Isolation of two distinct cold-inducible cDNAs encoding plant
uncoupling proteins from the spadix of skunk cabbage
(
Symplocarpus foetidus
)
Kikukatsu Ito *
Di6ision of Biosystem and Bioresource Technology,Cryobiosystem Research Center,Faculty of Agriculture,Iwate Uni6ersity,Morioka,
Iwate020-8550,Japan
Received 7 May 1999; received in revised form 2 August 1999; accepted 3 August 1999
Abstract
To know the involvement of mitochondrial uncoupling protein (UCP) in thermogenesis in plants, cDNA cloning of UCP-like gene was performed using cDNA library prepared from the spadix of skunk cabbage (Symplocarpus foetidus). Two novel cDNAs (SfUCPaandSfUCPb) encoding uncoupling proteins were isolated. TheSfUCPacDNA contained an open reading frame of 303 amino acids (predicted molecular weight 32.6 kDa) while the SfUCPb cDNA encoded 268 amino acids (predicted molecular weight 29.0 kDa). While the SfUCPA protein had six transmembrane domains and three mitochondrial energy-transfer protein signatures that are characteristic of all known UCPs, SfUCPB lacks the fifth transmembrane domain and the third energy-transfer protein signature, suggesting an altered topology in the inner membrane of the mitochondria. The expression ofSfUCPa and
SfUCPb mRNAs was detected only in the spadix but not in the leaf. Further, the extents of the expression of SfUCPa and
SfUCPb mRNAs were induced by low-temperature treatment. These data suggest that SfUCPA and SfUCPB may have an important role in thermogenesis in the spadix of skunk cabbage. © 1999 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Skunk cabbage; Low temperature; Thermogenesis; Mitochondria; Uncoupling protein
www.elsevier.com/locate/plantsci
1. Introduction
Low temperature, drought and salinity are com-mon adverse environmental factors encountered by land plants. Among these stresses, low temper-atures that cause chilling or freezing injury seem to be the most important factor limiting crop produc-tivity [1]. To cope with these low temperature stresses, cold-tolerant plants such as wheat and rye carry out a number of physiological and metabolic responses that lead to cold acclimation [2 – 5]. In contrast, it is well-known that some plants, includ-ing skunk cabbage, have developed specialized mechanism to avoid cooling by thermogeneration [6 – 8].
The temperature of the flowers on the spadices of skunk cabbage that appear in early spring remain above +10°C, even if the air temperature drops to −15°C [7]. This is achieved by doubling the respiration rate from 12°C to subfreezing tem-perature levels. The heat produced by a ther-mogenic plant has been believed to be associated with a large increase in the activity of the mito-chondrial cyanide-insensitive, nonphosphorylating electron transport pathway regulated by alterna-tive oxidase (AOX), which is unique to plant mitochondria [9 – 12].
In mammals, on the contrary, a mitochondrial protein called uncoupling protein (UCP) has been shown to play an important role in heat produc-tion. The UCPs that are found in the inner
mem-brane of the mitochondria, allow for a
transmembrane H+ influx, which uncouples
respi-ration from ATP synthesis permitting the dissipa-* Tel./fax: +81-19-6216253.
E-mail address:[email protected] (K. Ito)
tion of chemical energy into metabolic heat [13 – 15]. In animals, three UCPs have been found: UCP1 is distributed mainly in brown adipose tis-sue [16]; UCP2 is ubiquitously found in many tissues [17]; and UCP3 is highly specific for skele-tal muscle [18]. Like other mitochondrial carrier proteins, mammalian UCPs seem to be composed of six transmembrane segments whose net hydro-phobic character derives from paired amphiphilic helices [19,20]. Furthermore, the activity of these UCPs is regulated negatively by binding purine nucleotides (ATP, GTP, GDP and ADP) at the C-terminal region, whereas free fatty acids in-crease the activity [21 – 25].
Recently, two cDNAs encoding UCP-like
protein have been isolated from potato (StUCP)
[26] and Arabidopsis (AtPUMP) [20]. Because the
expression of StUCPwas detected mainly in flow-ers and fruit, it has been hypothesized that StUCP can be associated with a burst of respiration in flowering and fruit ripening in combination with AOX [26].
Although potato andArabidopsisare thought to be non-thermogenic plants, cold-inducible expres-sions of StUCP and AtPUMP have been suspected their involvement in thermogenesis [20,26]. In thermogenic plants such as skunk cabbage, how-ever, molecular mechanism underlying UCP-medi-ated heat production remains to be determined. The present report describes the isolation of two distinct spadix-specific and cold-inducible cDNAs
belonging to a novelUCPgene family from skunk
cabbage. This is the first report showing the
pres-ence of a UCP-like gene in thermogenic plants.
2. Materials and methods
2.1. Plant material and treatments
Skunk cabbage plants (Symplocarpus foetidus) were collected at Ikoino-Mori, a public park in Omonogawa Town located in Akita Prefecture, Japan. The plantlets were subjected to cold treat-ment by letting them in the cold room (4°C) for 12 h under continuous dim light.
2.2. cDNA library construction and screening
Total RNA was extracted from the spadix of skunk cabbage and the integrity of isolated RNA
was determined by 1.0% agarose gel
electrophore-sis [27]. Poly(A)+ RNA purified using an mRNA
isolation kit (Pharmacia) was subjected to reverse transcriptase-polymerase chain reaction (RT-PCR)
to isolate the related clones of the UCP gene
family. For the first-strand cDNA synthesis,
poly(A)+ RNA (0.1 mg) was annealed with 20
pmol of cDNA priming primer [27] and then extended using 10 units of reverse transcriptase (New England Biolab) at 37°C for 30 min in 20ml of 1×RT buffer containing 10 mM 1,4-dithiothre-itol and 0.5 mM dNTPs. The reaction mixture consisted of 10 mM Tris – HCl at pH 8.0, 50 mM
KCl, 1.5 mM MgCl2, 4 mM dNTPs, 0.2 unit of
EX Taq polymerase (Takara) and 10 pmol of two
degenerated primers, ZF1 (5%
-CCIYTIGAYACIG-CIAAR-3%) and ZR1 (5%
-ACWTTCCAISYIC-CIAWIC-3%) that correspond to the conserved
amino acid sequences of the UCP family. A major PCR product of approximately 0.8 kb was ob-tained after 35 cycles of amplification as follows: 0.5 min at 94°C; 1 min at 50°C and 1 min at 72°C. The amplified cDNA fragments were cloned into the T-vector, and one clone (p2-1) giving the UCP-related sequence was used as a probe for the following library screening. Poly(A)+ RNA (5 mg)
prepared from the spadix was processed to
con-struct the lgt11 phage library by standard DNA
techniques [28]. The positive clones were excised and subcloned into the pBluescript SK plasmid (Stratagene). Two plasmids, pz8-1 and pz8-2 that
carry full length SfUCPa and SfUCPb cDNAs,
respectively, were sequenced on both strands using
the BcaBest sequencing kit (Takara) and
ABI373A automated sequencer with T3, T7 and gene-specific primers. Sequence data were assem-bled and analyzed using a GENETYX-Homology
software system, version 2.2.0 (Software
Development).
2.3. In 6itro transcription and translation
Plasmids (pz8-1 and pz8-2) carrying full length SfUCPa and SfUCPb, respectively, were lin-earized, and sense or anti-sense RNA was tran-scribed in vitro using the protocol of the MAXIscript transcription kit (Ambion) using T7 or T3 RNA polymerase. Equal amounts of RNA (4 mg) were used in the in vitro translation reac-tion, using wheat germ extracts (Promega) in the
de-scribed by the manufacturer. The translation products were analyzed with SDS-PAGE. Before fluorography, the gels were fixed, incubated in Amplify (Amersham) and then dried.
2.4. Preparation of total RNA and Northern blot analysis
Total RNA was extracted as described by Ito et al. [27]. Northern blotting was performed as described previously [29].
3. Results
3.1. Isolation and nucleotide sequence analyses of SfUCPa and SfUCPb cDNA
To isolate a UCP-related gene in skunk
cab-bage, RT-PCR was initially performed using two degenerated primers, ZF1 and ZR1, that corre-spond to the conserved amino acid sequences of human and plant UCPs. One main product of
0.8 kb was detected using poly(A)+ RNA from
the spadix of skunk cabbage. Sequencing of the amplified fragment showed that the deduced amino acid sequence of one reading frame had a fairly high similarity with that of UCP genes. With this fragment as a probe, eight positive clones were isolated from the cDNA library. Af-ter deAf-termining the DNA sequences, it was found that they originated from two distinct genes. Two cDNAs, pz8-1 and pz8-2, carrying the full length of UCP-like gene were designated SfUCPa and SfUCPb, respectively, and were an-alyzed further.
The sizes of SfUCPa and SfUCPb cDNA
in-serts were 1525 and 2991 bp, respectively (Fig. 1). A putative polyadenylation signal (AATAAA) was found 236 bp upstream from the poly(A)
sequence in SfUCPa cDNA, while SfUCPb
cDNA contained two polyadenylation signals at the position of 1171 and 1243 bp. The fact
should be noted that SfUCPb cDNA contains a
long 3%-untranslated region compared with that
of SfUCPa. The SfUCPa cDNA contained a sin-gle open frame of 303 amino acids that encodes a putative protein with a predicted molecular
mass of 32.6 kDa (Fig. 1). The SfUCPb cDNA
contained an open reading frame of 268 amino acids that encodes a putative protein with a
pre-dicted molecular mass of 29.0 kDa (Fig. 1). Southern blot analyses showed that the genome of skunk cabbage contains a multi-copy for the SfUCPa gene and a single copy for the SfUCPb gene (data not shown).
3.2. In 6itro transcription/translation analysis of
SfUCPa and SfUCPb
To confirm the molecular mass of each
protein, in vitro transcription/translation were
conducted. The translation products of 35
S-la-beled SfUCPA or SfUCPB gave one main band corresponding to the estimated molecular mass (ca. 32 kDa for SfUCPA, ca. 29 kDa for SfUCPB) only when sense RNA was used as a template (Fig. 2).
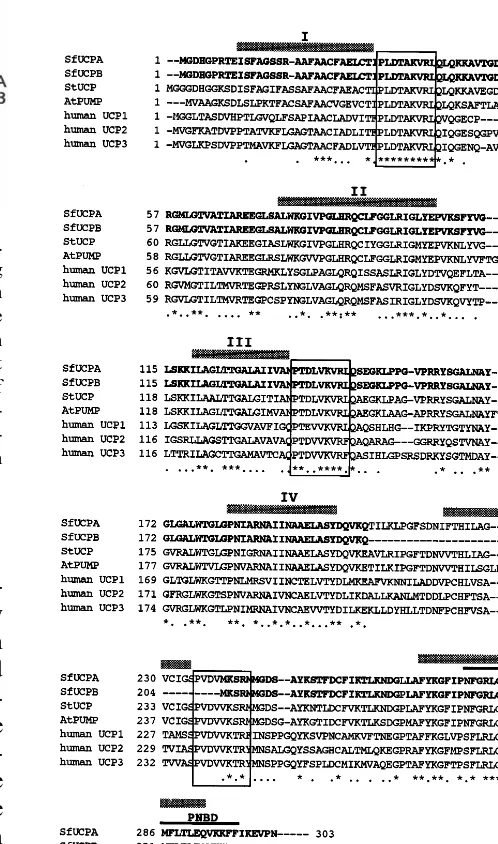
3.3. Amino acid sequence analyses of SfUCPA and SfUCPB
The amino acid sequences of SfUCPA and SfUCPB show higher similarity to plant UCPs than to human UCPs (Fig. 3). It should be note that one region corresponding to the amino acid sequences between Thr 204 and Val 238 of SfUCPA was completely deleted in SfUCPB (Fig. 3). In addition, Leu at position of 265 in SfUCPa was substituted by Pro in SfUCPB (Fig. 3). Like other mitochondrial UCP proteins, SfUCPA contained six predicted transmembrane domains and three typical mitochondrial energy-transfer-protein signature domains [18,20], while SfUCPB lacked the fifth transmembrane domain
and the third mitochondrial energy-transfer
protein signature domain (Fig. 3). Potential purine nucleotide binding domains (PNBD) were located at the C-terminal of both SfUCPA and SfUCPB proteins (Fig. 3).
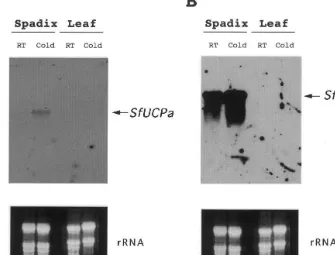
3.4. Spadix-specific and low temperature-induced expressions of SfUCPa and SfUCPb mRNAs
4. Discussion
In this study, two novel cDNA clones named SfUCPa and SfUCPb encoding putative mito-chondrial uncoupling proteins were isolated and characterized using skunk cabbage, a typical ther-mogenic plant species. The predicted polypeptides of SfUCPA and SfUCPB shared a high sequence similarity with potato UCP (StUCP) whose un-coupling activity has been shown in yeast mito-chondria [26]. Although the activity of SfUCPA and SfUCPB is not yet defined in molecular detail, amino acid sequence similarities between skunk cabbage and potato UCPs support the view that the SfUCPA and SfUCPB proteins have at least in part UCP-like function.
The structure-function relationship of mam-malian UCP1 has been extensively studied. The polypeptide chain has been shown to consist of three tandemly related domains of about 100 amino acids in length [23]. Each domain is charac-terized by the presence of two transmembrane regions and one mitochondrial energy transfer mo-tif. Topological studies showed that both the C-and N-terminal ends of UCP1 are oriented to-wards the cytosolic side of the inner mitochondrial membrane [30]. Further, the binding of purine nucleotide to the C-terminal region induces a con-formational change that leads to inhibition of H+
permeability [14].
Although SfUCPA has six predicted transmem-brane domains, three mitochondrial energy
Fig. 2. In vitro translation of SfUCPa and SfUCPb genes. Sense or anti-sense RNA was transcribed in vitro using
SfUCPaorSfUCPbcDNA templates as described in Section 2. Four micrograms of sense (S) or anti-sense (AS) RNA were subjected to in vitro translation using wheat germ extracts in the presence of 35S-methionine. Control reaction without
RNA temperate was also performed (−). Positions of SfUCPA and SfUCPB are indicated by arrows. Bands indi-cated by asterisks are non-specific products. Open circle de-notes the position of low-molecular-weight translation artifacts synthesized by small ORFs.
in skunk cabbage. However, considering that non-thermogenic plants such as Arabidopsisand potato
also contain UCP-like genes (StUCP and
At-PUMP, respectively), molecular mechanism
gov-Fig. 3. Alignment of the deduced amino acid sequences of the putative SfUCPA and SfUCPB proteins with the potato (StUCP), Arabipopsis (AtPUMP) and human UCPs. The sequences are in single letter codes. Asterisks indicate perfect matches and dot represents conservative changes within all sequences. Bold letters depict identical sequences between SfUCPA and SfUCPB. The gaps introduced to optimize the sequence alignment are represented by dashes. The alignment was performed with the CLUSTAL W program. The typical mitochondrial energy-transfer protein signature domains are boxed. Six predicted transmembrane regions (I – VI) are indi-cated by horizontal shadow lines. The potential purine-nucle-otide binding domain (PNBD) is overlined. Accession numbers are: StUCP (Y11220); human UCP1 (P25874); hu-man UCP2 (P55851); huhu-man UCP3 (P55916).
fer motifs and one nucleotide binding region sug-gesting that the SfUCPA has a similar topology to human UCP1, SfUCPB lacks both the fifth potential transmembrane region and the third part of the mitochondrial energy transfer signa-ture. Because the sixth predicted transmembrane domain and the putative purine nucleotide bind-ing domain are intact in the SfUCPB protein, the topological feature of the SfUCPB seems to be different from UCP family members reported in the literatures. Namely, the C-terminal end of the SfUCPB including the purine nucleotide binding domain is probably oriented towards the matrix
side of the inner mitochondrial membrane.
Therefore, SfUCPB would exert its biological ef-fect without any control by the cytosolic purine nucleotides.
The discovery of genes encoding SfUCPA and SfUCPB from the spadix of skunk cabbage seems to support the idea that the existence of UCPs, as first described in brown adipocytes in mammals, represents a general phenomenon in thermogenesis that also occur outside the mammalians. Espe-cially, cold-inducible and spadix-specific mRNA
expressions ofSfUCPa andSfUCPb seem to
Fig. 4. Spadix-specific and low-temperature-inducible expressions of SfUCPa and SfUCPb mRNAs. Expression profiles of
SfUCPaandSfUCPbtranscripts in the spadix and leaves of skunk cabbage at room temperature (RT) and cold condition (4°C, 12 h). Each lane was loaded with 20mg of total RNA. [32P]-labeled cDNA probe for SfUCPa(A) orSfUCPb (B) was used as
a probe. Positions ofSfUCPaandSfUCPbtranscripts are indicated by arrows. An ethidium bromide-stained gel pictures showing undegraded rRNA are also shown at the bottom of plates.
erning plant thermogenesis seen in skunk cabbage seems to be more complex than those of animals. One could postulate that two energy-dissipating systems, both AOX and UCP, are involved in heat production in plants. In accordance with this, partial purification of AOX from the spadix of skunk cabbage has been succeeded [9]. However, it has been recently shown that AOX and UCP never seem to work simultaneously at their maxi-mal activity [24]. Namely, high levels of free fatty acid-induced UCP activity excludes AOX activity. Therefore, it seems to be necessary to assign each functional relationship during the thermogenesis in the spadix of skunk cabbage.
In conclusion, identification of two distinct genes encoding UCP from skunk cabbage provides novel insights on the molecular mechanism of heat production in plants. Especially, SfUCPB, topo-logically different isoform in the UCP family, may have an important role in the thermogenesis, pre-sumably being escaped from the negative regula-tion by the cytosolic purine nucleotide. Funcregula-tional analyses of SfUCPA and SfUCPB are in progress.
Acknowledgements
This work was supported by the Kuribayashi Foundation. I am grateful to Koji Tomita, Mayor of Omonogawa Town, for his kind permission to sample skunk cabbage at the ‘Ikoino-mori’, a pub-lic park in Omonogawa Town. I thank Dr. M. Uemura for his valuable discussions and critical reading of the manuscript, Y. Konishi for his kind advice.
References
[1] J. Levitt, Responses of Plants to Environmental Stresses, second ed., Academic press, New York, 1980.
[2] P.L. Steponkus, Role of the plasma membrane in freez-ing injury and cold acclimation, Annu. Rev. Plant Phys-iol. 35 (1984) 543 – 581.
[3] A. Sakai, W. Larcher, Frost Survival of Plants: Re-sponses and Adaptations to Freezing Stress, Springer-Verlag, Berlin and New York, 1987.
(Eds.), Plant Cold Hardiness: Molecular Biology, Bio-chemistry, and Physiology, Plenum Press, 1997, pp. 171 – 179.
[5] M.F. Thomashow, Role of cold-responsive genes in plant freezing tolerance, Plant Physiol. 118 (1998) 1 – 7. [6] K.A. Nagy, D.K. Odell, R.S. Seymour, Temperature
regulation by the inflorescence of Philodendron, Science 178 (1972) 1195 – 1197.
[7] R.M. Knutson, Heat production and temperature regula-tion in eastern skunk cabbage, Science 186 (1974) 746 – 747.
[8] E.L. Schneider, J.D. Buchanan, Morphological studies of the Nymphaeaceae. XI. The floral biology of Nelumbo pentapetala, Am. J. Bot. 67 (1980) 182 – 193.
[9] D.A. Berthold, J.N. Siedow, Partial purification of the cyanide-resistant alternative oxidase of skunk cabbage (Symplocarpus foetidus) mitochondria, Plant Physiol. 101 (1993) 113 – 119.
[10] L. McIntosh, Molecular biology of the alternative oxi-dase, Plant Physiol. 105 (1994) 781 – 786.
[11] A.M. Wagner, K. Krab, The alternative respiration path-way in plants: Role and regulation, Physiol. Plant. 95 (1995) 318 – 325.
[12] Y. Ito, D. Saisho, M. Nakazono, N. Tsutsumi, A. Hirai, Transcript levels of tandem-arranged alternative oxidase genes in rice are increased by low temperature, Gene 12 (1997) 121 – 129.
[13] M. Klingenberg, E. Winkler, The reconstituted isolated uncoupling protein is a membrane potential driven H+
translocator, EMBO J. 4 (1985) 3087 – 3092.
[14] S. Klaus, L. Casteilla, F. Bouillaud, D. Ricquier, The uncoupling protein UCP: a membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue, Int. J. Biochem. 23 (1991) 791 – 810.
[15] D. Ricquier, L. Casteilla, F. Bouillaud, Molecular studies of the uncoupling protein, FASEB J. 5 (1991) 2237 – 2242.
[16] D.G. Nicholls, R.M. Locke, Thermogenic mechanisms in brown fat, Physiol. Rev. 64 (1984) 1 – 64.
[17] C. Fleury, M. Neverova, S. Collins, S. Raimbault, O. Champigny, C. Levi-Meyrueis, F. Bouilland, M.F. Seldin, R.S. Surwit, D. Ricquier, C.H. Warden, Uncou-pling protein-2: a novel gene linked to obesity and hyper-insulinemia, Nature Genet. 15 (1997) 269 – 272.
[18] O. Boss, S. Samec, A. Paoloni-Giacobino, C. Rossier, A. Dulloo, J. Seydoux, P. Muzzin, J.-P. Giacobino, Uncou-pling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression, FEBS Lett. 408 (1997) 39 – 42.
[19] X. Liu, A.W. Bell, K.B. Freeman, G.C. Shore, Topogen-esis of mitochondrial inner membrane uncoupling protein. Rerouting transmembrane segments to the solu-ble matrix compartment, J. Cell Biol. 107 (1988) 503 – 509.
[20] I.G. Maia, C.E. Benedetti, A. Leite, S.R. Turcinelli, A.E. Vercesi, P. Arruda, AtPUMP: an Arabidopsis gene en-coding a plant uncoupling mitochondrial protein, FEBS Lett. 429 (1998) 403 – 406.
[21] C.-S. Lin, M. Klingenberg, Characteristics of the isolated purine nucleotide binding protein from brown fat mito-chondria, Biochemistry 21 (1982) 2950 – 2956.
[22] E. Rial, A. Poustie, D.G. Nicholls, Brown-adipose-tissue mitochondria: the regulation of the 32000-Mruncoupling
protein by fatty acids and purine nucleotides, Eur. J. Biochem. 137 (1983) (2000) 197 – 203.
[23] P. Jezek, H. Engstova, M. Zackova, A.E. Vercesi, A.D.T. Costa, P. Arruda, K.D. Garlid, Fatty acid cy-cling mechanism and mitochondrial uncoupling proteins, Biochim. Biophys. Acta 1365 (1998) 319 – 327.
[24] F.E. Sluse, A.M. Almeida, W. Jarmuszkiewicz, A.E. Vercesi, Free fatty acids regulate the uncoupling protein and alternative oxidase activities in plant mitochondria, FEBS Lett. 433 (1998) 237 – 240.
[25] S. Katiyar, E. Shrago, Reconstitution of purified brown adipose tissue mitochondria uncoupling protein: Demon-stration of separate identity of nucleotide binding and proton translocation sites by chemical probes, Proc. Natl. Acad. Sci. USA 86 (1989) 2559 – 2562.
[26] M. Laloi, M. Klein, J.W. Riesmeier, B. Mu¨ller-Ro¨ber, C. Fleury, F. Bouillaud, D. Ricqier, A plant cold-induced uncoupling protein, Nature 389 (1997) 135 – 136. [27] K. Ito, T. Kusano, K. Tsutsumi, A cold-inducible bZIP
protein gene in radish root regulated by calcium- and cycloheximide-mediated signals, Plant Sci. 142 (1999) 57 – 65.
[28] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning: a Laboratory Manual, second ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989.
[29] K. Ito, K. Tsutsumi, T. Kuzumaki, P.F. Gomez, K. Otsu, K. Ishikawa, A novel growth-inducible gene that encodes a protein with a conserved cold-shock domain, Nucl. Acids Res. 22 (1994) 2036 – 2041.
[30] B. Miroux, V. Frossard, S. Raimbault, D. Ricquier, F. Bouillaud, The topology of the brown adipose tissue mitochondrial uncoupling protein determined with anti-bodies against its antigenic sites revealed by a library of fusion proteins, EMBO J. 12 (1993) 3739 – 3745.
.