B
RIEF
R
EPORT
Acute Effects of Mirtazapine on Sleep Continuity and
Sleep Architecture in Depressed Patients: A Pilot Study
Andrew Winokur, Michael J. Sateia, J. Boyd Hayes, Wendy Bayles-Dazet,
Mary M. MacDonald, and Keith A. Gary
Background: Mirtazapine, a clinically effective antide-pressant, acts by antagonizing centrala
2-adrenergic and 5-HT2/5-HT3receptors. No data are available regarding mirtazapine’s effects on sleep architecture in patients with major depressive disorder.
Methods:Six patients meeting criteria for major depres-sive disorder and scoring $4 on the three Hamilton Depression Rating Scale sleep items were studied. Poly-somnographic evaluations were performed at baseline and after 1 (15 mg at bedtime) and 2 weeks (30 mg at bedtime) of open-label mirtazapine treatment.
Results:Mirtazapine significantly decreased sleep latency and significantly increased total sleep time and sleep efficiency from baseline levels during week 1, with similar results observed after week 2. Mirtazapine did not signif-icantly alter rapid eye movement sleep parameters. Clin-ically, Hamilton Depression Rating Scale and sleep dis-turbance ratings improved after treatment.
Conclusions: Mirtazapine significantly improves sleep continuity in major depressive disorder patients with poor sleep quality at weeks 1 and 2 of treatment, while preserving sleep architecture. Biol Psychiatry 2000;48: 75–78 © 2000 Society of Biological Psychiatry
Key Words: Polysomnography, depression, insomnia
Introduction
T
he majority of major depressive disorder (MDD) patients subjectively report prolonged sleep onset and/or disturbed sleep continuity as the most prevalent symptoms (Winokur and Reynolds 1994). Objective poly-somnographic (PSG) analysis of depressed patients re-veals disrupted sleep continuity, altered rapid eye move-ment (REM) sleep timing, and reductions in slow-wave sleep (SWS) quantity (Kupfer and Reynolds 1992). Addi-tionally, antidepressant drug administration elicits pro-nounced differences in PSG measures of sleep continuityand sleep architecture (Winokur and Reynolds 1994). Sedating antidepressants shorten sleep latency and im-prove sleep continuity, whereas more activating antide-pressants prolong sleep latency and impair sleep continu-ity measures. Sleep architecture also may be differentially altered by antidepressant drugs (e.g., whereas many anti-depressants potently suppress REM sleep, a few do not, despite similar efficacies in treating depression).
Effects of antidepressant drugs on sleep parameters are clinically relevant due to the frequency of sleep com-plaints in depressed patients. Although controlled studies have not evaluated sleep disorder symptoms as criteria in antidepressant drug selection for specific patients to en-hance treatment outcome, clinical experience suggests this may represent an important basis for treatment selection.
The novel a
2-adrenergic and 5-HT2/5-HT3 receptor
antagonist, mirtazapine, is a clinically effective antide-pressant drug (Claghorn et al 1987; Pinder 1997). Subjec-tive complaints of sleep disturbances improved signifi-cantly and rapidly in placebo-controlled studies of depressed patients treated with mirtazapine. Conversely, over half of patients receiving mirtazapine treatment reported daytime somnolence, as compared with 18% in patients randomized to a placebo (Organon 1996). These observations suggest that laboratory studies of mirtaza-pine’s effects on sleep physiology are important in guiding optimal use of this compound; yet, to date, only a single PSG study has examined mirtazapine. Ruigt et al (1990) administered mirtazapine (30 mg) or a placebo at 9:00PM
to six healthy subjects and observed decreased sleep latency, increased total sleep time, and reduced stage 1 sleep and increased stage 3 sleep, indicative of deeper sleep in treated subjects.
In this open-label study we examine the acute effects of 1- and 2-week mirtazapine administration on sleep conti-nuity and sleep architecture variables by employing PSG techniques and subjective sleep measures in six patients with MDD accompanied by subjective sleep complaints.
Methods and Materials
Subjects were recruited from the Psychiatry Department at Dart-mouth Hitchcock Medical Center and from respondents to
newspa-From the Department of Psychiatry, Dartmouth Medical School, Hanover, New Hampshire.
Address reprint requests to Andrew Winokur, M.D., Ph.D., University of Connect-icut Health Center, Department of Psychiatry, 263 Farmington Avenue, Farmington CT 06030-1410.
Received October 12, 1999; revised March 28, 2000; accepted March 30, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
per advertisements. Six patients provided informed consent, en-rolled, and successfully completed the study. Patients were required to meet the following criteria: 18 – 65 years of age with a DSM-IV diagnosis of MDD based upon a semistructured interview; a score of
$18 on the 17-item Hamilton Depression Rating Scale (HDRS), and a score of$4 on the three HDRS sleep items (Hamilton 1960). Patients with a history of primary sleep disorder, significant medical problems, current alcohol or substance abuse, psychosis, suicidal ideation, and those performing shift work were excluded from the study. Patients were screened for “normal” sleep/wake schedules, with typical bed times no earlier than 10:00PMor risings earlier than 6:00AM, and identical schedules maintained in the sleep laboratory. Psychotropic drugs were discontinued at least 1 week before study
initiation, and no subject had been taking fluoxetine or other central nervous system agents with prolonged effects in the previous month. Polysomnography consisted of bilateral monopolar central electroencephalogram (EEG) recording and occipital EEG (C3/ A2, C4/A1, and Oz/A1), bilateral electrooculogram, and sub-mental electromylogram. Single-channel electrocardiogram was monitored and sleep studies scored per standard Rechtschaffen and Kales criteria (Rechtschaffen and Kales 1968). After initial clinical assessment and HDRS administration subjects under-went 2 nights of baseline PSG monitoring. Mirtazapine admin-istration (15 mg at bedtime [h.s.]) was initiated and 2 nights of PSG repeated at the end of week 1. Clinical assessment, adverse event evaluation, and HDRS ratings were repeated, and the Clinical Global Improvement (CGI) scale employed, between the 2 PSG nights (Guy 1976). The CGI reflects clinical global improvement on a scale of 0, no change; 1, slight improve-ment; 2, moderate improveimprove-ment; and 3, marked improvement. Mirtazapine dosage was then increased to 30 mg h.s., and 2-night PSG, clinical assessment, HDRS, and CGI ratings repeated at the end of week two. Clinical treatment response was determined by clinical assessments, HDRS, and CGI performed at weeks 4 and 6.
Polysomnographic data included total sleep time, sleep effi-ciency (total sleep time:time in bed), and sleep latency (onset to first stage 1) asa prioriprimary variables, and total sleep time and percentage of total sleep time for stages 1, 2, 3, and 4; stages 3 and 4 combined to represent SWS; REM; and REM latency as
a priorisecondary variables. Total sleep time measures for stages 1– 4 were recorded to assess potential sleep stage–specific alterations induced by mirtazapine. Mean, SD, and SEM were calculated for each parameter across subjects. The means were then compared at baseline, week 1 (15 mg), and week 2 (30 mg) by one-way repeated-measures analysis of variance (SigmaStat, SPSS, Chicago). Measures achieving statistical significance (p,
.05) were analyzed post hoc by the Bonferroni method due to the relatively small number of comparisons and its increased strin-gency in achieving statistical significance.
Results
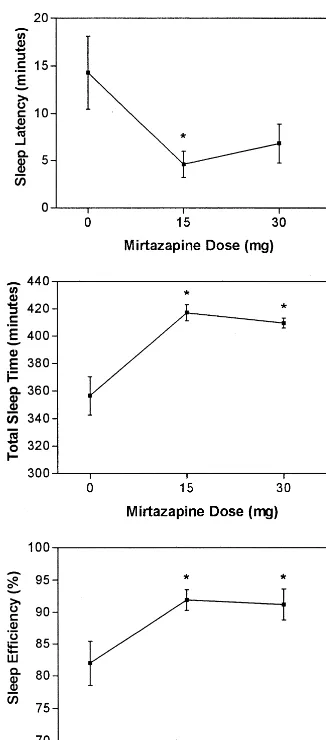
The PSG data demonstrated rapid improvement in sleep continuity measures, with significant changes from base-line observed after both treatment weeks in sleep latency [F(2,17) 5 4.79,p5 .009], total sleep time [F(2,17) 5
16.3,p5.004], and sleep efficiency [F(2,17)510.1,p5
.0003; Figure 1]. Objective improvements in sleep conti-nuity were accompanied by improvements in HDRS and sleep disturbance scores (Table 1). Three of six subjects reported mild daytime sedation and one rated the sedation as moderate, but sedation did not worsen with dosage increase or time and was not cause for study discontinu-ation for any subject.
No significant changes in sleep stage distribution were observed and, specifically, no increase in SWS [F(2,17)
51.87,p5.204], which was low at baseline and remained
low over the 2 weeks of PSG study. No significant
Figure 1. Sleep continuity in six subjects compared with base-line after 1 and 2 weeks of receiving 15 and 30 mg mirtazapine, respectively. The polysomnography data demonstrate rapid and robust improvement in sleep disturbance. Significant reduction in sleep latency was observed, wheras total sleep time and sleep efficiency increased significantly at week 1 (15 mg) and week 2 (30 mg). Data represent the mean (6SD) from all subjects for
each measure and time point. *Statistical significance at thep,
.05 level.
76 BIOL PSYCHIATRY A. Winokur et al
changes in stage 1 percentage [F(2,17)50.505,p5.62],
stage 2 percentage [F(2,17) 5 0.222, p 5 .806], REM
percentage [F(2,17) 5 1.12, p5 .365], total REM time
[F(2,17)5 3.93,p5.059], or REM latency [F(2,17)5
2.92,p5.112; Table 1) were identified.
At the end of week 2 (30 mg mirtazapine h.s.), signif-icant improvements continued in sleep latency, total sleep time, and sleep efficiency, and improvements in HDRS ratings and sleep disturbance scores were also sustained. Despite the increased mirtazapine dose, daytime somno-lence complaints diminished or abated, with only two subjects continuing to report daytime sleepiness at levels of “mild.” No significant alterations in sleep architecture were observed at the week 2 testing period, though a trend for prolonged REM latency was evident.
Discussion
Our results suggest that acute mirtazapine administration rapidly improves both subjective ratings of depression and objective physiologic parameters of sleep disturbance in patients with MDD accompanied by disturbed sleep. Although four of six subjects reported daytime somno-lence during week 1 (15 mg), these complaints resolved by the end of week 2 (30 mg). Thus, patients may develop tolerance to mirtazapine’s sedating effects within a few days of treatment initiation. Conversely, no loss of effi-cacy was observed with respect to the sleep continuity measures across the 2-week period assessed.
The mechanisms underlying mirtazapine’s sleep-pro-moting effects have not been clarified, although its phar-macologic properties suggest several possibilities (Pinder 1997). Both antihistaminic and a-adrenolytic actions of
mirtazapine might contribute to its sleep-enhancing ef-fects. Alternatively, the potent effects of mirtazapine may result from 5-HT2receptor inhibition (de Boer 1996), as
the 5-HT2receptor is an important site implicated in sleep
initiation and/or maintenance with particular relevance to SWS regulation (Idzikowski et al 1989). Moreover, the 5-HT2receptor may mediate the activating effects of some
selective serotonin reuptake (Stahl 1996). Further studies will be needed to explore the possible relationship be-tween mirtazapine and the 5-HT2receptor with respect to
sleep physiology.
Our study was limited by several factors. First, inclu-sion of only six patients places these findings in the context of a pilot study, requiring replication and exten-sion with a considerably larger sample size. Nevertheless, these findings constitute the first published report of mirtazapine’s effects on sleep physiology in depressed patients. Second, the treatment protocol was conducted in an open fashion without a placebo control group, limiting the interpretability of the treatment response data (though extensive double-blind treatment response data for mir-tazapine have previously been presented) and, arguably, of the findings pertaining to the sleep physiology parameters. Finally, mirtazapine’s effects on daytime sleepiness were obtained exclusively from subjective patient reports, with-out objective assessments of daytime sleepiness.
Despite study limitations, the results suggest several intruiging implications. Both depression ratings and sleep disturbance symptoms improved significantly in this pa-tient population after 1 week of mirtazapine administra-tion. Conceivably, rapid relief of insomnia symptoms may have contributed to the rapidity of antidepressant response. The magnitude of objective changes in sleep continuity
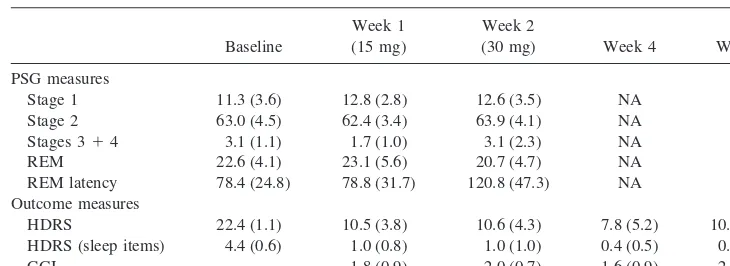
Table 1. Sleep Architecture and Treatment Outcome Measures Obtained from Patients before and after Mirtazapine Treatment
Baseline
Week 1 (15 mg)
Week 2
(30 mg) Week 4 Week 8 PSG measures
Stage 1 11.3 (3.6) 12.8 (2.8) 12.6 (3.5) NA NA Stage 2 63.0 (4.5) 62.4 (3.4) 63.9 (4.1) NA NA Stages 314 3.1 (1.1) 1.7 (1.0) 3.1 (2.3) NA NA
REM 22.6 (4.1) 23.1 (5.6) 20.7 (4.7) NA NA REM latency 78.4 (24.8) 78.8 (31.7) 120.8 (47.3) NA NA Outcome measures
HDRS 22.4 (1.1) 10.5 (3.8) 10.6 (4.3) 7.8 (5.2) 10.4 (5.1) HDRS (sleep items) 4.4 (0.6) 1.0 (0.8) 1.0 (1.0) 0.4 (0.5) 0.2 (0.4) CGI — 1.8 (0.9) 2.0 (0.7) 1.6 (0.9) 2.0 (0.7)
Sleep architecture observed in six subjects compared with baseline after 1 and 2 weeks of receiving 15 and 30 mg mirtazapine, respectively. Drug treatment did not significantly alter sleep-stage distribution, slow-wave sleep (stages 3:4 percentage), or percentage of sleep spent in rapid eye movement (REM). There was a nonsignificant trend toward increased REM latency by week 2 of mirtazapine treatment. Data represent the mean (6SD) from all subjects for each measure and time point. Treatment outcomes data represent the mean (6SD) from all subjects for each measure and time point. HDRS reflects total score on the 17-item Hamilton Depression Rating Scale. HDRS (sleep items) reflects the total score for the three sleep items on the HDRS 17-item scale. CGI reflects clinical global improvement on a scale of 0, no change; 1, slight improvement; 2, moderate improvement; 3, marked improvement. PSG, polysomnography.
Effects of Mirtazapine on Sleep in Depression BIOL PSYCHIATRY 77
parameters produced by mirtazapine was strikingly large, with total sleep time increased by over 1 hour after week 1 and associated with a 9% increase in sleep efficiency. These increases in sleep continuity parameters are larger than generally reported in PSG-monitored studies of anti-depressant drug treatment (Rush et al 1998) and compare favorably to effects reported for specific hypnotic agents developed for primary insomnia treatment. Thus, addi-tional studies examining mirtazapine’s short- and long-term effects on sleep parameters both in depressed patients with coexisting insomnia and in individuals with subjec-tive complaints of insomnia for other reasons are warranted.
Funding provided by an unrestricted educational grant from Organon Inc. (AW).
References
Claghorn JL, Johnstone EE, Studebaker SL, Ajeman SE (1987): The effectiveness of Org 3770 in depressed patients. Psycho-pharmacol Bull23:160 –161.
de Boer T (1996): The pharmacologic profile of mirtazapine.
J Clin Psychiatry57:19 –25.
Guy W (1976):ECDEU Assessment Manual for Psychopharma-cology.Rockville, MD: National Institute of Mental Health.
Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry23:56 – 62.
Idzikowski C, James R, Burton SW (1989): Human SWS is increased dose-dependently by seganserine and ritanserine.
Sleep Res18:55.
Kupfer DJ, Reynolds CF III (1992): Sleep and affective disor-ders. In: Paykel ES, editor.Handbook of Affective Disorders,
2nd ed. Edinburgh: Churchill Livingstone, 311–323. Organon (1996):Remeron—a Novel Pharmacological Treatment
for Depression.West Orange, NJ: Organon.
Pinder RM (1997): The pharmacology of mirtazapine. J Clin Psychiatry58:501–502.
Rechtschaffen A, Kales A (1968):A Manual of Standardized Terminology, Techniques, and Scoring for Sleep Stages of Human Subjects. Bethesda, MD: National Institutes of Health, Neurologic Information Network.
Ruigt GS, Kemp B, Groenhout CM, Kamphuisen HA (1990): Effect of the antidepressant Org 3770 on human sleep.Eur J Clin Pharmacol38:551–554.
Rush AJ, Armitage R, Gillin JC, Yonkers KA, Winokur A, Moldofsky H, et al (1998): Comparative effects of nefaz-odone and fluoxetine on sleep in outpatients with major depressive disorder.Biol Psychiatry44:3–14.
Stahl S (1996):Essential Psychopharmacology.Cambridge, UK: Cambridge University Press.
Winokur A, Reynolds CF III (1994): The effects of antidepres-sants on sleep physiology.Primary Psychiatry1:22–27.
78 BIOL PSYCHIATRY A. Winokur et al