www.elsevier.comrlocateranireprosci
Comparative aspects of equine embryonic
development
Keith J. Betteridge
)Animal Biotechnology – Embryo Laboratory, Department of Biomedical Sciences, Ontario Veterinary College, UniÕersity of Guelph, Building 165-ABEL, Guelph Ontario, Canada, N1G 2W1
Abstract
The developmental changes in the equine conceptus, its maternal environment and their interaction during the first 4 weeks following fertilization are reviewed. Attention is drawn to species-specific events to show why the horse is such a valuable model in which to study early pregnancy.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Horse; Embryo; Pregnancy; Capsule; Blastocyst; Endometrium; Oviduct
1. Introduction
Ž .
The three purposes of this paper are to: 1 review briefly our knowledge of the embryonic development in the horse during the 4 weeks following fertilization, paying special attention to features that distinguish early equine ontogeny from analogous
Ž .
events in other mammals; 2 argue that many reproductive characteristics of horses provide us with unusual, and under-used, opportunities to contribute to comparative
Ž .
studies of early mammalian pregnancy; 3 draw attention to the practical importance of understanding early pregnancy in the mare.
The advocacy of the horse as a model in studying early pregnancy is by no means
Ž .
new. More than a century ago, Ewart 1897 beautifully illustrated how the early equine
Ž .
conceptus resembles that of the Virginian opossum and reasoned probably wrongly that pregnancy failure at about 42 days in horses could reflect an evolutionary call for the conceptus to be ‘‘born’’ at the time appropriate to such a marsupial. More recently,
Ž .
Renfree 1982 has drawn attention to the similarity of blastocyst expansion in the horse
)Tel.:q1-519-824-4120; fax:q1-519-824-1643.
Ž .
E-mail address: [email protected] K.J. Betteridge .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
Ž .
and another marsupial, the tammar wallaby. Betteridge 1989,1995a,b and Denker Ž2000 have reviewed how horse studies have taught us much about comparative aspects. of early development, and of blastocyst coverings in particular.
2. Developmental events
2.1. General
Ž
It should be emphasized at the outset that the conceptus i.e., the embryo proper and .
its associated membranes and fluid needs always to be considered as only one partner in the physiological dialogue between it and its environment. Focusing on the conceptus is both useful and convenient but can be misleading if this interaction is forgotten. Clinically, such a focus is much easier in horses than in other domestic animals because the whole conceptus, and even the embryo itself, are so easy to monitor by
ultrasonogra-Ž
phy Palmer and Driancourt, 1980; Chevalier and Palmer, 1982; Simpson et al., 1982; .
Ginther, 1986 . On the other hand, developmental studies in horses have suffered in comparison with those of other species in which embryo production in vitro has progressed so very much further. These considerations should be kept in mind in the following commentary on the future foal’s acquisition of steadily more complex
Ž .
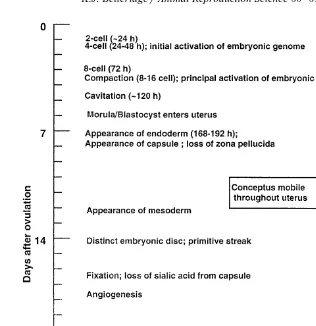
conformation and activity in the mare’s reproductive tract Fig. 1 .
2.2. DeÕelopment in the oÕiduct
2.2.1. The oÕiductal enÕironment
Released as it is from a follicle containing in excess of 20 ml of rich liquor, it might be expected that the equine oocyte’s final preparation for fertilization would be strongly influenced by follicular fluid. Strangely, this may not be the case; Townson and Ginther Ž1989 found no corresponding distension of the oviduct immediately after ovulation,.
Ž .
and Palmer et al. 1997 demonstrated that follicular fluid, while probably helpful, is not absolutely essential to convey the oocyte to the ampulla. Nevertheless, fibroblasts apparently do enter the oviduct at ovulation and produce collagen masses in its lumen,
Ž .
which may affect early development Lantz et al., 1998 . Considering that fertilized and
Ž .
unfertilized equine eggs are transported differentially in the mare see below , it seems unlikely that follicular contents influence embryo transport in the mare, as has been
Ž .
proposed to occur in cattle Wijayagunawardane et al., 1999 .
The reciprocal nature of the dialogue between the embryo and the mare’s reproduc-tive tract almost certainly begins immediately in the oviduct. The histochemical charac-teristics of the oviductal epithelium vary with reproductive status and from region to
Ž .
region Ball et al., 1997 . Consequently, it should be remembered that the equine embryo spends most of its 6-day sojourn in the oviduct near the ampullary–isthmic
Ž .
junction of that organ Weber et al., 1996 . The oviductal secretory products are plentiful but, paradoxically — in view of the histochemical findings, show no clear
Ž .
Fig. 1. Approximate timing of some key developmental events during the first 4 weeks of gestation of the equine conceptus.
Functional characteristics of the oviduct, manifest as effects on embryo survival,
Ž .
evidently vary with age Carnevale et al., 1993 . The clearest evidence, however, that the oviductal epithelium affects embryo development comes from in vitro culture studies, which have demonstrated the beneficial effects of co-culture of cleaving
Ž .
embryos with oviductal epithelial cells Ball and Miller, 1992 . On the other hand, the equine endosalpinx cannot be absolutely essential for normal development, given that
Ž
viable horse embryos can develop in the oviduct of nonpregnant ewes Fehilly and .
Willadsen, 1986 . In cattle, at least, the oviductal epithelium produces endothelin, which Ž
may affect gamete transport either directly, by local effects on contractility Rosselli et
. Ž .
al., 1994 , or indirectly, by affecting local blood flow Garcia-Pascual et al., 1996 .
2.2.2. Embryonic deÕelopment in the oÕiduct
‘overtaken’ by developing embryos resulting from subsequent ovulations. Stemming
Ž .
from the original observation by Van Niekerk and Gerneke 1966 , experiments have shown that this is likely to involve the embryonic production of prostaglandin E2 in
Ž .
horses Weber and Woods, 1993; Weber et al., 1991a,b, 1995; Freeman et al., 1992 . Acceptance of the reality of this phenomenon in horses led to analogous observations in
Ž .
other species bat, Rasweiler, 1979; hamster, Ortiz et al., 1986; rats, Ortiz et al., 1989 . Ž
Completing the circle, it is interesting to note that hamster studies Velasquez et al., .
1995, 1997 now suggest that other embryonic products, such as platelet activating factor, may also deserve investigation in the horse. In this context, it should be noted that activation of the embryonic genome occurs while the embryo is still in the oviduct
Ž
and is a gradual process at the four- to eight-cell stage Ball et al., 1993; Grøndahl et al., .
1993; Brinsko et al., 1995 .
In the mouse at least, the structure of the oviduct wall is affected locally by the
Ž . Ž .
presence of the oocyte s in the cumulus mass soon after ovulation Sato et al., 1995 . The mouse has also provided us with fascinating indications that the interaction between developing embryos and the oviduct affects the subsequent interactions between
em-Ž .
bryos and the uterus, and thereby pregnancy rates Wakuda et al., 1999 .
Several comprehensive descriptions of fertilization and embryo cleavage in the horse have been summarized and compared with the timing of successive divisions in other
Ž .
domestic species Betteridge, 1995a . One unusual feature of early equine embryogene-sis is the polarity and opacity of the oocyte and zygote, a topic of increasing interest and
Ž .
practical significance in other mammals, including humans Fulka et al., 1998 . Others include the contents of the perivitelline space, the often ellipsoidal shape of the cleaving egg, and the fact that the cleaving embryo acquires additional coats on the outside of its zona pellucida. However, these have received relatively little attention, largely because of the logistical difficulties and expense of obtaining embryos from the oviduct at surgery or slaughter. Attempts to gain access to cleaving embryos by hastening their transport through the oviduct by application of a gel preparation of prostaglandin E2 to the parietal surface of the oviduct through an endoscope have met with some success ŽRobinson et al., 1998 . Unfortunately, however, it is suspected that an irritant substance.
Ž in the gel vehicle may cause inflammation and lead to scarring of the mesosalpinx W.R.
. Allen, personal communication .
The time taken for the cleaving embryo to traverse the oviduct, previously estimated
Ž .
to be 5.5–6.0 days, has recently been shown to be 6.0–6.5 days Battut et al., 1997 . The last part of the journey, through the isthmus, is accomplished relatively rapidly ŽWeber et al., 1996 . By the time the embryo enters the uterus, development has. progressed to the late morula or early blastocyst stage. Much more is known about development from this point onwards because of the ease with which pre-attachment conceptuses can be recovered from the uterus by transcervical lavage.
2.3. DeÕelopment in the uterus
2.3.1. The uterine enÕironment
Ž .
corpus luteum CL to be maintained; the other is associated with the nutrition of the developing conceptus. Physiologically, however, these two functions of the pregnant uterus are inextricably mixed and dependent on interaction with the conceptus, as has already been stressed. Indeed, the uterine environment, as reflected in uterine flushings
Ž
during pregnancy, often includes materials produced by the conceptus Zavy et al., 1982, .
1984 .
The mechanisms by which luteolysis is abrogated in the pregnant mare are still far Ž from being completely understood. It is best viewed as being a gradual process Sharp et
.
al., 1989 and clearly involves down-regulation of receptors for oxytocin in the endometrium so that luteolytic releases of endometrial prostaglandin F2a are prevented Žsee Stout et al., 1999 for references . Oxytocin itself, neurophysin, and the messages for. both have been demonstrated in the endometrium by immunohistochemistry and RT-PCR,
Ž .
respectively Watson et al., 1998; Behrendt-Adam et al., 1999 . Both oxytocin and arginine vasopressin have also been detected in the yolk sac fluid, their concentrations
Ž .
increasing after day 12 of gestation Waelchli et al., 2000a .
Numerous progesterone-dependent proteins, presumably vital to the nutrition of the conceptus, have been shown to be produced by the uterus. Of these, by far the best
Ž .
characterized is the lipocalin P19 Stewart et al., 1995; Crossett et al., 1996, 1998 , Ž
which probably helps transport small, hydrophobic molecules e.g., steroids and
. Ž .
eicosanoids into the yolk sac of the conceptus see below . A distinct endometrial
Ž .
retinol-binding protein has also been cloned in the horse McDowell et al., 1995 and can be presumed to play a vital role in embryonic development by transporting morphogenic retinoids.
Ž .
Numerous proteins found in the embryonic capsule see below are under investiga-tion to determine which are of maternal origin and which come from the conceptus.
Ž
Several of these are growth factors and their binding proteins Herrler et al., 1998, 1999; .
Herrler and Beier, 2000 .
2.3.2. Conceptus deÕelopment in the uterus
It is in the uterus that developmental characteristics of the conceptus in the horse become especially distinct from those of other domestic species. First of all, the equine embryo, its fetal membranes and its circulatory system develop to an extraordinary
Ž .
extent before even the first signs of a functional yolk sac choriovitelline placenta at
Ž .
day 22 Enders et al., 1993 and allantochorionic placenta at about 40 days after ovulation. Second, the endodermal wall of the yolk sac is completed from cells that are unusually broadly distributed around the interior of the blastocyst, and which seem to
Ž .
have unusual functional characteristics Enders et al., 1993 . Third, although as in the other species the zona pellucida is shed from the blastocyst, it is replaced in the horse by a glycoprotein capsule of considerable functional importance between approximately days 7 and 21. Fourth, the encapsulated conceptus interacts with its environment during two distinct phases; it is extremely mobile until about days 16–17 and then becomes
Ž .
There are numerous structural changes associated with conceptus development during Ž
the second and third weeks of pregnancy Betteridge et al., 1982; Flood et al., 1982; .
Enders and Liu, 1991; Enders et al., 1988, 1993 . They become of major importance in interpreting the corresponding functional changes that are undergone during this period,
Ž .
as is especially well explained for the cellular tissues by Enders et al. 1993 . The formation and dissolution of the acellular capsule have also been the subject of much attention. This interest is likely to increase, given the importance of the capsule as a
Ž .
‘‘mailbox’’ for proteins and perhaps other molecules? being exchanged between the Ž
conceptus and the endometrium Crossett et al., 1998; Herrler et al., 1998, 1999; Herrler .
and Beier, 2000 . Furthermore, the development of the capsule soon after the blastocyst enters the uterus is of practical significance. It radically changes the ease with which equine blastocysts can be cryopreserved, presumably because of an effect on permeation
Ž .
by cryoprotective agents Bruyas, 1998; Hochi, 1998 and also makes the
micromanipu-Ž .
lation of the embryo very difficult Huhtinen et al., 1997 .
The capsule is composed of a mucin-like glycoprotein, produced largely by the
Ž .
trophoblast Oriol et al., 1993a,b . However, the presence of mRNA from the mucin
Ž .
gene MUC1 in both the trophoblast and endometrium Gillies et al., 1999 suggests that an endometrial contribution to its formation should not yet be ruled out. It is interesting that sialic acid is lost from the capsule at the time of conceptus fixation, raising the
Ž .
possibility of changes in charge being involved in the mechanism s of attachment to the endometrium. The loss of sialic acid does not occur if the mare is treated experimentally
Ž .
with PGF2a at day 14 Chu et al., 1996 . The capsule is a strong and resilient structure and it is thought to play a protective role and to facilitate migration throughout the
Ž .
uterine lumen before day 16 Betteridge, 1989 . This migration is essential to the
Ž .
maintenance of pregnancy Sharp et al., 1989 . After fixation, capsule production appears to decline until it ruptures at about day 21 through mechanisms, perhaps
Ž .
enzymatic, that remain unclear Denker, 2000 .
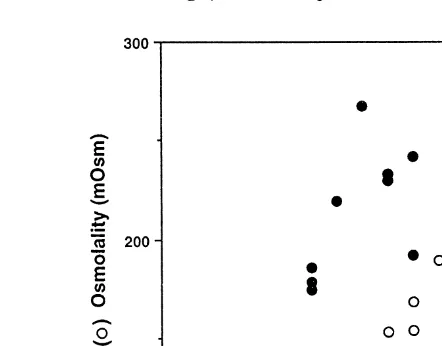
Blastocyst expansion mechanisms in horses seem to differ markedly from those observed in other species; the fluid within the expanding yolk sac is markedly hypotonic Ž;120 mosMrkg until, after day 16, it begins to rise slowly to. ;250 mosMrkg at
Ž .
about day 22 Waelchli and Betteridge, 1996; Waelchli et al., 1997 . How fluid can be
Ž .
imbibed from a uterine fluid environment of higher osmolality Waelchli et al., 2000b is still under investigation. The gradual rise in osmolality after day 16 is reflected in a
Ž
parallel increase in the concentration of fructose within the yolk sac Ruddock et al., .
2000; Fig. 2 .
There are two basic approaches to investigating secretory products of the conceptus that are likely to be important to its interaction with the mare. The first is to hypothesize the existence of a substance and then to search for it either in the blastocyst fluid or in medium used to culture conceptus tissues. The second is to use various analytical techniques to detect an array of products, then to identify members of the array and hypothesize about their functions. The approaches are complementary but the advent of
Ž .
Fig. 2. Changes in the osmolality and fructose concentrations of equine yolk sac fluid during the second to fourth weeks of pregnancy.
blastocyst fluid could have been conveyed there by transport proteins such as P19 ŽCrossett et al., 1998 . Identifying the origin of a putative product must, therefore,. involve culture in vitro andror tissue analysis by immunological or molecular probes. Since the first demonstrations of estrogen and androgen production by the equine
Ž .
conceptus in the 1970s Flood et al., 1979; Zavy et al., 1979 , the most intensive study of steroidogenesis in these tissues has been by Goff and his colleagues. They have shown that 17a-hydroxyprogesterone, and not oestradiol as had been expected, is the major steroid synthesized by the equine conceptus between days 7 and 14 of pregnancy. They also showed that this steroid is further metabolized to an unidentified steroid by the endometrium and reasoned that these steroids could play a role in conceptus
Ž .
development or the prevention of luteolysis Goff et al., 1993 . Earlier, the same group had shown that endoderm and trophectoderm cells from the conceptus exhibit different steroidogenic profiles in vitro, leading them to suggest that the two tissues need to act in
Ž .
concert if pregnancy is to proceed Marsan et al., 1987 .
With regard to the eicosanoids, the equine conceptus continues to produce PGE2 following its passage into the uterus, production increasing between days 11 and 15 ŽVanderwall et al., 1993 . The conceptus also produces PGF2. a by day 14 Watson andŽ
. Sertich, 1989 .
The production of proteins and peptides by the conceptus has been the subject of
Ž .
many investigations. McDowell et al. 1990 demonstrated that the array of proteins produced by conceptuses before day 14 differed from the array produced by older conceptuses in which a pattern characteristic of yolk sac tissue emerged. Searching for a conceptus secretory product capable of suppressing PGF2a production from
en-Ž .
dometrium in vitro, Sharp et al. 1989 used dialysis membranes with different molecu-lar exclusion properties to conclude that a molecule of Mr)1000 and -6000 is
Ž .
Ž
conceptuses. They have identified cDNA clones for calcyclin a calcium binding
. Ž .
protein and phospholipase A2 involved in eicosanoid metabolism to be differentially
Ž .
expressed at these times. Green et al. 1999 have identified a new aspartic proteinase that is produced by the equine trophoblast as early as day 15 and which would seem to be related to the pregnancy-associated glycoproteins of the order Artiodactyla.
3. The value of studies of equine embryology
3.1. The horse as a model species in embryology
In no other domestic species can entire conceptuses, including embryos undergoing advanced organogenesis, be obtained from the uterus so easily, repeatedly, atraumati-cally, and free of maternal tissues. This fact, together with the unusual features summarized above, underline just how valuable horses are as a model species in which
Ž .
to investigate early pregnancy Betteridge, 1995b . In my view, the demonstrated and potential advantages of the model go a long way toward offsetting costs.
3.2. Practical implications of equine embryology
Early pregnancy is a time of considerable embryonic death and economic loss in the equine breeding industry. Following high fertilization rates, an estimated 30–40% of
Ž
pregnancies are lost within 2 weeks Ball, 1988; Baker, 1995, cited by Simpson et al., .
1999 . Paradoxically, this is fortunate in that it provides a practical incentive to undertake the research necessary to combat the problem. That research will quite certainly also pay dividends in unanticipated ways in the broader field of reproductive biology.
3.3. Future needs and prospects
The advantages of horses as a model in which to study uterine conceptuses is severely offset by the difficulty of obtaining younger embryos from the oviduct ex vivo. The analogous problem in other farm species has been largely circumvented by efficient production of embryos in vitro. Although ICSI is providing some encouraging results in
Ž horses, there is a real need to improve methods of producing embryos in vitro Guignot
.
et al., 1998; Hochi, 1998 . A related problem is that of conceptus culture; although cleavage stage embryos can be cultured successfully in vitro, there is not yet a system that will meet the requirements of the expanding blastocyst and allow it to grow and produce a capsule as it does in the uterus.
4. Concluding remarks
analysis of uterine flushings to molecular dissection of the time at which genes controlling the production of an infinite variety of products become transcriptionally active in the conceptus and the mare’s reproductive tract. These advances create a crucial role for reproductive biologists with a broad enough background to synthesize useful biological ‘stories’ from masses of detailed but isolated findings. Providing the training required by such individuals constitutes a challenge that needs to be met.
Acknowledgements
I am grateful to Drs. J.I. Raeside and R.O. Waelchli, and to P. Nevins, W.D.J. Ruddock and L.J.E. Crews for their help in the preparation of this manuscript. The financial support of NSERC Canada and OMAFRA is also much appreciated.
References
Ball, B.A., 1988. Embryonic loss in mares: incidence, possible causes, and diagnostic considerations. Vet. Clin. North Am.: Equine Pract. 4, 263–290.
Ball, B.A., Dobrinski, I., Fagnan, M.S., Thomas, P.G., 1997. Distribution of glycoconjugates in the uterine
Ž .
tube oviduct of horses. Am. J. Vet. Res. 58, 816–822.
Ball, B.A., Ignotz, G.G., Brinsko, S.P., Thomas, P.G.A., Miller, P.G., Ellington, J.E., Currie, W.B., 1993. The in-vitro block to development and initiation of transcription in early equine embryos. Equine Vet. J.
ŽSuppl. 15, 87–90..
Ball, B.A., Miller, P.G., 1992. Survival of equine embryos co-cultured with equine oviductal epithelium from the four- to eight-cell to the blastocyst stage after transfer to synchronous recipient mares. Theriogenology 37, 979–991.
Battut, I., Colchen, S., Fieni, F., Tainturier, D., Bruyas, J.F., 1997. Success rates when attempting to
Ž .
nonsurgically collect equine embryos at 144, 156 or 168 hours after ovulation. Equine Vet. J. Suppl. 25, 60–62.
Behrendt-Adam, C.Y., Adams, M.H., Simpson, K.S., McDowell, K.J., 1999. Oxytocin-neurophysin I mRNA abundance in equine uterine endometrium. Domest. Anim. Endocrinol. 16, 183–192.
Betteridge, K.J., 1989. Structure and function of the equine capsule in relation to embryo manipulation and
Ž .
transfer. Equine vet. J. Suppl. 8, 92–100.
Betteridge, K.J., 1995a. Phylogeny, ontogeny and embryo transfer. Theriogenology 44, 1061–1098. Betteridge, K.J., 1995b. Equine pregnancy: the road from Caxambu?. Biol. Reprod. Mono. Ser. 1, 115–123. Betteridge, K.J., Eaglesome, M.D., Mitchell, D., Flood, P.F., Beriault, R., 1982. Development of horse´ embryos up to twenty-two days after ovulation: observations on fresh specimens. J. Anat. 135, 191–209. Brinsko, S.P., Ball, B.A., Ignotz, G.G., Thomas, P.G., Currie, W.B., Ellington, J.E., 1995. Initiation of
transcription and nucleologenesis in equine embryos. Mol. Reprod. Dev. 42, 298–302.
Bruyas, J.-F., 1998. Contribution a l’etude de la congelation des embryons equins: une approche metabolique` ´ ´ ´ ´ et cellulaire. Thesis, ENSA, Rennes.
Carnevale, E.M., Griffin, P.G., Ginther, O.J., 1993. Age-associated subfertility before entry of embryos into
Ž .
the uterus of the mare. Equine Vet. J. Suppl. 15, 31–35.
Chevalier, F., Palmer, E., 1982. Ultrasonic echography in the mare. J. Reprod. Fertil., Suppl. 32, 423–430. Chu, J.W.K., Sharom, F.J., Oriol, J.G., Betteridge, K.J., Cleaver, B.D., Sharp, D.C., 1996. Biochemical changes in the equine capsule following prostaglandin-induced pregnancy failure. Mol. Reprod. Dev. 46, 286–295.
Crossett, B., Suire, S., Herrler, A., Allen, W.R., Stewart, F., 1998. Transfer of a uterine lipocalin from the endometrium of the mare to the developing equine conceptus. Biol. Reprod. 59, 483–490.
Denker, H.-W., 2000. Structural dynamics and function of early embryonic coats. Cell Tiss. Org. 166, 180–207.
Enders, A.C., Liu, I.K., 1991. Lodgement of the equine blastocyst in the uterus from fixation through endometrial cup formation. J. Reprod. Fertil., Suppl. 44, 427–438.
Enders, A.C., Lantz, K.C., Liu, I.K., Schlafke, S., 1988. Loss of polar trophoblast during differentiation of the blastocyst of the horse. J Reprod. Fertil. 83, 447–460.
Enders, A.C., Schlafke, S., Lantz, K.C., Liu, I.K., 1993. Endoderm cells of the equine yolk sac from day 7
Ž .
until formation of the definitive yolk sac placenta. Equine Vet. J. Suppl. 15, 3–9.
Ewart, J.C., 1897. A Critical Period in the Development of the Horse. Adam and Charles Black, London.
Ž .
Fehilly, C., Willadsen, S.M., 1986. Embryo manipulation in farm animals. In: Clarke, J.R. Ed. , Oxford Reviews of Reproductive Biology. Clarendon Press, Oxford, pp. 379–413.
Flood, P.F., Betteridge, K.J., Irvine, D.S., 1979. Oestrogens and androgens in blastocoelic fluid and cultures of cells from equine conceptuses of 10–22 days gestation. J. Reprod. Fertil., Suppl. 27, 413–420.
Flood, P.F., Betteridge, K.J., Diocee, M.S., 1982. Transmission electron microscopy of horse embryos 3–16 days after ovulation. J. Reprod. Fertil., Suppl. 32, 319–327.
Freeman, D.A., Woods, G.L., Vanderwall, D.K., Weber, J.A., 1992. Embryo-initiated oviductal transport in the mare. J. Reprod. Fertil. 95, 535–538.
Fulka, J. Jr., Karnikova, L., Moor, R.M., 1998. Oocyte polarity: ICSI, cloning and related techniques. Hum. Reprod. 13, 3303–3305.
Garcia-Pascual, A., Labadia, A., Triguero, D., Costa, G., 1996. Local regulation of oviductal blood flow. Gen. Pharmacol. 27, 1303–1310.
Gillies, L., Waelchli, R.O., Ruddock, W.D.J., Betteridge, K.J., LaMarre, J., 1999. Patterns of MUC1
Ž .
expression in the equine endometrium and trophoblast during early pregnancy. Theriogenology Abstract 51, 225.
Ginther, O.J., 1986. Ultrasonic Imaging and Reproductive Events in the Mare. Equiservices, Cross Plains, WI. Goff, A.K., Leduc, S., Poitras, P., Vaillancourt, D., 1993. Steroid synthesis by equine conceptuses between
days 7 and 14 and endometrial steroid metabolism. Domest. Anim. Endocrinol. 10, 229–236.
Green, J.A., Xie, S., Szafranska, B., Gan, X., Newman, A.G., McDowell, K., Roberts, R.M., 1999. Identification of a new aspartic proteinase expressed by the outer chorionic layer of the equine placenta. Biol. Reprod. 60, 1069–1077.
Grøndahl, C., Grøndahl Nielsen, C., Eriksen, T., Greve, T., Hyttel, P., 1993. In-vivo fertilisation and initial
Ž .
embryogenesis in the mare. Equine Vet. J. Suppl. 15, 79–83.
Guignot, F., Ottogalli, M., Yvon, J.-M., Magistrini, M., 1998. Preliminary observations in in vitro develop-ment of equine embryo after ICSI. Reprod. Nutr. Dev. 38, 653–663.
Herrler, A., Stewart, F., Crossett, B., Pell, J.M., Ellis, P.D., Brown, K.D., Beier, H.M., Allen, W.R., 1998. Maternal and embryonic proteins in the equine blastocyst capsule. In: Proc. 7th Int. Symp. Eq. Reprod., Pretoria. pp. 157–158, Abstract.
Herrler, A., Stewart, F., Allen, W.R., Beier, H.M., 1999. Proteins detectable in the coats of preimplantation
Ž .
rabbit and horse embryos. J. Reprod. Fertil. Abstr. Ser. 23, 33, Abstract 80 .
Herrler, A., Beier, H.M., 2000. Early embryonic coats: morphology, function, practical applications. Cell Tiss. Org. 166, 233–246.
Hochi, S., 1998. Current status of IVMrIVFrIVC technology and embryo cryopreservation in the equine species. J. Mammal. Ova Res. 15, 117–127.
Huhtinen, M., Peippo, J., Bredbacka, P., 1997. Successful transfer of biopsied equine embryos. Theriogenol-ogy 48, 361–367.
Lantz, K.C., Enders, A.C., Liu, I.K., 1998. Possible significance of cells within intraluminal collagen masses in equine oviducts. Anat. Rec. 252, 568–579.
Marsan, C., Goff, A.K., Sirois, J., Betteridge, K.J., 1987. Steroid secretion by different cell types of the horse conceptus. J. Reprod. Fertil., Suppl. 35, 363–369.
McDowell, K.J., Adams, M.H., Williams, N.M., 1993. Characterization of equine oviductal proteins synthe-sized and released at estrus and at day 4 after ovulation in bred and nonbred mares. J. Exp. Zool. 267, 217–224.
McDowell, K.J., Adams, M.H., Franklin, K.M., Baker, C.B., 1995. Changes in equine endometrial retinol-bi-nding protein RNA during the estrous cycle and early pregnancy and with exogenous steroids. Biol. Reprod. 52, 438–443.
Oriol, J.G., Betteridge, K.J., Clarke, A.J., Sharom, F.J., 1993a. Mucin-like glycoproteins in the equine embryonic capsule. Mol. Reprod. Dev. 34, 255–265.
Oriol, J.G., Sharom, F.J., Betteridge, K.J., 1993b. Developmentally regulated changes of the equine embryonic capsule. J. Reprod. Fertil. 99, 653–664.
Ortiz, M.E., Bedregal, P., Carvajal, M.I., Croxatto, H.B., 1986. Fertilized and unfertilized ova are transported at different rates by the hamster oviduct. Biol. Reprod. 34, 777–781.
Ortiz, M.E., Llados, C., Croxatto, H.B., 1989. Embryos of different ages transferred to the rat oviduct enter the uterus at different times. Biol. Reprod. 41, 381–384.
Palmer, E., Driancourt, M.A., 1980. Use of ultrasonic echography in equine gynecology. Theriogenology 13, 203–216.
Palmer, E., Duchamp, G., Cribiu, E.P., Mahla, R., Boyazoglu, S., Bezard, J., 1997. Follicular fluid is not a´
Ž .
compulsory carrier of the oocyte at ovulation in the mare. Equine Vet. J. Suppl. 25, 22–24.
Rasweiler, J.J. IV, 1979. Differential transport of embryos and degenerating ova by the oviducts of the long-tongued bat, Glossophaga soricina. J. Reprod. Fertil. 55, 329–334.
Ž .
Renfree, M.B., 1982. Implantation and placentation. In: Austin, C.R., Short, R.V. Eds. , Reproduction in Mammals. 2nd edition Cambridge Univ. Press, Cambridge, pp. 26–69.
Robinson, S.J., Neal, H., Allen, W.R., 1998. Modulation of oviductal transport in the mare by local application of prostaglandin E2. In: Proc. 7th Int. Symp. Eq. Reprod., Pretoria. pp. 153–154, Abstract.
Rosselli, M., Imthurn, B., Macas, E., Keller, P.J., 1994. Endothelin production by bovine oviduct epithelial cells. J. Reprod. Fertil. 101, 27–30.
Ruddock, W.D.J., Crews, L.J.E., Waelchli, R.O., Betteridge, K.J., 2000. Fructose accumulation by the equine
Ž .
conceptus during the second to fourth weeks of gestation. Theriogenology Abstract 53, 286.
Sato, E., Ando, N., Takahashi, Y., Miyamoto, H., Toyoda, Y., 1995. Structural changes in the oviductal wall during the passage of unfertilized cumulus-oocyte complexes in mice. Anat. Rec. 241, 363–368. Sharp, D.C., McDowell, K.J., Weithenauer, J., Thatcher, W.W., 1989. The continuum of events leading to
maternal recognition of pregnancy in mares. J. Reprod. Fertil., Suppl. 37, 101–107.
Simpson, D.J., Greenwood, R.E.S., Ricketts, S.W., Rossdale, P.D., Sanderson, M., Allen, W.R., 1982. Use of ultrasound echography for early diagnosis of single and twin pregnancy in the mare. J. Reprod. Fertil., Suppl. 32, 431–439.
Simpson, K.S., Adams, M.H., Behrendt-Adam, C.Y., Baker, C.B., McDowell, K.J., 1999. Identification and initial characterization of calcyclin and phospholipase A2 in equine conceptuses. Mol. Reprod. Dev. 53, 179–187.
Stewart, F., Charleston, B., Crossett, B., Barker, P.J., Allen, W.R., 1995. A novel uterine protein that associates with the embryonic capsule in equids. J. Reprod. Fertil. 105, 65–70.
Stout, T.A.E., Lamming, G.E., Allen, W.R., 1999. Oxytocin administration prolongs luteal function in cyclic mares. J. Reprod. Fertil. 116, 3115–3320.
Townson, D.H., Ginther, O.J., 1989. Ultrasonic characterization of follicular evacuation during ovulation and fate of the discharged follicular fluid in mares. Anim. Reprod. Sci. 20, 131–141.
Vanderwall, D.K., Woods, G.L., Weber, J.A., Lichtenwalner, A.B., 1993. PGE2 secretion by the conceptus
Ž .
and binding by the non-pregnant endometrium in the horse. Equine Vet. J. Suppl. 15, 24–27.
Van Niekerk, C.H., Gerneke, W.H., 1966. Persistence and parthenogenetic cleavage of tubal ova in the mare. Onderstepoort J. Vet. Res. 31, 195–232.
Velasquez, L.A., Aguilera, J.G., Croxatto, H.B., 1995. Possible role of platelet-activating factor in embryonic signaling during oviductal transport in the hamster. Biol. Reprod. 52, 1302–1306.
Velasquez, L.A., Ojeda, S.R., Croxatto, H.B., 1997. Expression of platelet-activating factor receptor in the hamster oviduct: localization to the endosalpinx. J. Reprod. Fertil. 109, 349–354.
Waelchli, R.O., Jaworski, T., Ruddock, W.D.J., Betteridge, K.J., 2000b. Estimation of sodium and potassium concentrations in uterine fluid of mare by microdialysis and ion chromatography. J. Reprod. Fertil., Suppl. 55, in press.
Waelchli, R.O., MacPhee, D.J., Kidder, G.M., Betteridge, K.J., 1997. Evidence for the presence of
NarK-Ž
ATPase a1 andb1 subunit isoforms and their probable role in blastocyst expansion in the preattachment horse conceptus. Biol. Reprod. 57, 630–639.
Waelchli, R.O., Shand, N.A., Roud, H.K., Alexander, S.L., Betteridge, K.J., 2000a. Oxytocin and arginine vasopressin accumulation in the equine conceptus during the second to fifth weeks of pregnancy.
Ž .
Theriogenology Abstract 53, 287.
Wakuda, K., Takakura, K., Nakanishi, K., Kita, N., Shi, H., Hirose, M., Noda, Y., 1999. Embryo-dependent induction of embryo receptivity in the mouse endometrium. J. Reprod. Fertil. 115, 315–324.
Watson, E.D., Sertich, P.L., 1989. Prostaglandin production by horse embryos and the effect of co-culture of embryos with endometrium from pregnant mares. J. Reprod. Fertil. 87, 331–336.
Watson, E.D., Bjorksten, T., Buckingham, J., Nikolakopoulos, E., 1998. Immunolocalisation of oxytocin and¨ ´ neurophysin in the uterus of the mare. In: Proc. 7th Int. Symp. Eq. Reprod., Pretoria. pp. 75–76, Abstract. Weber, J.A., Woods, G.L., 1993. Influence of embryonic secretory chemicals on selective oviductal transport
Ž .
in mares. Equine Vet. J. Suppl. 15, 36–38.
Weber, J.A., Freeman, D.A., Vanderwall, D.K., Woods, G.L., 1991a. Prostaglandin E2 secretion by oviductal transport-stage equine embryos. Biol. Reprod. 45, 540–543.
Weber, J.A., Freeman, D.A., Vanderwall, D.K., Woods, G.L., 1991b. Prostaglandin E2 hastens oviductal transport of equine embryos. Biol. Reprod. 45, 544–546.
Weber, J.A., Woods, G.L., Lichtenwalder, A.B., 1995. Relaxatory effects of prostaglandin E2 on circular smooth muscle isolated from the equine oviductal isthmus. Biol. Reprod. Mono. Ser. 1, 125–130. Weber, J.A., Woods, G.L., Aigular, J.J., 1996. Location of equine oviductal embryos on day 5 post ovulation
and oviductal transport time of day 5 embryos autotransferred to the contralateral oviduct. Theriogenology 46, 1477–1483.
Wijayagunawardane, M.P., Choi, Y.H., Miyamoto, A., Kamishita, H., Fujimoto, S., Takagi, M., Sato, K., 1999. Effect of ovarian steroids and oxytocin on the production of prostaglandin E2, prostaglandin F2alpha and endothelin-1 from cow oviductal epithelial cell monolayers in vitro. Anim. Reprod. Sci. 56, 11–17. Zavy, J.T., Mayer, R., Vernon, M.W., Bazer, F.W., Sharp, D.C., 1979. An investigation of the uterine luminal
environment of non-pregnant and pregnant pony mares. J. Reprod. Fertil., Suppl. 27, 403–411.
Zavy, M.T., Clark, W.R., Sharp, D.C., Roberts, R.M., Bazer, F.W., 1982. Comparison of glucose, fructose, ascorbic acid and glucosephosphate isomerase enzymatic activity in uterine flushings from nonpregnant and pregnant gilts and pony mares. Biol. Reprod. 27, 1147–1158.