www.elsevier.comrlocateranireprosci
In vitro culture and embryo metabolism of cattle
and sheep embryos — a decade of achievement
J.G. Thompson
ReproductiÕe Technologies Group, AgResearch Ruakura Research Centre, PB 3123, Hamilton, New Zealand
Abstract
At the beginning of the 1990s, co-culture of cattle and sheep embryos was the most favoured method to support embryo development, but the use of this system has hampered progress in raising the efficiency of embryo production. Furthermore, little was known of the requirements of embryos and the biochemistry of early embryo development. As the decade progressed, energy metabolism studies improved our understanding of the energy substrate requirements for embryo development. Furthermore, an appreciation of the reproductive tract environment increased. This resulted in more ‘‘defined’’ systems, which have evolved further in the development of ‘‘sequen-tial’’ media systems, where components change in accordance to the needs of the embryo. Nevertheless, wholly defined systems, such as the replacement of albumin with PVA, are less able to support similar levels of development as protein-containing medium, and the resulting embryos are metabolically compromised. This highlights the nutritive role of albumin. One area in which much work has been conducted, but yet no unifying theory has emerged, is that of the interactive
Ž .
roles of growth factors including autocrinerparacrine , cytokines and extra-cellular matrix molecules in the development of a viable embryo. A new concept is that of regulation of energy
Ž .
metabolism. Compounds such as ethylenediamine tetraacetic acid EDTA , NaN and 2,4-dinitro-3 phenol have been shown to increase embryo development and quality of resulting embryos. This demonstrates that the process of ATP production is a key regulator of in vitro embryo develop-ment.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Sheep; Cattle; Embryo; Development; In vitro; Metabolism
1. Overview of the development of cattle and sheep embryo culture systems
At the beginning of the last decade of the 2nd millennium, very little was known about the metabolic requirements of ruminant embryos. There was a general assumption that they were similar to the metabolic patterns of other mammalian embryos, especially the mouse. The pioneering work of Brinster, Biggers, Wales, Whittingham and others
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
Ž .
had shown that early development 1- to 8-cell stage in the mouse was supported by the oxidation of carboxylic acids pyruvate and lactate, especially pyruvate. Glucose was poorly utilised, which was attributed to a block to a regulatory glycolytic enzyme,
Ž .
phosphofructokinase Barbehenn et al., 1974 . During progression through compaction and blastulation, this block was removed and glucose contributed increasingly to ATP production via oxidation, so that mouse morula development to the blastocyst stage
Ž .
could be supported by glucose alone. Formulations, such as M16 Whittingham, 1971 , which included glucose, pyruvate and lactate, were found to be effective in the
Ž
development of 2-cell embryos of most mouse strains and 1-cell embryos from hybrid .
strains .
Against this background, the pioneers of ruminant embryo culture struggled to
Ž .
develop comparably successful systems e.g. Wright and Bondioli, 1981 . Tervit et al. Ž1972, 1974. described ‘‘Synthetic Oviduct Fluid’’ ŽSOF , a culture system that. produced viable blastocysts from early cleavage stage embryos produced in vivo from both cattle and sheep. However, little uptake of this system was initially attempted, with
Ž .
the exception of Walker et al. 1989 . Indeed, for ruminant embryos, there were conflicting reports as to the appropriate in vitro requirements. For example, Betterbed
Ž .
and Wright 1985 advocated the use of concentrations of glucose of 5.6 mM and an atmospheric oxygen concentration for the in vitro development of sheep embryos,
Ž .
whereas Tervit et al. 1972 had advocated a low O tension and energy substrate levels2
based on oviduct concentrations. Underpinning these conflicting results was the inability to reliably culture ruminant embryos through the so-called ‘‘8- to 16-cell developmental block’’, a culture-induced phenomenon which was irreversible but did not result in
Ž .
immediate embryonic death Wright and Bondioli, 1981; Eyestone and First, 1991 . Due to a general lack of understanding as to what caused the block to development and the few but highly variable results obtained with SOF and other simple media, an alternative
Ž
strategy was sought which led to the advent of co-culture systems Gandolfi and Moor, .
1987; Eyestone and First, 1989 . Co-culture significantly advanced the application of in vitro embryo production, in as much as viable blastocysts were obtained. Nevertheless, in terms of our understanding of factors influencing embryo development, much less information has been generated using co-culture systems compared to defined systems ŽBavister, 1995 . Furthermore, positive factors influencing embryo development, such as. a low O environment and low glucose levels, which have been attributed to the success2
Ž .
of co-culture conditions Watson et al., 1994b; Edwards et al., 1997 , were first identified using defined systems.
2. Oxygen, carboxylic acids, carbohydrates and lipids
Ž .
Utilising both radioisotope-labelled substrates e.g. Rieger and Guay, 1988 and Ž non-invasive quantitative fluorescence-linked substrate uptakerproduction assays e.g.
.
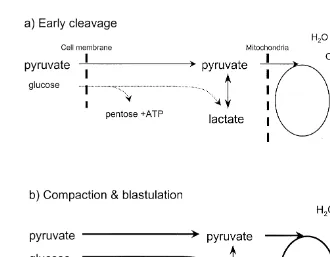
Fig. 1. Simple representation of the subjective differences in metabolic pathway preferences of carboxylic
Ž . Ž .
acids and glucose between the early cleavage stages a and compaction and blastulation stages b in cattle
Ž .
and sheep embryos. During early stages, uptake of glucose is low dotted line , whereas uptake of pyruvate
Ž .
and lactate provide the fuel for the TCA-cycle solid line , which predominates. In general, rate of ATP production is low during the early cleavage stages, as demand is not high. In comparison, ATP demand
Ž .
increases as compaction and blastulation proceeds heavy lines . In addition, the contribution to ATP production from glycolysis also increases with development, causing a significant increase in glucose consumption. However, little of the glucose carbon is oxidised through the TCA cycle. Rather, it appears to preferentially be directed to lactate production.
embryos. In general, embryos throughout pre-elongation development are reliant on
Ž .
oxidative phosphorylation via oxidation of pyruvate and amino acids for the generation Ž
of ATP for embryo development Javed and Wright, 1991; Rieger et al., 1992a,b; .
Gardner et al., 1993; Thompson et al., 1996, 2000 . However, there is a switch to an Ž
increased contribution of glycolysis during compaction and blastulation Gardner et al., .
1993; Thompson et al., 1996, 2000 . Failure to depress glycolysis during pre-compaction Ž
is one factor associated with the ‘‘8- to 16-cell’’ developmental block Gardner et al., .
and RNA synthesis, which is essential for embryonic development. Such metabolic
w 14 x
intermediates have been detected in sheep embryos following incubation with U C -Ž
glucose, as well as incorporation into non-glycogen macromolecules Thompson et al., .
1995a .
There remains one major question regarding the metabolism of these substrates. Why is glucose poorly oxidized through the tricarboxylic acid cycle, when exogenous pyruvate appears to be readily oxidised via this pathway? The current and yet inade-quately tested hypothesis is that there is a block to the NADqrNADH shuttle
mecha-nism across the mitochondrial membrane. Thus, to maintain an appropriate redox equilibrium, cytoplasmic NADH must be oxidised in order to maintain glycolytic flux ŽThompson et al.,1993; Edwards et al., 1997 . Thus, glucose is nearly 100% converted.
Ž
to L-lactate, especially at the blastocyst stage Gardner et al., 1993; Thompson et al., .
1996 . A possible explanation for this phenomenon is that there is substrate compart-mentalisation within the cytoplasm of the developing embryo. Glucose-derived pyruvate is almost entirely converted to lactate, possibly through a mechanism such as substrate channelling.
Another major question still unresolved within ruminant embryos is the role of intracellular lipid in ATP production and other cellular functions. There is ample
Ž .
evidence that lipid is present in reproductive tract secretions e.g. Grippo et al., 1994 , as Ž there is evidence that lipid can accumulate within embryos cultured in vitro e.g.
.
Thompson et al., 1995b; Ferguson and Leese, 1999 . More recently, attempts have been Ž
initiated to characterise the lipid content of cattle and sheep embryos e.g. Ferguson and .
Leese, 1999 . It is hoped that further analysis and improved understanding will answer the question of lipid metabolism.
3. Amino acids
If one had to choose the most significant ‘‘new’’ medium component affecting ruminant embryo development in vitro identified this decade, then the addition of pooled amino acids would rate very highly. This is because their impact has been the removal
Ž of serum from culture systems while maintaining high levels of development e.g.
.
Rosenkrans and First, 1994; Gardner et al., 1994 . It was the pioneering work of
Ž .
Bavister and colleagues in the hamster e.g. Bavister and Arlotto, 1990 that first showed the benefits of amino acids during in vitro development. The significance of pooled groups of amino acids, especially the non-essential amino acids was demonstrated in
Ž .
sheep embryos by Thompson et al. 1992b and then more thoroughly by Gardner et al. Ž1994 . Furthermore, the latter authors, based on their experience with mouse embryo. development in vitro, demonstrated that embryos were sensitive to the production of ammonia, generated from the spontaneous degradation of amino acids during culture Žespecially glutamine and amino acid metabolism Gardner et al., 1994 . This led to the. Ž . strategy of replacing media with fresh every 2nd day, to optimise development rates ŽGardner et al., 1994 . The importance of removing ammonia is significant, as it is a. primary candidate for the induction of ‘‘fetal oversize syndrome’’ induced during
Ž .
A clear understanding of the metabolism of amino acids is still lacking, although slow progress is taking place. Glutamine is known to contribute to ATP production ŽRieger et al., 1992b . Uptake studies of pooled amino acids have revealed that uptake.
Ž .
levels and preferences change during development Partridge and Leese, 1996 . How-ever, it is recognised that the amino acid concentrations are likely to differ between those commercially supplied as ‘‘pooled’’ preparations and that found within the oviduct and uterine secretions. Indeed, culture in ‘‘in vivo-like’’ concentrations of
Ž
amino acids has been shown to improve development Moore and Bondioli, 1993; Hill .
et al., 1997 . Some of this may be attributed to the intracellular osmolyte function of Ž those amino acids found in relatively high concentration in vivo, such as glycine e.g.
. Dawson and Baltz, 1997; Dawson et al., 1998 .
4. Growth factors, cytokines and extra-cellular matrix molecules
During the 1980s, co-culture, especially with primary cultures of oviduct epithelial cells, emerged as the most effective method for ‘‘overcoming’’ the so-called 8- to16-cell
Ž .
development block Gandolfi and Moor, 1987 . These observations stimulated the search for embryotrophic factors that were provided by the epithelial cells. Indeed, one such
Ž
factor was identified, a tissue inhibitor of metalloproteinase TIMP-1, Satoh et al., .
1994 . Nevertheless, the focus of research was chiefly directed towards secreted growth
Ž .
factors Gandolfi et al., 1989, 1992 . Furthermore, it became evident that culturing in ‘‘groups’’ of embryos under serum-free conditions, in relatively small volumes of
Ž
defined medium, conferred benefits to embryo development e.g. Gardner et al., 1994; .
Keefer et al., 1994 . Only under conditions of serum supplementation can single culture
Ž .
systems perform as well as group culture Hagemann et al., 1998 . Thus, the importance of autocrinerparacrine growth factors has emerged. For example, by use of antibodies and single or group culture, PDGF was shown to act as a paracrine growth factor ŽThibodeaux et al., 1995 . Furthermore, a number of mRNAs for several growth factors.
Ž and their receptors are expressed during cattle and sheep embryo development Watson
.
et al., 1992, 1994a . More recently, attention has focussed not so much on mitogenicr dif-Ž ferentiation properties, but rather that of anti-apoptotic, or ‘‘survival factors’’ Conlon
.
and Raff, 1999 . For example, it has been found that transforming growth factor b ŽBrison and Shultz, 1997 and platelet activating factor PAF, O’Neill, 1998 act as. Ž . autocrinerparacrine survival factors for mouse embryos, by reducing levels of apoptosis
Ž
within the inner cell mass. Cytokines, such as leukemia inhibitory factor Fry et al.,
. Ž .
1992 , granulocyte-macrophage colony stimulating factor de Moraes and Hansen, 1997
Ž .
and extracellular matrix molecules such as hyaluronic acid Furnus et al., 1998 also appear to influence embryo development. However, most in vitro studies performed are restricted to examining the action of a single factor. Furthermore, to avoid the confound-ing influence of growth factor-contaminated biologicals, the medium is usually
protein-Ž .
free which adds its own complications to interpretation of data, see Section 5 . Much of the research ignores the interplay between these classes of molecules, as found within
Ž .
excep-Ž . tion being those which act as survival factors Brison and Shultz, 1997; O’Neill, 1998 . To date, there is no unifying model that describes the various roles of growth factors, cytokines and extra-cellular matrix molecules that contribute to the development of a viable embryo. Indeed, the growth factor cocktail that exists within serum, remains a
Ž
powerful promoter of peri-compaction embryo development in vitro e.g. Hagemann et .
al., 1998 , despite its non-uniform constitution, adverse effects on embryo morphology,
Ž .
biochemistry and post transfer consequences see review by Thompson, 1997 . This has led some investigators to study signal transduction mechanisms and their influence on
Ž .
embryo development e.g. Fahy and Kane, 1993; Grealy and Sreenan, 1999 . This approach should assist our understanding of the complexities of embryo–growth factor interactions.
5. The need for albumin
Biologically derived media components, such as serum albumin and serum itself, have been widely used throughout the history of the development of embryo production systems. However, due to the undefined nature and variability in composition, the often less-than-desirable effects on embryos, plus the risk of infection from contaminated biological fluids, much research has been conducted into embryo culture using
‘‘de-Ž . Ž .
fined’’ media Bavister, 1995 . In particular, the use of polyvinyl alcohol PVA has been mooted as a preferred additive, especially as this provides a surfactant activity similar to albumin. However, albumin is the most prevalent protein in the mammalian reproductive tract, and evidence from the mouse suggests that albumin may have intracellular roles, as well as the additional extracellular support roles that it is thought
Ž .
to play Dunglison and Kaye, 1993; Dunglison et al., 1995 . However, albumin is not an
Ž .
absolute requirement for cattle embryo development in vitro Keskintepe et al., 1995 . Nevertheless, apart from the excellent results achieved by the laboratory of Brackett and
Ž .
colleagues e.g. Keskintepe et al., 1995 , most others have reported that levels of development are generally lower following in vitro development in PVA — than in
Ž
albumin-supplemented medium Eckert et al., 1999; Krisher et al., 1999; Lonergan et al., .
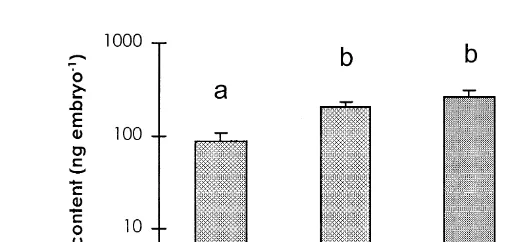
1999 . An examination of the total protein content of in vitro derived embryos ŽThompson et al., 1998 revealed that during early cleavage, protein content decreases.. This is followed by an increase in content during compaction and blastulation. Hence, protein degradation exceeds protein synthesis during early cleavage. Furthermore, total protein content was lower in blastocysts cultured in PVA-supplemented medium, compared to those produced in vivo or in culture medium supplemented with either BSA
Ž .
or fetal calf serum Fig. 2; Thompson et al., 1998 . This was not due to a difference in Ž
protein synthesis rates but the action of protein uptake via pinocytosis Thompson et al., .
1998 . Furthermore, a profound effect on energy substrate utilisation was found in blastocyst stage embryos cultured in PVA medium compared to BSA medium. In
Ž
PVA-medium, pyruvate uptake was considerably higher than in BSA medium Eckert et .
al., 1999 although oxygen uptake was reduced. This would normally be regarded as contradictory, because increased pyruvate uptake would normally be associated with
Ž .
10 Ž .
Fig. 2. Log plot histograms of protein content "S.E.M. of Day 7 blastocyst stage embryos produced in
Ž .
vitro and incubated in medium supplemented with either: polyvinyl alcohol PVA ; bovine serum albumin
ŽBSA ; fetal calf serum. ŽFCS-D1 , BSA from Days 1–5 of development and FCS for Days 5–7 of. Ž
development. A further group, Day 7 blastocysts derived in vivo following superovulation and embryo
. Ž . Ž .
collection were also included in vivo . Different superscripts signify significant differences P-0.001 .
ŽReprinted with permission from Wiley, New York..
pyruvate was significantly lower in blastocysts derived from culture in PVA medium compared to BSA medium, in agreement with the oxygen uptake data of Eckert et al. Ž1999 . Thus, despite an increase in pyruvate uptake, oxidation of this substrate was. decreased. Therefore, it appears that pyruvate plays other roles in blastocysts derived from PVA media. One such role is possibly the amination of pyruvate to alanine in the detoxification of NH , generated by endogenous protein metabolism, which may occur3 in the absence of exogenous protein, such as albumin. Based on the data from the mouse ŽDunglison and Kaye 1993; Dunglison et al., 1995 and from our own observations, it. seems likely that albumin has a significant nutritive role to play during embryo development, especially post-compaction. It is clear that blastocysts derived from PVA-supplemented medium have an altered metabolic profile compared to those cultured in the presence of albumin, or those derived in vivo. Whether other protein sources can replace albumin during in vitro development has yet to be established. In any case, the trend towards ‘‘defined’’ systems must be tempered with the physiological requirements of the embryo during development.
6. Sequential media, perfusion and metabolic regulation
Ž . Ž .
As reviewed by Thompson 1996 and Gardner and Lane 1997 , our understanding of the requirements of early embryos as they progress through development and the temporal relationship between the reproductive tract fluid milieu and embryo develop-ment, has led to the concept that media components and physical conditions should be altered during culture to achieve improved development. This concept has subsequently
Ž .
been termed sequential media systems Gardner and Lane, 1997 and has led to at least Ž
two human embryo culture systems now commercially available G1rG2, Scandinavian .
IVF and Cook human embryo culture medium, Cook Australia . For cattle embryos, our Ruakura laboratory has developed a sequential media system for fertilisation and culture
Ž .
development of this concept is the use of perfusion culture as the vehicle to introduce
Ž .
changes in media composition Thompson, 1996 . Work on the development of the Ž
equipment necessary for perfusion culture is well underway Lim et al., 1996; McGowan .
and Thompson, 1997 . The introduction of perfusion culture should enable more elegant experimentation on altering concentrations and introducing new components into media, while causing minimal disturbance to embryos during development.
Metabolic regulation is a relatively new concept, but has its origins before the beginning of this decade. Perhaps, the best known example of metabolic regulation is
Ž .
the use of ethylenediamine tetraacetic acid EDTA , a non-selective chelator of divalent cations during embryo development. Several studies have shown that when in vitro glucose concentrations are higher than in vivo, early embryo development is retarded or
Ž
even blocked Seshagiri and Bavister, 1989; Chatot et al., 1989; Thompson et al., .
1992a . This has been likened to the ‘‘Crabtree-effect’’, whereby oxidative phosphoryla-Ž
tion is depressed due to an abnormally high glycolytic rate Seshagiri and Bavister, .
1989 . Until recently, this was generally controlled by reducing the concentration of, or
Ž .
even removing, glucose within the culture medium e.g. Chatot et al., 1989 . However, such conditions are unphysiological, as glucose is found in lumen fluids of the
Ž .
reproductive tract Gardner, 1998 . A previously unrelated observation is that EDTA addition during mouse in vitro embryo development overcame culture-induced
develop-Ž . Ž
ment ‘‘2-cell’’ blocks Abramczuk et al., 1977; Mehta and Kiessling, 1990; Nasr-.
Esfahini et al., 1992 . It is believed that EDTA sequesters the toxic effects of contaminating heavy metal cations, most likely by inhibiting the production of reactive
2q 2q Ž
oxygen species, catalysed by ions such as Fe and Cu reviewed by Johnson and
. Ž .
Nasr-Esfahini, 1994 . However, Lane and Gardner 1997 have demonstrated that, at a cellular level, EDTA depresses glycolytic rates within pre-compaction mouse embryos, a
Ž .
result also demonstrated for cattle embryos Gardner et al., 1997 . These authors believe that in the mouse, at least, the mechanism of EDTA is via the intracellular chelation of
2q Ž .
Mg , a necessary co-factor for several glycolytic enzymes Lane and Gardner, 1997 . The beneficial effect of EDTA is optimal only when introduced during early develop-ment, when culture-induced high glycolytic rates will compromise development. Thus EDTA is used to manipulate the metabolic profile of embryos during stage-specific developmental points, in order to optimise energy metabolism, leading to improved development. We have also used metabolic manipulation strategies during peri-r post-compaction development to significantly improve cattle embryo production yields. This has been achieved by inducing the ‘‘opposite’’ effect of EDTA, i.e. stimulating glycolysis by partially inhibiting oxidative phosphorylation during post-compaction
Ž .
development Thompson et al., 2000 . Non-toxic concentrations of metabolic inhibitors
Ž .
such as NaN and 2,4-dinitrophenol 5–103 mM present in SOF-98 medium from Day 5–7 of development have been found to stimulate embryo development, increasing the proportion of embryos developing to compact morulae and blastocysts from 50% to
Ž . Ž
60%, with a similar increase in transferable quality embryos 40% to 50% Thompson .
et al., 2000 . Furthermore, both cell number and glucose uptake increase in NaN -treated3
Ž .
embryos Thompson et al., 2000 . The exact mechanism by which such compounds increase development has yet to be determined. Nevertheless, previously it was thought
.
Van Soom et al., 1997 . However, use of peri-rpost-compaction metabolic regulation can also ‘‘rescue’’ embryos that normally would not undergo further development.
7. Concluding remarks and the future
Our understanding of the requirements for the development of cattle and sheep embryos in vitro to the blastocyst stage from in vitro or in vivo produced zygotes has progressed enormously in this final decade of the 2nd millennium. Nevertheless, there remains a clear distinction between the embryos derived in vivo and those produced in vitro. In particular, subtle differences exist at the cellular level in terms of metabolic
Ž .
profiles and morphology Thompson, 1997; Leese et al., 1998 . Of great interest has been the demonstration that gene expression differences exist between in vivo and in
Ž .
vitro derived embryos Wrenzycki et al., 1996 and between different methods of in
Ž .
vitro culture Wrenzycki et al., 1999 . The array of post-transfer abnormalities, such as
Ž .
fetal oversize syndrome e.g. Kruip and den Daas 1997 and the high incidence of late
Ž .
embryo-early fetal loss e.g. Reichenbach et al., 1992 require further understanding of the relationship between development and sub-optimal culture environments. However, there is also many other puzzling questions and challenges confronting embryologists in this field. First, embryos of other ruminants do not necessarily develop in systems
Ž .
developed for the cattle or sheep. The red deer CerÕus elaphus embryo, for example, is
Ž .
extremely difficult to culture to the blastocyst stage in vitro Berg et al., 1995 . Second, an inability to support normal development post-hatching, and even the processes of hatching, are areas of significant interest. Understanding the role of intracellular pH and cell volume regulation, signal transduction mechanisms and mRNA transcription regula-tion in embryo development should provide answers to many of these quesregula-tions. Of particular interest will be a greater understanding of the regulation of expression of those
Ž .
genes related to ‘‘stress’’ responses Leese et al., 1998 and blastocyst formation ŽWatson et al., 1999 . With advances in technology, it has become increasingly possible. to investigate many of these facets within individual developing embryos. However, just as Brinster, Biggers and company found with the mouse embryo in the 1960s and early 1970s, the key regulator of cattle and sheep in vitro embryo development is the way in which the embryo produces ATP.
Acknowledgements
Many thanks to Dr. Don Rieger for his assistance in preparing this manuscript.
References
Abramczuk, J., Solter, D., Koprowski, H., 1977. The beneficial effect of EDTA on development of mouse one cell embryos in chemically defined medium. Dev. Biol. 61, 378–383.
Barbehenn, E.K., Wales, R.G., Lowry, O.H., 1974. The explanation for the blockade in glycolysis in early mouse embryos. Proc. Natl. Acad. Sci. USA 71, 1056–1060.
Bavister, B.D., Arlotto, T., 1990. Influence of single amino acids on the development of hamster one-cell embryo in vitro. Mol. Reprod. Dev. 25, 45–51.
Berg, D.K., Thompson, J.G., Pugh, P.A., Tervit, H.R., Asher, G.W., 1995. Successful in vitro culture of early cleavage stage embryos recovered from superovulated red deer. Theriogenology 44, 247–254.
Betterbed, B., Wright, R.W. Jr., 1985. Development of one-cell, ovine embryos in two culture media under two gas atomsphere. Theriogenology 23, 547–553.
Brison, D.R., Schultz, R.M., 1997. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor ?. Biol. Reprod. 56, 1088–1096.
Chatot, C.L., Ziomek, C.A., Bavister, B.D., Lewis, J.L., Torres, I., 1989. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 86, 679–688. Conlon, I., Raff, M., 1999. Size control in animal development. Cell 96, 235–244.
Dawson, K.M., Baltz, J.M., 1997. Organic osmolytes and embryos: Substrates of the Gly and b transport systemsprotect mouse zygotes against the effects of raised osmolarity. Biol. Reprod. 56, 1550–1558. Dawson, K.M., Collins, J.L., Baltz, J.M., 1998. Osmolarity-dependent glycine accumulation indicates a role
for glycine as an organic osmolyte in early preimplantation mouse embryos. Biol. Reprod. 59, 225–232. de Moraes, A.A.S., Hansen, P.J., 1997. Granulocyte-macrophage colony stimulating factor promotes
develop-ment of in vitro produced bovine embryos. Biol. Reprod. 57, 1060–1065.
Dunglison, G.F., Kaye, P.L., 1993. Insulin regulates protein metabolism in mouse blastocysts. Mol. Reprod. Dev. 36, 42–48.
Dunglison, G.F., Jane, S.D., McCaul, T.F., Chad, J.E., Fleming, T.P., Kaye, P.L., 1995. Stimulation of endocytosis in mouse blastocysts by insulin: a quantitative morphological analysis. J. Reprod. Fertil. 105, 115–123.
Eckert, J., Pugh, P.A., Thompson, J.G., Niemann, H., Tervit, H.R., 1999. Exogenous protein affects developmental competence and metabolic activity of bovine preimplantation embryos in vitro. Reprod. Fertil. Dev. 10, 327–332.
Edwards, L.J., Batt, P.A., Gandolfi, F., Gardner, D.K., 1997. Modifications made to culture medium by bovine oviduct epithelial cells: Changes to carbohydrates stimulate bovine embryo development. Mol. Reprod. Dev. 46, 146–154.
Eyestone, W.H., First, N.L., 1989. Co-culture of early cattle embryos to the blastocyst stage with oviductal tissue or in conditional media. J. Reprod. Fertil. 85, 715–720.
Eyestone, W.H., First, N.L., 1991. Characterisation of developmental arrest in early bovine embryos cultured in vitro. Theriogenology 35, 613–623.
Ferguson, E.M., Leese, H.J., 1999. Triglyceride content of bovine oocytes and early embryos. J. Reprod. Fertil. 116, 373–378.
Gandolfi, F., Brevini, T.A.L., Modina, S., Passoni, L., 1992. Early embryonic signals: embryo maternal interactions before implantation. Anim. Reprod. Sci. 28, 269–276.
Gandolfi, F., Moor, R., 1987. Stimulation of early embryonic development in sheep by co-culture with oviduct epthelial cells. J. Reprod. Fertil. 81, 23–28.
Gandolfi, F., Tiziana, A.L., Richardson, L., Brown, C.R., Moor, R., 1989. Characterisation of proteins secreted by sheep oviduct epithelial cells and their function in embryonic development. Development 106, 303–312.
Ž .
Gardner, D.K., 1998. Embryo development and culture techniques. In: Clark, J. Ed. , Animal Breeding. Technology for the 21st Century. Harwood Academic Publishers, Amsterdam, pp. 13–46.
Gardner, D.K., Lane, M., 1997. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum. Reprod. Update 3, 367–382.
Gardner, D.K., Lane, M., Batt, P., 1993. Uptake and metabolism of pyruvate and glucose by individual sheep preattachment embryos developed in vivo. Mol. Reprod. Dev. 36, 313–319.
Gardner, D.K., Lane, M., Spitzer, A., Batt, P., 1994. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate development. Biol. Reprod. 50, 390–400.
Gardner, D.K., Lane, M.W., Lane, M., 1997. Bovine blastocyst cell number is increased by culture with EDTA for the first 72 hours of development from the zygote. Theriogenology 47, 278.
Grippo, A.A., Anderson, S.H., Chapman, D.A., Henault, M.A., Killian, G.J., 1994. Cholesterol, phospholipid and phospholipase activity of ampullary and isthmic fluid from the bovine oviduct. J. Reprod. Fertil. 102, 87–93.
w x
Fahy, M.M., Kane, M.T., 1993. Incorporation of 3H -inositol into phosphoinositides and inositol phosphates by rabbit blastocysts. Mol. Reprod. Dev. 34, 391–395.
Fry, R.C., Batt, P.A., Fairclough, R.J., Parr, R.A., 1992. Human leukaemia inhibitory factor improves the viability of cultured ovine embryos. Biol. Reprod. 46, 470–474.
Furnus, C.C., de Matos, D.G., Martinez, A.G., 1998. Effect of hyaluronic acid on development of in vitro produced bovine embryos. Theriogenology 49, 1489–1499.
Hagemann, L.J., Weilert, L.L., Beaumont, S.E., Tervit, H.R., 1998. Development of bovine embryos in single
Ž .
in vitro production sIVP system. Mol. Reprod. Dev. 51, 143–147.
Hill, J.L., Wade, M.G., Nancarrow, C.D., Kelleher, D.L., Boland, M.P., 1997. Influence of ovine oviducal amino acid concentrations and an ovine oestrus-associated glycoprotein on development and viability of bovine embryos. Mol. Reprod. Dev. 47, 164–169.
Javed, M.H., Wright, R.W. Jr., 1991. Determination of pentose phosphate and embden-meyerhoff pathway activities in bovine embryos. Theriogenology 35, 1029–1037.
Johnson, M.H., Nasr-Esfahini, M.H., 1994. Radical solutions to cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro?. Bioessays 16, 31–38.
Kane, M.T., Morgan, P.M., Coonan, C., 1997. Peptide growth factors and preimplantation development. Hum. Reprod. Update 3, 137–157.
Keskintepe, L., Burnley, C.A., Brackett, B.G., 1995. Production of viable bovine blastocysts in defined in vitro conditions. Biol. Reprod. 52, 1410–1417.
Keefer, C.L., Stice, S.L., Paprocki, A.M., Golueka, P., 1994. In vitro culture of bovine IVM-IVF embryos: cooperative interaction among embryos and the role of the growth factors. Theriogenology 41, 1323–1331. Krisher, R.L., Lane, M., Bavister, B.D., 1999. Developmental competence and metabolism of bovine embryos
cultured in semi-defined and defined culture media. Biol. Reprod. 60, 1345–1352.
Kruip, T.A.M., den Daas, J.H.G., 1997. In vitro produced and cloned embryos: Effects on pregnancy, parturition and offspring. Theriogenology 47, 43–52.
Lane, M., Gardner, D.K., 1997. EDTA stimulates development of cleavage stage mouse embryos by inhibiting
Ž .
the glycolytic enzyme 3-phosphoglycerate kinase. Biol. Reprod. 56 Suppl. 1 , 193, Abstr. 193.
w 3 x w 14 x
Lee, E.S., Pugh, P.A., Allen, N., Fukui, Y., Thompson, J.G., 1998. 5- H -glucose and 1- C -pyruvate utilization by fresh and frozen-thawed Day 7 IVP bovine blastocysts cultured in PVA-or
BSA-supple-Ž .
mented medium. Theriogenology 49, 233, abstr .
Leese, H.J., Donnay, I., Thompson, J.G., 1998. Human assisted conception: a cautionary tale. Lessons from
Ž .
Domestic Animals. Hum. Reprod. 13 Suppl. 4 , 184–202.
Lim, J.M., Reggio, B.C., Godke, R.A., Hansel, W.A., 1996. A continuous flow, perifusion culture system for 8-to 16-cell bovine embryos derived from in vitro culture. Theriogenology 46, 1441–1450.
Lonergan, P., O’Kearney-Flynn, M., Boland, M.P., 1999. Effect of protein supplementation and the presence of an antioxidant on the development of bovine zygotes in synthetic oviduct fluid medium under high or low oxygen concentration. Theriogenology 51, 1565–1576.
McEvoy, T.G., Robinson, J.J., Aitken, R.P., Findlay, P.A., Robertson, I.S., 1997. Dietary excess of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Anim. Reprod. Sci. 47, 71–90.
McEvoy, T.G., Robinson, J.J., Carolan, C., Staines, M.E., Broadbent, P.J., Sinclair, K.D., 1999. Ovine fetal development following embryo culture in synthetic oviduct fluid with added ammonium. Theriogenology 51, 247.
McGowan, L.T., Thompson, J.G., 1997. Perfusion culture of bovine in vitro produced embryos. Proc. Aust. Soc. Reprod. Biol. 29, 24.
Mehta, T.S., Kiessling, A.A., 1990. Development potential of mouse embryos conceived in-vitro and cultured in ethylenediaminetetraacetic acid with or without amino acids or serum. Biol. Reprod. 43, 600–606. Moore, K., Bondioli, K.R., 1993. Glycine and alanine supplementation of culture medium enhances
develop-ment of in vitro matured and fertilised cattle embryos. Biol. Reprod. 48, 833–840.
acetic acid and oxygen tension on the concentration of reactive oxygen species and on development of the mouse preimplantation embryos in-vitro. J. Reprod. Fertil. 96, 219–231.
O’Neill, C., 1998. Autocrine mediators are required to act on the embryos by the 2-cell stage to promote normal development and survival of mouse preimplantation embryos in vitro. Biol. Reprod. 58, 1303–1309. Partridge, R.J., Leese, H.J., 1996. Consumption of amino acids by bovine preimplantation embryos. Reprod.
Fertil. Dev. 8, 945–950.
Reichenbach, H.D., Liebrich, J., Berg, U., Brem, G., 1992. Pregnancy rates and births after unilateral or bilateral transfer of bovine embryos produced in-vitro. J. Reprod. Fertil. 95, 363–370.
Rieger, D., Guay, P., 1988. Measurement of the metabolism of energy substrates in individual bovine blastocysts. J. Reprod. Fertil. 83, 585–591.
Rieger, D., Loskutoff, N.M., Betteridge, K.J., 1992a. Developmentally related changes in the metabolism of glucose, glutamine by cattle embryos produced and co-cultured in-vitro. J. Reprod. Fertil. 95, 585–595. Rieger, D., Loskutoff, N.M., Betteridge, K.J., 1992b. Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in-vitro. Reprod. Fertil. Dev. 4, 547–557.
Rosenkrans, C.F. Jr., First, N.L., 1994. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J. Anim. Sci. 72, 434–437.
Satoh, T., Kobayashi, K., Yamashita, S., Kikuchi, M., Sendai, Y., Hoshi, H., 1994. Tissue inhibitor of
Ž .
metaloproteinases TIMP-1 produced by granulosa and oviduct cells enhances in vitro development of bovine embryo. Biol. Reprod. 50, 835–844.
Seshagiri, P.B., Bavister, B.D., 1989. Glucose inhibits development of hamster 8-cell embryos in-vitro. Biol. Reprod. 40, 599–606.
Tervit, H.R., Rowson, L.E.A., 1974. Birth of lambs after culture of sheep ova in-vitro for up to six days. J. Reprod. Fertil. 38, 177–179.
Tervit, H.R., Whittingham, D.G., Rowson, L.E.A., 1972. Successful culture in-vitro of sheep and cattle ova. J. Reprod. Fertil. 30, 493–497.
Thibodeaux, J.K., Myers, M.W., Hansel, W., 1995. The beneficial effect of incubating bovine embryos in groups are due to platelet-derived growth factor. Theriogenology 43, 336.
Thompson, J.G., 1996. Defining the requirements for bovine embryo culture. Theriogenology 45, 27–40. Thompson, J.G., 1997. Comparison between in vivo derived and in vitro produced pre-elongation embryos
from domestic ruminants. Reprod. Fertil. Dev. 9, 254–341.
Thompson, J.G., Allen, N.W., McGowan, L.T., Bell, A.C.S., Lambert, G., Tervit, H.R., 1998. The effect of delayed supplementation of fetal calf serum to culture medium on bovine embryo development and following transfer. Theriogenology 49, 1239–1249.
Thompson, J.G., Bell, A.C.S., Pugh, P.A., Tervit, H.R., 1993. Metabolism of pyruvate by pre-elongation sheep embryos and effect of pyruvate and lactate concentrations during in vitro culture. Reprod. Fertil. Dev. 5, 417–423.
Thompson, J.G., Bell, A.C.S., Tervit, H.R., 1995a. Partitioning of glucose carbon in post-compaction ovine embryos. Anim. Reprod. Sci. 38, 119–126.
Thompson, J.G., Gardner, D.K., Pugh, P.A., McMillan, W.H., Tervit, H.R., 1995b. Lamb birth weight is affected by culture system utilized during in vitro pre-elongation development of ovine embryos. Biol. Reprod. 53, 1385–1391.
Thompson, J.G., McNaughton, C., Gasparinni, B., McGowan, L.T., Tervit, H.R., 2000. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos. J.
Ž .
Reprod.. Fertil. 118, in press .
Thompson, J.G., Partridge, R.J., Houghton, F.D., Cox, C.I., Leese, H.J., 1996. Oxygen uptake and carbo-hydrate metabolism by in vitro derived bovine embryos. J. Reprod. Fertil. 106, 299–306.
Thompson, J.G., Sherman, A., Allen, N.W., McGowan, L.T., Tervit, H.R., 1998. Total protein content and protein synthesis within pre-elongation stage bovine embryos. Mol. Reprod. Dev. 50, 139–145. Thompson, J.G., Simpson, A.C., Pugh, P.A., Tervit, H.R., 1992a. Requirement for glucose during in vitro
culture of sheep preimplantation embryos. Mol. Reprod. Dev. 31, 253–257.
Van Soom, A., Ysebaert, M.-T., De Kruif, A., 1997. Relationship between timing of development, morula morphology, and cell allocation to the inner cell mass and trophectoderm in in-vitro produced bovine embryos. Mol. Reprod. Dev. 47, 47–56.
Walker, S.K., Lampe, R.J., Seamark, R.F., 1989. Culture of sheep zygotes in synthetic oviduct fluid medium with different concentrations of sodium bicarbonate and hepes. Theriogenology 32, 797–804.
Watson, A.J., Hogan, A., Hahnel, A., Wiemer, K.E., Schultz, G.A., 1992. Expression of growth factor ligand and receptor genes in the preimplantation bovine embryo. Mol. Reprod. Dev. 31, 87–95.
Watson, A.J., Watson, P.H., Arcellana-Panillio, M., Warnes, D., Walker, S.K., Schultz, G.A., Armstrong, D.G., Seamark, R.F., 1994a. A growth factor phenotype map for ovine preimplantation development. Biol. Reprod. 50, 725–733.
Watson, A.J., Watson, P.H., Warnes, D., Walker, S.K., Armstrong, D.T., Seamark, R.F., 1994b. Preimplanta-tion development of in vitro-matured and in vitro-fertilized ovine zygotes: comparison between coculture on oviduct epithelial cell monolayers and culture under low oxygen atmospheres. Biol. Reprod. 50, 715–724.
Watson, A.J., Westhusin, M.E., DeSousa, P.A., Betts, D.H., Barcroft, L.C., 1999. Gene expression regulating blastocyst formation. Theriogenology 51, 117–133.
Whittingham, D.G., 1971. Culture of mouse ova. J. Reprod. Fertil. 14, 7–21.
Wrenzycki, C., Herrman, D., Carnwarth, J.W., Niemann, H., 1996. Expression of the gap junction gene
Ž .
connexin43 C=43 in preimplantation bovine embryos derived in vitro or in vivo. J. Reprod. Fertil. 108
Ž .1 , 17–24.
Wrenzycki, C., Herrman, D., Carnwarth, J.W., Niemann, H., 1999. Alterations in the relative abundance of gene transcripts in preimplantation bovine embryos cultured in medium supplemented with either serum or PVA. Mol. Reprod. Dev. 53, 8–18.