the level of OA may be related to the increased behavioral activity that is characteristic of adult insects.
levels of neurotransmitters such as OA. In order to understand the mechanisms controlling the levels of this amine, we have investigated its biosynthesis in the sphinx moth, Manduca sexta.
OA is biosynthesized from tyrosine in the insect ner-vous system, and the studies of Livingstone and Temple (1983) suggested that OA biosynthesis requires two enzymatic steps: decarboxylation of tyrosine by tyrosine decarboxylase to form tyramine, followed by hydroxyl-ation of tyramine by tyramine-β-hydroxylase (TβH) to produce OA. Little information is available about either of these enzymes in insects. Tyrosine decarboxylase activity has not been characterized or identified in any arthropod, but it appears to be distinct from DOPA decarboxylase, a well-studied enzyme required for the conversion of l-DOPA to dopamine in the insect ner-vous system and cuticle (Livingstone and Temple, 1983; Hirsh, 1989). In the only biochemical characterization of TβH, Wallace (1976) showed that extracts of nervous tissue from the American lobster, Homarus americanus, can hydroxlyate tyramine to produce OA, and that this conversion is dependent upon pH, ascorbic acid, copper, and catalase. These properties suggested that TβH is similar to dopamine-β-hydroxylase (DβH), which in mammals is required for the hydroxylation of dopamine to form norepinephrine. Indeed, Wallace (1976) demon-strated that purified lobster TβH can produce norepi-nephrine via the β-hydroxlyation of dopamine. More-over, DβH exhibits relatively broad substrate specificity and can hydroxylate tyramine to form OA (Creveling et al., 1962; Goldstein and Contrera, 1962; reviewed by Kaufman and Friedman, 1965). These results indicate that lobster TβH is functionally related to mammalian DβH.
In this study we focused on TβH because it is the last and putatively rate-limiting enzyme in the biosynthetic pathway leading to OA. We document that, as in Mames-tra configurata (Bodnaryk, 1980), levels of OA increase during metamorphosis in M. sexta. To determine if this OA increase is associated with an increased rate of syn-thesis, we characterized TβH activity in extracts of M. sexta CNS tissue and showed that during metamor-phosis, there is a direct correlation between increased levels of TβH activity and levels of OA. Based upon apparent TβH kinetic constants measured in extracts of late-larval and adult CNS tissue, we conclude that increased levels of TβH protein account for the increase in TβH activity during metamorphosis. The possible cellular and molecular mechanisms that may be respon-sible for a long-term increase in TβH are discussed.
2. Materials and methods
2.1. Experimental animals
Larvae of M. sexta (Lepidoptera: Sphingidae) were reared on an artificial diet (modified from that of Bell
and Joachim, 1976) and maintained on a long-day photo-period regimen (17L:7D) at 25–26°C and 50–60% rela-tive humidity. Pharate adults were staged according to previously published criteria (Sanes and Hildebrand, 1976; Tolbert et al., 1983), with adult development occurring in 18 stages and completed about 21 days after pupation.
2.2. Reagents
Radiochemicals, [ring-3H]tyramine and S-[methyl-3
H]adenosyl-l-methionine (SAM), were purchased from New England Nuclear (Boston, MA), and OA and N-ethylmaleimide were obtained from Research Biochemi-cals Inc. (Natick, MA). Unless otherwise noted, all other chemicals were reagent grade, and those products as well as catalase and phenylethanolamine-N-methyl transfer-ase (PNMT) used in assays were purchtransfer-ased from Sigma Chemical Co. (St. Louis, MO).
2.3. Protein assay
The amount of soluble protein in samples was esti-mated using a bicinchoninic acid kit obtained from Pierce Chemical Co. (Rockford, IL). The kit was used according to the manufacturer’s instructions, and bovine serum albumin was used to prepare the standard curves.
2.4. Octopamine assay
The assay used to estimate OA concentrations in sol-ution was modified from the procedures of Molinoff et al., 1979. Brains (comprising both the supraoesophageal ganglion, including optic lobes, and the suboesophageal ganglion) and abdominal ganglia (AG; third, fourth, fifth, and terminal abdominal ganglia) were dissected at various stages of adult development and immersed in 80% ice-cold acetone. Each tissue sample was homogen-ized, centrifuged (10 min, 10,000 rpm), and re-extracted with aqueous acetone. The supernatant solution was con-centrated to dryness in a vacuum centrifuge (Savant Industries, Farmingdale, NY) and resuspended in 100µl of 0.1 M Tris buffer (pH 8.6). PNMT (25 units/mg; 0.2 units) in 0.1 M Tris was added to each tube along with 5.0 µCi of [methyl-3H]SAM (55–85 Ci/mmol). The
tubes were incubated for 2 h at 37°C. The reaction was stopped by the addition of 200µl of 0.5 M sodium borate (pH 10). The N-[3H]methyl OA (i.e. [3H]synephrine)
con-ditions described below. More than 90% of the radioac-tivity extracted with toluene–isoamyl alcohol co-eluted with synephrine. Unknowns were compared to a stan-dard curve produced with OA stanstan-dards. The curve was linear (r=0.95) over the range 0.1–2.5 pmol.
2.5. Enzyme extracts
CNS tissue from M. sexta was dissected under ice-cold normal saline solution (150 mM NaCl, 3 mM KCl, 3 mM CaCl2, 20 mM MgCl2, and 10 mM
N-tris[hyd-roxymethyl]methyl-2-aminoethanesulfonic acid (TES), pH 6.9; Christensen et al., 1991), frozen on dry ice, and stored at 280°C. This material was kept for up to 2 weeks without loss of enzyme activity. Tissue was thawed immediately prior to use, placed in a ground-glass homogenizer containing normal saline solution with catalase (19,000 units/mg; 10.0 mg/ml), and homo-genized with 20 strokes by hand. The homogenate was centrifuged (10,000 rpm, 10 min) at 4°C, and the resulting supernatant fraction was used as the crude enzyme source.
2.6. Tyramine-b-hydroxylase assay
The assay we developed to measure TβH activity was based upon the hydroxylation of [ring-3H]tyramine to
form [ring-3H]OA. First, [ring-3H]tyramine (20–40
Ci/mmol) was pre-purified on a C-18 HPLC column (Nova-Pak, Waters Assoc., Milford, MA) by isocratic elution with a solution of 10% methanol, 0.1 M potass-ium phosphate, and 5.0 mM octanesulfonic acid (pH 3.8). Radioactivity co-eluting with tyramine was col-lected, desalted with a Sep-Pak (Waters Assoc., Milford, MA), and concentrated on a vacuum concentrator. Then 5.0 µCi of [ring-3H]tyramine was added to 90 µl of an
enzyme reaction mixture containing (in final concentrations): 0.1 M potassium phosphate (pH 6.9), 1.0 mg catalase, 0.1 mM N-ethylmaleimide, 0.05 mM CuSO4, 5.0 mM disodium fumarate, and 5.0 mM
ascor-bic acid. The concentration of tyramine used in most assays was lower than that required to saturate the enzyme, but these levels of [ring-3H]tyramine offered
the highest sensitivity and therefore were used in assays necessary to characterize the enzyme. Greater concen-trations of tyramine (3.5 times the KM) were used to
esti-mate the levels of TβH during development (see below). The reaction was initiated by the addition of 100µg (to give a final protein concentration of 1µg/µl) of the crude enzyme (tissue homogenate), and then the mixture was incubated at room temperature (ca. 22°C) for 15 min without shaking. Background radioactivity was determ-ined by using an enzyme mixture containing heat-inacti-vated enzyme. The reaction was stopped by boiling the incubation mixture for 5 min. It then was centrifuged for 5 min at 14,000 rpm, the supernatant solution was
collected, and the reaction products were separated and identified by C-18 reverse-phase HPLC as described above. The solvent was pumped at a rate of 1 ml/min, and 1 min fractions were collected. Each fraction was combined with 5 ml of scintillation fluid (ScintiVerse LC, Fisher Scientific, Pittsburgh, PA), and radioactivity was quantified by scintillation counting.
The identity of [3H]OA produced in the TβH assay
was verified by three methods. First, the HPLC elution times of radioactive enzyme products were compared to the elution time of synthetic OA added to the enzyme reaction mixture immediately prior to termination of the reaction; detection was by UV absorbance (223 nm). Second, [3H]OA produced in the TβH assay was
methyl-ated by incubating the purified, desalted [3H]OA with
0.2 units of PNMT and 5.0 µCi of [methyl-3H]SAM for
2 h at 37°C. The reaction was stopped by boiling, and the elution time of the reaction product was then com-pared to that of synthetic N-methyl OA (synephrine) by HPLC using the column and solvents described above. Third, [3H]OA produced in the TβH assay was oxidized
with NaIO4to form p-hydroxybenzaldehyde, which then
was detected with sulfanilic acid (Touchstone and Dob-bins, 1983) as follows: the [3
H]OA was collected from HPLC, Sep-Pak purified, dried, resuspended in 20 µl of H2O, and incubated with 20µl of 2% NaIO4 for 30 min
at room temperature; the reaction was stopped by addition of 20 µl of 10% sodium metabisulfite, silica TLC plates (LK-6, Whatman) were spotted with 20 µl of the reaction mixture, and finally the TLC plates were developed in n-butanol/acetic acid/water (4:1:2), dried, scraped into scintillation vials, combined with scintil-lation fluid, and assayed by scintilscintil-lation counting. The Rf values of radioactivity produced by treatment with
NaIO4were compared to the mobilities of synthetic OA,
tyramine, and p-hydroxybenzaldehyde detected with sul-fanilic acid.
Kinetic parameters of the crude enzyme for tyramine were determined from the data in Fig. 5. The data were displayed as double-reciprocal plots, and estimates of Vmax and KM were obtained from the slopes and
inter-cepts of the straight lines generated in these plots.
2.7. Developmental studies
Levels of TβH in the developing brain and abdominal ganglia were estimated by assaying enzyme activity in homogenates of CNS tissue at various stages of adult development. Brains and abdominal ganglia were dis-sected separately from developing adults at stages P2, P6, P10, P14, and P18 and subjected to the TβH assay procedure. Homogenates were processed as described above, 1µg/µl of protein was added to the TβH reaction mixture containing 0.76 mM [ring-3H]tyramine (0.007
developmental stage was quantified by HPLC and scin-tillation counting.
3. Results
3.1. Tyramine-b-hydroxylase assay
We developed an assay for TβH activity as a first step in an analysis of mechanisms that are responsible for the increases in the levels of OA in the CNS during adult development. Incubation of [ring-3H]tyramine with
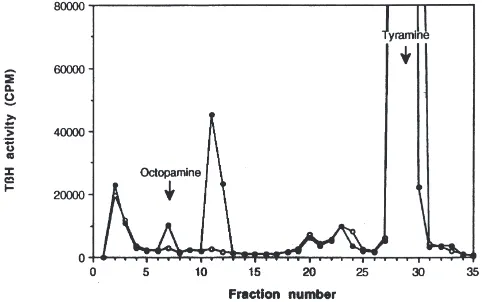
crude brain extracts resulted in the formation of two radiolabeled products that were distinguished by reverse-phase HPLC (Fig. 1). The total amount of radioactivity collected from these two fractions was less than 5% of that in the [ring-3H]tyramine used as substrate. The
identity of the compound that eluted in HPLC fraction 7 was determined by comparing its elution time to that of unlabeled OA (Figs. 1 and 2). In addition, the elution of the methylated fraction 7 was compared to the elution of N-methyl OA (synephrine) on HPLC, and the mobility of fraction 7 treated with sodium periodate was compared to the mobility of p-hydroxybenzaldehyde on TLC (data not shown). In each instance the product reco-vered from HPLC fraction 7 was indistinguishable from synthetic OA.
The conversion of tyramine to OA by brain homogen-ates depended upon incubation time, protein concen-tration, and pH (Fig. 3). Production of [3H]OA was linear
for at least 20 min (Fig. 3A). Little [3H]OA synthesis
was detected at protein concentrations below 0.2 µg/µl, but at higher protein concentrations (0.5–2.0 µg/µl), a linear rate of synthesis of [3H]OA was observed. At
pro-tein concentrations greater than 2.0µg/µl, additional pro-duction of [3H]OA was not detected, and the enzymatic
Fig. 1. Reverse-phase HPLC radiochromatograms of the enzymatic products from incubation of M. sexta CNS extracts with [ring-3
H]tyra-mine. Arrows indicate the elution of synthetic OA and tyramine detected by UV absorbance (223 nm).I: Radiochromatogram of the
enzymatic products from untreated nervous system extracts.s:
Com-pounds recovered from boiled enzyme reaction.
Fig. 2. Identification of OA as a product of a standard tyramine-β -hydroxylase assay. Following a standard tyramine-β-hydroxylase assay, 10µl of 1025M synthetic OA was added to the reaction
pro-ducts and the mixture was separated by reverse-phase HPLC. Each fraction was tested for the presence of OA using PNMT and
[methyl-3H]SAM as described in the text.I: Radiochromatogram of the
enzy-matic products from untreated CNS extracts.s: Radiochromatogram
of the enzymatic products from PNMT and [methyl-3H]SAM.
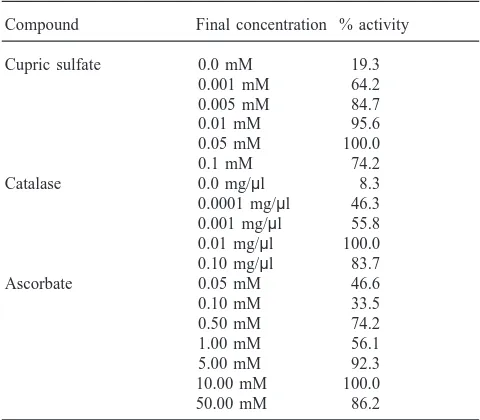
reaction appeared to be substrate-limited (Fig. 3B). The optimum pH for the conversion of tyramine to OA was 7.0 (Fig. 3C). TβH activity also depended on the pres-ence of copper, catalase, and ascorbate (Table 1). Copper concentrations greater than 0.001 mM were required for activity, and maximal activity was achieved with 0.05 mM cupric sulfate. Metal-complexing agents, including 1.0 µM diethyldithiocarbamate and 0.4 mM KCN, reduced enzyme activity 95% (data not shown). Catal-ase, at concentrations in the range 0.0001–0.1 mg/µl, was required for TβH activity; the optimal concentration was 0.01 mg/µl. Finally, maximal TβH activity was measured in enzyme incubation mixtures containing 5.0 mM ascorbic acid; higher concentrations appeared to be saturating.
In addition to OA, one other enzymatic product was routinely detected in the TβH assay. This unidentified product, which eluted in HPLC fractions 11 and 12, was re-incubated with 1.0 µg/µl of brain homogenate for 15 min at room temperature, and the reaction mixture was analyzed by HPLC to determine if its radioactivity could contribute to the radioactivity co-eluting with OA. The retention times of the metabolites of fractions 11 and 12 were compared to the retention times of OA and tyram-ine. Under these conditions, the unidentified product did not co-elute with OA. In similar experiments, [3H]OA
Fig. 3. Effects of incubation time, protein concentration, and pH on activity of M. sexta tyramine-β-hydroxylase. The reaction mixture contained 5.0µCi [ring-3H]tyramine, 1.0 mg catalase, 0.1 mM N-ethylmaleimide, 0.05 mM CuSO
4, 5.0 mM disodium fumarate, and 5.0 mM ascorbic acid.
Hydroxylase activity was estimated by the standard assay procedure as described in Materials and methods except for the parameter under study. (A) Incubation time varied from 0 to 20 min. (B) Protein concentration varied from 25 to 500 µg/100µl. (C) Constant-ionic-strength sodium phosphate buffer was used over the pH range 4–8.
Table 1
Requirements for tyramineβ-hydroxylase activity
Compound Final concentration % activity
Cupric sulfate 0.0 mM 19.3
0.001 mM 64.2 0.005 mM 84.7
0.01 mM 95.6
0.05 mM 100.0
0.1 mM 74.2
Catalase 0.0 mg/µl 8.3
0.0001 mg/µl 46.3 0.001 mg/µl 55.8 0.01 mg/µl 100.0 0.10 mg/µl 83.7
Ascorbate 0.05 mM 46.6
0.10 mM 33.5
0.50 mM 74.2
1.00 mM 56.1
5.00 mM 92.3
10.00 mM 100.0 50.00 mM 86.2
3.2. Tyramine-b-hydroxylase kinetics
The rate of synthesis of [3
H]OA was a function of the concentration of tyramine in the incubation mixture. The apparent KMfor tyramine, calculated from reaction
mix-tures of stage-18 brains and abdominal ganglia, was 0.22±0.04 mM, and the calculated Vmaxwas 17.86±1.85
pmol/min/mg protein (Fig. 4A). The apparent KM for
ascorbate was 2.75 mM. With reaction mixtures contain-ing stage-P0 CNS homogenates, the apparent KMfor
tyr-amine was 0.30±0.05 mM, and the calculated Vmaxwas
2.05±0.14 pmol/min/mg protein (Fig. 4B). Although the apparent Vmax of the stage-P18 homogenates was
sig-nificantly greater than that of stage-P0 homogenates, the apparent tyramine KM values estimated with the two
homogenates were not significantly different (Student’s t-test, P,0.05).
3.3. Developmental changes in tyramine-b-hydroxylase activity
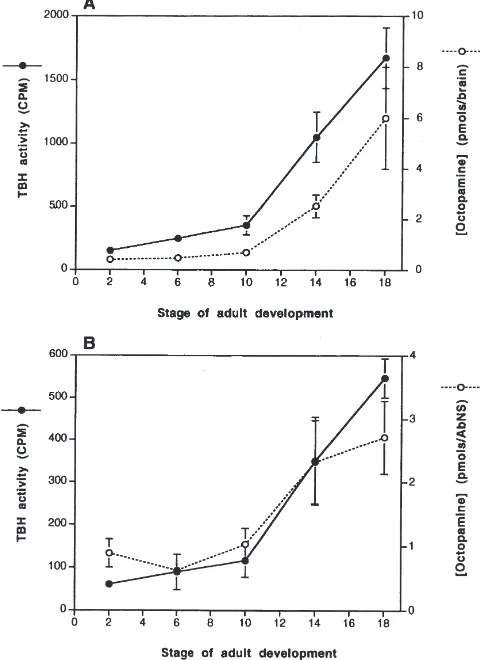
The levels of TβH activity in the brain and abdominal ganglia varied with the developmental stage of the moth. Minimal TβH activity was measured in brain extracts from animals early in adult development (stage P2, 149.6±29.3; stage P6, 246.0±34.4; stage P10, 352.6±75.4 cpm), whereas later in adult development OA synthesis was elevated (stage P14, 1051.6±197.6; stage P18, 1672.6±238.0) (Fig. 5A). Significant differences in TβH activity were detected between stage P2 and stages P14 and P18, although no significant differences in TβH activity were measured between stage P2 and stages P6 and P10 (ANOVA, P,0.05). A similar increase in TβH activity occurred in the abdominal ganglia. Relatively little production of [3H]OA was detected with
homogen-ates of abdominal ganglia taken from pupae at stages P2, P6, and P10 (61.0±8.9, 89.6±41.3, 116.6±38.3 cpm, respectively). Later in adult development much greater [3H]OA synthesis occurred (351.0±103.9 and
547.0±46.2 cpm from stages P14 and P18, respectively) (Fig. 5B). Whereas no significant differences in TβH activity were observed between stage P2 and stages P6 and P10, significant differences in TβH activity were detected between stage P2 and stages P14 and P18 (ANOVA, P,0.05).
3.4. Developmental changes in octopamine levels
Fig. 4. Effect of varying concentrations of tyramine on tyramine-β -hydroxylase activity from CNS homogenates obtained from stage-P18 and P0 animals. (A) Kinetic constants estimated from CNS homogen-ates obtained from stage-P18 animals. Assay was performed under standard conditions except that the concentration of unlabeled tyramine was varied from 0.007 to 2.4 mM. Inset: double reciprocal plot; abscissa=1/S (mM), ordinate=1/V (pmol/min/mg). Apparent kinetic constants were: Vmax, 17.86±1.85 pmol/min/mg; KM, 0.22±0.04 mM
(n=3, mean±SEM). (B) Kinetic constants estimated from CNS homo-genates obtained from stage-P0 animals. Assay was performed under standard conditions except that the concentration of unlabeled tyramine was varied from 0.007 to 1.0 mM. Inset: Double reciprocal plot; abscissa=1/S (mM), ordinate=1/V (pmol/min/mg). Apparent kinetic constants were: Vmax, 2.05±0.14 pmol/min/mg; KM, 0.30±0.05 mM
(n=3, mean±SEM).
of OA through stage P14 were not statistically signifi-cant, but the levels measured at stages 2 and 18 were significantly different (ANOVA, P,0.05). The level of OA in the abdominal ganglia also increased. In P2 ani-mals, OA levels were 0.89±0.22 pmol/AG. Beginning at stage P10, the level of OA increased from 1.03±0.25 pmol/AG to 2.71±0.57 pmol/AG by stage P18 (Fig. 5B). No significant differences in OA levels were observed at stages P2, P6, and P10, but the differences between stage P2 and stages P14 and P18 were significant (ANOVA, P,0.05).
Fig. 5. Tyramine-β-hydroxylase activity and octopamine levels in the brain and abdominal ganglia (AG) of M. sexta during adult develop-ment. (A) Tyramine-β-hydroxylase activity and OA levels detected from brain extracts at 5 different stages of adult development.I: TβH
activity. s: OA levels. Each point represents the mean of 3 replicates±SEM. (B) Tyramine-β-hydroxylase activity and OA levels detected in extracts of abdominal ganglia at five different stages of adult development. I: TβH activity.s: OA levels. Each point
rep-resents the mean of 3 replicates±SEM.
4. Discussion
This study had three objectives. First, we aimed to develop an assay for, and to characterize, TβH, the puta-tively rate-limiting enzyme for OA biosynthesis. Second, using the newly developed TβH assay, we asked whether TβH activity in the developing CNS of M. sexta increased during metamorphosis. Finally, we sought to determine whether changes in the levels of OA were associated with changes in TβH activity during meta-morphic adult development of this species of moth.
This study provides the first partial biochemical characterization of TβH from insect nervous tissue. The assay we developed to measure TβH activity relies upon the conversion of [ring-3H]tyramine to [ring-3H]OA and
ident-ified as OA, based upon coelution with OA standards, reaction with PNMT, and reaction with NaIO4. The other
product has not been identified. OA reportedly is inacti-vated by two mechanisms, acetylation and conjugation (reviewed by Evans, 1985; Wierenga and Hollingworth, 1990). Based on the lack of action of N-acetylation inhibitors in this study, we suspect that the unidentified compound is a tyramine metabolite, perhaps either a sulfate or amino-acid conjugate (Kennedy, 1978; Maxwell et al., 1980). Regardless of the identity, the unidentified compound did not interfere with measure-ments of TβH activity, because it did not contribute to the radioactivity co-eluting with OA, nor did the radioac-tivity co-eluting with OA break down to form the unidentified compound. Other compounds that appeared in boiled enzyme extracts were not enzymatic products and were probably oxidation products of tyramine.
M. sexta TβH has many similarities to lobster TβH and the mammalian enzyme, DβH. M. sexta TβH activity depended on incubation time, protein concen-tration, had a pH optimum of 7.0 and requires exogenous copper and catalase. These requirements are identical to the requirements for lobster TβH and DβH (Wallace, 1976; reviewed by Stewart and Klinman, 1988). In addition, ascorbate is required for M. sexta TβH activity. Our estimate of KM, ascorbate (2.75 mM) is similar to
values reported for lobster TβH (0.2 mM; Wallace, 1976) and mammalian DβH (0.57–23.5 mM; Annis et al., 1973; Saxena et al., 1985; Dhawan et al., 1986; Ste-wart and Klinman, 1991; Wimalasena and Wimalasena, 1991; Fortin et al., 1993). Finally, the apparent KM,
tyram-ine of M. sexta TβH in adult CNS extracts is similar to
the values for lobster TβH and mammalian DβH. We have estimated that the apparent KM, tyramine of M. sexta
TβH is 0.22±0.047 mM. By contrast, the apparent KM
of lobster TβH reportedly is 0.15±0.015 mM (Wallace, 1976), and that of mammalian DβH ranges from 0.55 to 2.8 mM (Annis et al., 1973; Saxena et al., 1985; Dhawan et al., 1986; Stewart and Klinman, 1991; Wimalasena and Wimalasena, 1991; Fortin et al., 1993). Recently, Monastirioti et al. (1996) have cloned the TβH gene from Drosophila melanogaster and have shown that the deduced amino-acid sequence of TβH is 39% identical to that of DβH. Thus, we conclude that M. sexta TβH is functionally and structurally similar to mammalian DβH. The endogenous substrates, however, distinguish TβH from DβH. For example, OA and dopamine are found in separate insect neurons (Homberg, 1994); therefore, if TβH and DβH occur in insects, TβH would convert tyramine to octopamine and DβH would convert dopam-ine to norepdopam-inephrdopam-ine. Apparently, however, norepi-nephrine does not occur in insects (Sparks and Geng, 1992). Furthermore, the TβH gene from D. melanogaster has been identified, cloned, and sequenced, but homolo-gous genes have not been reported (Monastirioti et al.,
1996). Thus, evidence to date suggests that insects lack DβH and that TβH is specific to octopaminergic neurons. The levels of OA in the adult brain and abdominal ganglia of M. sexta are consistent with the published lev-els of OA in adult insects. We observed 6.00±2.00 pmol/brain and 2.71±0.57 pmol/AG, whereas other investigators have reported 13.6 pmol/brain in the cricket Gryllus bimaculatus (Nagao and Tanimura, 1988); 3.2 pmol/brain in the blowfly Calliphora erythrocephala (Na¨ssel and Laxmyr, 1983); 23.5 pmol/central ganglia in the cockroach Periplaneta americana (Sloley and Okikaza, 1988); 12.6 pmol/brain in the moth M. config-urata (Bodnaryk, 1980); 13–14 pmol/brain in the corn earworm moth Helicoverpa zea (Linn and Roelofs, 1993); 5.8 pmol/cerebral ganglia, and 5.3 pmol/optic lobe in the moth Spodoptera littoralis (Davenport and Wright, 1986); and 3.9 pmol/protocerebrum and 3.8 pmol/optic lobe in M. sexta (Evans, 1985). In addition, we found that levels of OA in M. sexta increased approximately 15-fold in the brain and 3-fold in the abdominal ganglia during metamorphic adult develop-ment, observations similar to those in Mamestra config-urata by Bodnaryk (1980). Thus, OA levels are develop-mentally regulated. Because the levels of OA depend on the rate of biosynthesis, we developed an assay to meas-ure TβH activity and characterized TβH from the brain and abdominal ganglia of M. sexta during metamorphic adult development in order to explore the mechanisms responsible for the developmental regulation of OA.
An increase of TβH activity during metamorphosis correlates with the increase in OA levels in the brain of M. sexta. Relatively low levels of TβH activity were measured in the brain early in adult development and increased approximately 10-fold later in adult develop-ment. The brain of holometabolous insects undergoes a dramatic increase in size and complexity during meta-morphosis (Nordlander and Edwards, 1968; Copenhaver and Truman, 1986; Levine et al., 1995). Although there has been no direct examination of OA-containing neu-rons in the brain of M. sexta during development, the results of this study suggest that most of the OA present in the adult brain arises during metamorphosis. It is unknown if a few neurons with extensive arborizations are added (e.g. neurons innervating the medulla of the optic lobe; Homberg et al., 1992; Homberg, 1994) or if a large number of octopaminergic neurons are added, but whether there is postembryonic neurogenesis, reorgani-zation, or both, our findings suggest that OA contributes to the formation and function of the adult brain of M. sexta.
Barker, 1975; Eckert et al., 1992; Pflu¨ger et al., 1993). In moths, two VUM neurons occur in each abdominal ganglion and project to skeletal muscles (Brookes and Weevers, 1988). Pflu¨ger et al. (1993) examined the mor-phology and development of these neurons in an abdominal ganglion and have showed that octopami-nergic neurons in the fourth abdominal segment, despite loss of some of their muscle targets, show little reorgani-zation or growth during adult development and that new octopaminergic neurons do not appear in this ganglion during metamorphosis. By contrast, we have observed a significant increase in the levels of OA and TβH activity in extracts of abdominal ganglia during metamorphosis. Two possible explanations could account for the appar-ent discrepancy between these studies: rates of pro-duction of OA might increase in existing neurons with-out changes in morphology, or additional octopaminergic neurons might be added to segmental ganglia that were not observed by Pflu¨ger et al. (1993). In locusts, for example, the terminal abdominal ganglion contains a larger number of OA-immunoreactive neu-rons than do other ganglia (Stevenson et al., 1994). In our experiments, extraction of abdominal ganglia included the terminal abdominal ganglion, which con-tains DUM and VUM neurons that mature during meta-morphosis and innervate adult reproductive structures (Christensen et al., 1991; Thorn and Truman, 1989). Thus, while the number of octopaminergic neurons in the fourth abdominal ganglion might remain constant during metamorphosis (Pflu¨ger et al., 1993), we suspect that new octopaminergic neurons are added in the ter-minal abdoter-minal ganglion during adult development.
We observed that the levels of TβH activity rise coincidently with the levels of OA in all regions of the CNS during adult development. Therefore, increased levels of OA in the developing CNS appear to be due to increased activity of its synthetic enzyme, TβH. Two mechanisms may account for increased activity of TβH during development: more enzyme may be synthesized, or TβH may be modified post-translationally to produce a more active enzyme. We compared the kinetic con-stants of TβH from CNS homogenates from stages P0 and P18, and although a significant difference was detected in the apparent Vmax, no significant change in
the apparent KMwas observed. We conclude, therefore,
that increased levels of TβH protein may account for the increase in TβH activity during metamorphosis. More-over, OA levels and TβH activity begin to increase shortly after the titer of the steroid hormone 20-hydrox-yecdysone in the hemolymph reaches its maximum level in the developing moth (compare Fig. 5 to Bollenbacher et al., 1981). Ecdysteroids play important roles in the developing nervous system, and their actions include regulation of dendritic growth and regression, synapse formation, and apoptosis (Weeks and Truman 1985, 1986; Weeks and Levine, 1990; Prugh et al., 1992).
Also, steroids have an important role in the expression of neurotransmitter phenotype. Specifically, a small commitment peak of steroid released prior to larval ecdysis regulates the decline of cardioacceleratory pep-tide 2 bioactivity, and a later prepupal peak of steroid triggers the appearance of bursicon in lateral neurosecre-tory cells of the ventral nerve cord of M. sexta (Loi and Tublitz, 1993; Tublitz and Loi, 1993). The commitment peak and prepupal peak of steroid appear to mediate the decline of FMRFamide-like immunoreactivity in motor neurons during metamorphosis in M. sexta (Witten and Truman, 1996). Finally, the prepupal peak of ecdystero-ids regulates the expression of preecdysis-triggering hor-mone and ecdysis-triggering horhor-mone (ETH), apparently though the activation of ecdysteroid-receptor response elements found within the promoter region of the ETH gene (Zitnan et al., 1999). Thus, 20-hydroxyecdysone may play an important role in regulation of TβH syn-thesis in the nervous system of M. sexta during adult development. Studies are under way to test this possi-bility.
Acknowledgements
We thank Drs Stephen Carper, Norman Davis, and David Morton for helpful comments on the original manuscript, and Dr. A.A. Osman for insect rearing. This research was supported by grants from NIH (AI-23253 to JGH) and NSF (IBN-9496168 to HKL). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NSF.
References
Annis, D., Miras-Portugal, M.-T., Mandel, P., 1973. Bovine adrenal medullary dopamine β-hydroxylase: purification by affinity chro-matography, kinetic studies and presence of essential histidyl resi-dues. Biochim. Biophys. Acta 327, 313–327.
Bacon, J.P., Thompson, K.S.J., Stern, M., 1995. Identified octopami-nergic neurons provide an arousal mechanism in the locust brain. J. Neurophysiol. 74, 2739–2743.
Bell, R.A., Joachim, F.G., 1976. Techniques for rearing laboratory col-onies of tobacco hornworms and pink bollworms. Ann. Ent. Soc. Am. 69, 365–373.
Bodnaryk, R.P., 1980. Changes in brain octopamine levels during metamorphosis of the moth Mamestra configurata Wlk. Insect Biochem. 10, 169–173.
Bollenbacher, W.E., Smith, W.E., Goodman, W., Gilbert, L., 1981. Ecdysteroid titer during larval–pupal adult development of the tob-acco hornworm Manduca sexta. Gen. Comp. Endocrinol. 44, 302–306.
Brookes, S.J.H., Weevers, R.deG., 1988. Unpaired median neurones in a lepidopteran larva (Antheraea pernyi) I. anatomy and physi-ology. J. Exp. Biol. 136, 311–332.
physiological action of octopamine in the female sex-pheromone glands of heliothine moths. Insect Biochem. Molec. Biol. 22, 841–849.
Copenhaver, P.F., Truman, J.W., 1986. Metamorphosis of the central neuroendocrine system in the moth Manduca sexta. J. Comp. Neu-rol. 249, 186–204.
Creveling, C.R., Daly, J.W., Witkop, B., Udenfriend, S., 1962. Sub-strates and inhibitors of dopamine-β-oxidase. Biochim. Biophys. Acta 64, 125–134.
Davenport, A.P., Wright, D.J., 1986. Octopamine distribution in the larvae and adults of two species of moth, Spodoptera littoralis and Manduca sexta. J. Insect Physiol. 32, 987–993.
David, J.-C., Coulon, J.-F., 1985. Octopamine in invertebrates and ver-tebrates. A review. Prog. Neurobiol. 24, 141–185.
Dhawan, S., Hensley, P., Osborne, J.C. Jr., Fleming, P., 1986. Adenos-ine 59-diphosphate-dependent subunit dissociation of bovine dopa-mineβ-hydroxylase. J. Biol. Chem. 261, 7680–7684.
Dudai, Y., Buxbaum, J., Corfas, G., Ofarim, M., 1987. Formamidines interact with Drosophila octopamine receptors alter the flies’ behavior and reduce their learning ability. J. Comp. Physiol. A 161, 739–746.
Eckert, M., Rapus, J., Nu¨rnberger, A., Penzlin, H., 1992. A new spe-cific antibody reveals octopamine-like immunoreactivity in the cockroach ventral nerve cord. J. Comp. Neurol. 322, 1–15. Evans, P.D., 1985. Octopamine. In:. Kerkut, G.A., Gilbert, L.I. (Eds.),
Comprehensive Insect Physiology, Biochemistry, and Pharma-cology, vol. 11. Pergamon Press, New York, pp. 499–530. Fortin, D., Coulon, J.-F., Roberge, A.G., 1993. Comparative study of
biochemical parameters and kinetic properties of dopamine β -hydroxylase activity from cat and rat adrenals. Comp. Biochem. Physiol. 104 B, 567–575.
Goldstein, M., Contrera, J.F., 1962. The substrate specificity of phe-nylamine-β-hydroxylase. J. Biol. Chem. 237, 1898–1902. Hirsh, J., 1989. Molecular genetics of DOPA decarboxylase and
bio-genic amines in Drosophila. Dev. Genetics 10, 232–238. Homberg, U., Binkle, U., Lehman, H.K., Vullings, H.G.B., Eckert,
M., Rapus, J., Hildebrand, J.G., 1992. Octopamine-immunoreactive neurons in the brain of two insect species. In: Elsner, N., Richter, D.W. (Eds.) Rhythmogenesis in Neurons and Networks. Proc. 20th Go¨ttingen Neurobiology Conference. Thieme Verlag, Stuttgart, p. 477.
Homberg, U., 1994. Distribution of neurotransmitters in the insect brain. In:. Progress in Zoology, vol. 40. Gustav Fischer Verlag, Stuttgart.
Hoyle, G., Barker, D.L., 1975. Synthesis of octopamine by insect dor-sal median unpaired neurons. J. Exp. Zool. 193, 433–439. Kaufman, S., Friedman, S., 1965. Dopamine-β-hydroxylase.
Pharma-col. Rev. 17, 71–100.
Kennedy, M.B., 1978. Products of biogenic amine metabolism in the lobster: sulphate conjugates. J. Neurochem. 30, 315–320. Levine, R.B., Morton, D.B., Restifo, L.L., 1995. Remodeling of the
insect nervous system. Curr. Opinion Neurobiol. 5, 28–35. Linn, C.E., Roelofs, W.L., 1993. Levels of biogenic amines and
pep-tides in individual corn earworm moths, Helicoverpa zea, using high performance liquid chromatography with electrochemical detection. Insect Biochem. Molec. Biol. 23, 367–373.
Livingstone, M.S., Temple, B.L., 1983. Genetic dissection of monoam-ine neurotransmitter synthesis in Drosophila. Nature 303, 67–70. Loi, P.K., Tublitz, N.J., 1993. Hormonal control of transmitter
plas-ticity in insect peptidergic neurons. I. Steroid regulation of the decline in cardioacceleratory peptide 2 (CAP2) expression. J. Exp.
Biol. 181, 175–194.
Maxwell, G.D., Moore, M.M., Hildebrand, J.G., 1980. Metabolism of tyramine in the central nervous system of the moth, Manduca sexta. Insect Biochem. 10, 657–665.
Molinoff, P.B., Landsberg, L., Axelrod, J., 1979. An enzymatic assay
for octopamine and other β-hydroxylated phenylethylamines. J. Pharmacol. Exp. Ther. 170, 253–261.
Monastirioti, M., Linn, C.E., White, K., 1996. Characterization of Dro-sophila tyramineβ-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 16, 3900–3911.
Nagao, T., Tanimura, T., 1988. Distribution of biogenic amines in the cricket central nervous system. Analyt. Biochem. 171, 33–40. Na¨ssel, D.R., Laxmyr, L., 1983. Quantitative determination of biogenic
amines and DOPA in the CNS of adult and larval blowflies, Calli-phora erythrocephala. Comp. Biochem. Physiol. 75C, 259–265. Nordlander, R.H., Edwards, J.S., 1968. Morphology of the larval and
adult brain of the monarch butterfly Danaus plexippus L. J. Mor-phol. 126, 67–93.
Orchard, I., Ramirez, J.-M., Lange, A.B., 1993. A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 38, 227–249. Pflu¨ger, H.J., Witten, J.L., Levine, R.B., 1993. Fate of abdominal ven-tral unpaired median cells during metamorphosis of the hawkmoth, Manduca sexta. J. Comp. Neurol. 335, 508–522.
Prugh, J., Della Croce, K., Levine, R.B., 1992. Effects of steroid hor-mone, 20-hydoxyecdysone, on the growth of neurites by identified insect motoneurons in vitro. Dev. Biol. 154, 331–347.
Sanes, J.R., Hildebrand, J.G., 1976. Structure and development of the antennae in a moth Manduca sexta. Dev. Biol. 51, 282–299. Saxena, A., Hensley, P., Osborne, J.C. Jr., Fleming, P., 1985. The
pH-dependent subunit dissociation and catalytic activity of bovine dopamineβ-hydroxylase. J. Biol. Chem. 260, 3386–3392. Sloley, B.D., Okikaza, S., 1988. Selective depletion of dopamine,
octo-pamine and 5-hydroxytryptamine in the nervous tissue of the cock-roach, Periplaneta americana. J. Neurochem. 51, 535–541. Sparks, T.C., Geng, C., 1992. Analysis of the biogenic amines in the
central nervous system of the tobacco hornworm by high-perform-ance liquid chromatography with 16 sensor electrochemical detec-tion. Analyt. Biochem. 205, 319–325.
Stevenson, P.A., Pflu¨ger, H.-J., Eckert, M., Rapus, J., 1994. Octopam-ine-like immunoreactive neurones in locust genital abdominal gan-glia. Cell Tiss. Res. 275, 299–308.
Stewart, L.C., Klinman, J.P., 1988. Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function. Annu. Rev. Biochem. 57, 551–592.
Stewart, L.C., Klinman, J.P., 1991. Cooperativity in the dopamineβ -monooxygenase reaction. Evidence for ascorbate regulation of enzyme activity. J. Biol. Chem. 266, 11537–11543.
Thorn, R.S., Truman, J.W., 1989. Sex-specific neuronal respecification during the metamorphosis of the genital segments in the tobacco hornworm moth, Manduca sexta. J. Comp. Neurol. 284, 489–503. Tolbert, L.P., Matsumoto, S.G., Hildebrand, J.G., 1983. Development of synapses in the antennal lobes of the moth Manduca sexta during metamorphosis. J. Neurosci. 3, 1158–1175.
Touchstone, J.C., Dobbins, M.F., 1983. Practice of TLC, 2nd ed. Wiley, New York.
Tublitz, N.J., Loi, P.K., 1993. Hormonal control of transmitter plas-ticity in insect peptidergic neurons: II. Steroid control of the up-regulation of bursicon expression. J. Exp. Biol. 181, 195–212. Wallace, B.G., 1976. The biosynthesis of octopamine —
characteriz-ation of lobster tyramineβ-hydroxylase. J. Neurochem. 26, 761– 770.
Weeks, J.C., Levine, R.B., 1990. Postembryonic neuronal plasticity and its hormonal control during insect metamorphosis. Annu. Rev. Neurosci. 13, 183–194.
Weeks, J.C., Truman, J.W., 1985. Independent steroid control of the fates of motoneurons and their muscles during insect metamor-phosis. J. Neurosci. 5, 2290–2300.
Weeks, J.C., Truman, J.W., 1986. Steroid control of neuron and muscle development during the metamorphosis of an insect. J. Neurobiol. 17, 249–267.
metabolism in the insect nervous system. J. Neurochem. 54, 479–489.
Wimalasena, K., Wimalasena, D.S., 1991. Continuous spectrophoto-metric assays for dopamineβ-monooxygenase based on two novel electron donors: N,N-dimethyl-1,4-phenylenediamine and 2-ami-noascorbic acid. Analyt. Biochem. 197, 353–361.
Witten, J.L., Truman, J.W., 1996. Developmental plasticity of
neuro-peptide expression in motoneurons of the moth, Manduca sexta: steroid hormone regulation. J. Neurobiol. 29, 99–114.