www.elsevier.nlrlocateraqua-online
Ultrastructure and pathogenesis of Monodon
ž
/
baculo
Õ
irus Pm SNPV in cultured larvae and
natural brooders of Peneaus monodon
P. Ramasamy
a, P.R. Rajan
a, V. Purushothaman
b,
G.P. Brennan
c,)a
Department of Zoology, Life Sciences Building, UniÕersity of Madras, Chennai 600 025, India b
Department of Microbiology, Madras Veterinary College, TanuÕas, Chennai 600007, India c
School of Biology and Biochemistry, Medical Biology Centre, The Queen’s UniÕersity of Belfast, Belfast, BT9 7BL Northern Ireland, UK
Accepted 8 September 1999
Abstract
Ž .
Monodon baculoÕirus MBV occurred in the hepatopancreas of cultured larvae, zoea, mysis
and post-larvae and in wild caught juveniles, sub-adults and brood stocks of Penaeus monodon. Infected larvae exhibited lethargy and reduced feeding and preening activities while infected juveniles, sub-adults and brooders exhibited normal behaviour. MBV-infected hepatopancreatic cells exhibited a 300% volume increase in the hypertrophied nuclei, which contained eosinophilic, spherical, intranuclear occlusion bodies. At the apical cell surface, the number of microvilli were greatly reduced by cytolysis. The cytoplasm contained numerous vacuoles and few mitochondria. Two types of occlusion bodies were observed. Type 1 comprised a paracrystalline array of polyhedrin sub-units with a lattice spacing of 5–7 nm, with numerous occluded virions and a few non-occluded virions in the periphery within a double envelope measuring 267"2 nm long and 78"3 nm wide. Type 2 occlusion bodies consisted of non-crystalline, granulin-like sub-units each measuring 12 nm in diameter and contained mostly non-occluded virions measuring 326"4 nm long and 73"1 nm wide. In the zoea, mysis and post-larvae, the average infection rates for
)Corresponding author. Tel.:q44-1232-272083; fax:q44-12342-236505; e-mail: [email protected] 0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
MBV were 28%"2%, 57%"3% and 91%"1%, respectively. Mortality rates were greater in the infected than in the uninfected post-larvae, at 81%"0% and 11%"0%, respectively. The mortality rate in MBV infected zoea and mysis was 23%"3% and 49%"2%, respectively, while in the uninfected larvae, it was 5%"0% and 8%"0%, respectively. The mortality rate for the infected and uninfected nauplii was similar at 3%"0%. In the brood stocks, the infection rate was 21%"1%, while in the juveniles and sub-adults, it was lower at 4%"0% and 5%"1%. MBV infected larvae harboured 10 times more bacteria than uninfected larvae.q2000 Elsevier
Science B.V. All rights reserved.
Keywords: Monodon baculoÕirus; Penaeus monodon; Hepatopancreas
1. Introduction
Viral diseases are recognized as a major factor limiting the future of the shrimp aquaculture industry in India with consequent lost productivity estimated at 10,000–
Ž
12,000 metric tonnes at a cost of US$6–8 billion per annum Alagarswami, 1995; .
Ramasamy, 1995, 1999 . It was not until 1980, with the aid of electron microscopy, that research was directed at identifying and locating the infecting organisms. Numerous invertebrate viruses were identified, many of them in shrimp which were previously
Ž .
considered disease-free Ramasamy, 1995, 1996; Ramasamy et al., 1995 . Monodon
Ž . Ž .
baculoÕirus MBV was first described by Lightner and Redman 1981 in Taiwanese
Penaeus monodon and was thought to be specific to this species. However, it has since
been identified in several penaeid shrimp from different geographic regions. To date, 21 Ž
different viral species have been described from cultured penaeid shrimp Ramasamy, .
1999 . Only a few of these have been implicated in cases of heavy shrimp losses, while Ž
many remain a curiosity Sindermann, 1990; Spann and Lester, 1996, 1997; Ramasamy, .
1999 . From 1994 to 1999, aquaculture farms on the east and west coasts of India experienced repeated outbreaks of viral disease identified as a single outbreak of MBV
Ž .
present in post-larval P. monodon Ramasamy, 1995 . The distribution, pathogenesis Ž
and morphology of MBV have been reviewed Brock et al., 1983; Lightner, 1983, 1993; Lightner et al., 1983, 1985, 1990; Fukuda et al., 1988; Johnson and Lightner, 1988; Hung et al., 1991; Vega-Villasante and Puente, 1993; Chen et al., 1995; Flegel, 1997;
. Ž .
Ramasamy, 1999 . The prevalence and ultrastructure of MBV from post-larval PL
20-Ž .
to 37-day old cultured Indian penaeid shrimp was recorded by Ramasamy et al. 1995 ,
Ž .
while Sundararaj et al. 1996 reported MBV in 35- to 60-day old shrimp as determined
Ž .
at the light microscope level. Studies by Ramasamy et al. 1995 showed that MBV infections peaked in the post-larval stage with a mortality rate of 90% with subsequent heavy losses in early juveniles and senescent adults. At that time, however, MBV had not been demonstrated in the zoea, mysis, sub-adults or brooders from either wild or
Ž
cultured P. monodon Lin, 1989; Tangtongpiroj, 1989; Thikiew, 1990; Lightner, 1993; .
2. Materials and methods
Ž .
Eggs, larvae nauplii, mysis , post-larvae, juveniles, sub-adults and brooders of the penaeid shrimp, P. monodon, were collected from hatcheries, aquaculture farms and
Ž natural sources in the coastal regions of Andhra Pradesh and Tamil Nadu, India see
. Ž .
Scheme 1 , where outbreaks of viral disease had been reported Tables 1 and 3 . The hepatopancreas was removed and dissected from post-larvae, juveniles, sub-adults and brood stocks of P. monodon. A squash preparation, stained with 0.05% aqueous malachite green, was used to identify the presence of hypertrophied nuclei with spherical, multiple occlusion bodies. Whole mount squash preparations were also made
Ž .
from the eggs and larvae nauplii, mysis .
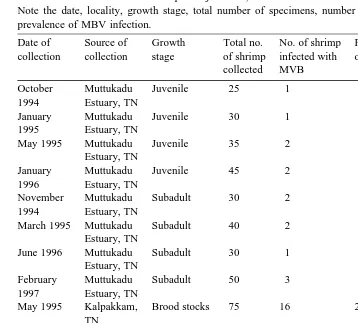
Table 1
Prevalence of MBV infection in captured juveniles, sub-adults and brood stocks of P. monodon
Note the date, locality, growth stage, total number of specimens, number of MBV-infected specimens and prevalence of MBV infection.
Date of Source of Growth Total no. No. of shrimp Percentage Mean percentage collection collection stage of shrimp infected with of infection of infection"SE
collected MVB
October Muttukadu Juvenile 25 1 4.0
1994 Estuary, TN
January Muttukadu Juvenile 30 1 3.3 4.4"0.4
1995 Estuary, TN
May 1995 Muttukadu Juvenile 35 2 5.7
Estuary, TN
January Muttukadu Juvenile 45 2 4.4
1996 Estuary, TN
November Muttukadu Subadult 30 2 6.7
1994 Estuary, TN
March 1995 Muttukadu Subadult 40 2 5.0 5.3"0.6
Estuary, TN
June 1996 Muttukadu Subadult 30 1 3.3
Estuary, TN
February Muttukadu Subadult 50 3 6.0
1997 Estuary, TN
May 1995 Kalpakkam, Brood stocks 75 16 21.3
TN
May 1995 Gudur, Brood stocks 55 19 20.0
AP
June 1995 Kovalam, Brood stocks 73 14 19.18 20.5"0.6
TN
June 1995 Nellore, Brood stocks 85 19 22.89
AP
May 1996 Tuticorin, Brood stocks 100 21 21.0
TN
May 1996 Gudur, Brood stocks 48 9 18.75
AP
TNsTamil Nadu. APsAndhra Pradesh.
2.1. Histopathology
For histopathology, the tissues selected for examination were hepatopancreas, midgut, hindgut, heart, haemopoetic tissue, nerve cord, lymphoid organs and muscle tissue from
Ž
infected and uninfected shrimp. The tissues were fixed in Davidson’s fixative Bell and .
Lightner, 1988 for 24–72 h, routinely processed for light microscopy and stained with haematoxylin and eosin. Changes in volume of MBV infected hepatopancreatic cells
was calculated by measuring the diameter of the nucleusrcell ratio using the formula
2.2. Ultrastructure
Ž .
For transmission electron microscopy TEM , MBV-infected tissues of P. monodon were fixed in 4% glutaraldehyde buffered to pH 7.2 with 0.1 M sodium cacodylate–HCl
containing 3% sucrose and 0.5% sodium chloride for 24 h at 48C. The tissues were
Ž .
washed in buffer, post-fixed in 1% aqueous osmium tetroxide OsO4 for 1 h,
dehy-drated through an ascending series of ethanols, infiltrated and embedded in Agar 100
Ž .
resin Agar Scientific, Stansted, England and polymerized for 48 h at 608C. Ultrathin
sections, 60–70 nm in thickness, were double stained with uranyl acetate and lead citrate and examined in a JEOL 100CX TEM for the presence of viral inclusions.
2.3. Field studiesrhatcheries
Ž .
Various stages of PL 7–20 P. monodon were periodically collected from four
Ž .
different hatcheries in Tamil Nadu between 1996 and 1997 Table 3 and examined for the presence of MBV infection, using a squash preparation. The post-larvae were maintained in well-aerated, filtered sea water and fed with Artemia nauplii. Their initial size was recorded, and abnormalities in behaviour or morphology were noted in individual shrimp, together with the mortality rate, over a 48-h period. The hepatopan-creas was dissected out and examined for histopathological changes and screened for MBV to determine the percentage infection. Similarly, wild caught juveniles and sub-adults of P. monodon collected from the Muttukadu estuary and brooders collected from various sites in Tamil Nadu and Andhra Pradesh, India were examined for the
Ž .
occurrence of MBV Table 1 .
Ž .
Using Bergey’s Manual of Determinative Bacteriology Holt et al., 1994 , the total
Ž .
viable bacteria and Vibrio per post-larvae infectedruninfected were determined. The
post-larvae were washed in several changes of autoclaved seawater, dipped in 70%
Ž .
ethanol, rinsed in autoclaved seawater 1218Cr15 min and homogenised using a sterile
homogeniser. The samples were then serially diluted and plated on Zobell’s marine
Ž . Ž .
2216E agar Hi Media, Bombay or thiosulphate–citrate–bile salt–sucrose agar TCBS
ŽHi Media, Bombay , incubated for 24 h at 28. 8C and the total colony forming units
ŽCFUrper larva of the heterotrophic bacteria and Vibrio were determined..
2.4. Experimental trials with nauplii, zoea, mysis and post-larÕae
The mortality rates for MBV-infected and uninfected nauplii, zoea, mysis and post-larvae from cultured P. monodon, recovered from a hatchery in Tamil Nadu were
determined. Nauplii were stocked at a concentration of 100 nauplii ly1, in concrete tanks
containing 5000 l of filtered, UV-irradiated water. Zoea were fed Chaetocerus, at a
density of 1–1.3 million cells mly1 tank water every 24 h. Mysis were fed Chaetocerus
at a density of 65,000–80,000 cells mly1 and 0.25 Artemia nauplii mly1 or
microen-Ž .
capsulated pellet feed Argent Chemicals, Redmond, WA, USA . The water was
density of the zoea, mysis and post-larval populations in each tank was estimated daily
Ž .
from aliquots removed from the tanks Ramasamy et al., 1996 . Dead and moribund larvae and post-larvae were removed from each tank and counted. Samples of nauplii, zoea, mysis and post-larva were collected routinely and examined for MBV infection. Mortalities and behavioural abnormalities, if any, were determined by observing the actions of the shrimp. In addition, squash preparations were also made to determine their infection status.
3. Results
Ž
MBV infection was recorded in the hepatopancreas of cultured larvae zoea and .
mysis , post-larvae, wild caught juveniles, sub-adults and brood stocks. Infected larvae and post-larvae exhibited lethargy and reduced feeding and preening activities, while juveniles, sub-adults and brooders showed no abnormal behaviour. In the later stages of infection MBV and bacteria occurred concurrently.
3.1. Histopathology
Within MBV-infected hepatopancreatic epithelial cells, the nuclei contained from 1 to 12 spherical, refractile occlusion bodies which varied in staining intensity from weak to
Ž .
strongly eosinophilic Figs. 1–5 . Many epithelial cells exhibited extensive degeneration characterised by an increased ratio in the nuclear volume to the cell cytoplasmic volume
Ž3:1 , and the presence of numerous vacuoles, 15. "1mm in diameter Figs. 2–5 . InŽ .
heavy infections, necrosis and lysis of the epithelial cells occurred with sloughing-off of
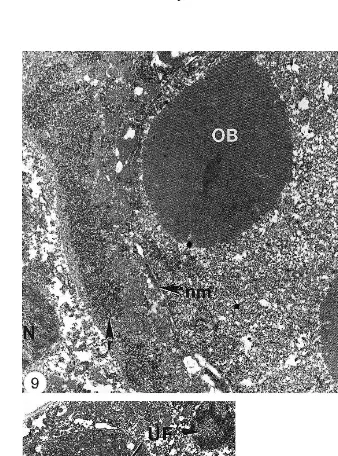
Fig. 1. Light photomicrograph of mysis P. monodon showing the nucleus of an MBV infected
hepatopancre-Ž . Ž .
atic cell containing an eosinophilic occlusion body OB . Two uninfected cells UF are also present. Nucleus
Ž .n . Scale bars5mm.
Ž .
Fig. 2. Light photomicrograph of mysis P. monodon. An hypertrophied nucleus N within an
hepatopancre-Ž . Ž .
atic cell containing intensely stained eosinophilic bodies OB . Numerous large vacuoles V are present in the cytoplasm. Scale bars4mm.
Fig. 3. Light photomicrograph of post-larval P. monodon. Section through the anterior midgut cells of P.
Ž . Ž .
monodon showing an MBV occlusion body OB . Numerous vacuoles V are present within the cytoplasm of Ž .
the infected cells. Gut lumen L . Scale bars15mm.
Ž .
Fig. 4. Light photomicrograph showing infected cells containing MBV occlusion bodies OB within the nuclei
Ž .
of the of juvenile P. monodon anterior midgut cells. Numerous uninfected cells UF are also present. Scale bars15mm.
Fig. 5. Section through a region of the anterior midgut of post-larval P. monodon showing MBV occlusion
Ž . Ž .
bodies OB within an hypertrophied nucleus. Gut lumen cells that have been sloughed off cl are lying free in
Ž .
Ž .
3.2. Ultrastructure
TEM studies of uninfected anterior midgut cells and hepatopancreatic epithelial cells
Ž . Ž
showed numerous mitochondria, endoplasmic reticulum ER and vesicles Figs. 6 and .
7 . Infected hepatopancreatic cells exhibited a thin band of cytoplasm, numerous vacuoles, Golgi, ER, polyribosomes and few mitochondria, with a considerable loss of
Ž .
microvilli from the apical cell surface Figs. 8–10 . The nuclei were hypertrophied and contained from one to three ovoid, crystalline, occlusion bodies, the nucleoplasm was less electron-dense while the chromatin appeared to disintegrate and electron-lucent
Ž .
areas were widespread Figs. 8–11 . The nucleolus was peripherally located, the nuclear membrane was dilated and nuclear pores were abundant. Two types of occlusion bodies were identified. Type 1 contained an electron-dense matrix with a paracrystalline array of polyhedrin subunits, 14.5 nm in diameter, with a lattice spacing of approximately 5–7 nm. The virions were rod-shaped, occluded and non-occluded with a double-layered
Ž .
envelope Figs. 10, 12 and 13 which measured 78"3 nm wide and 276"2 nm long,
Ž . Ž .
with a nucleocapsid 54"2 nm long and 161"3 nm wide ns100 Fig. 14 . Within
the virions, electron-dense nucleoproteins were present. In some larval stages, occluded virions were embedded within the paracrystalline matrix however, virions could not
Ž .
always be observed within the occlusion bodies Fig. 15 . The type 2 occlusion bodies consisted of non-crystalline, granulin-like sub-units, each measuring 12 nm in diameter
ŽFigs. 16 and 17 and contained numerous non-occluded virions aggregated close to the.
nuclear membrane, with only a few occluded virions present in the matrix. The virions
possessed a single-layered envelope and measured 326"4 nm long and 73"1 nm wide
Ž .
with a nucleocapsid 295"1 nm long and 67"1 nm wide ns100 .
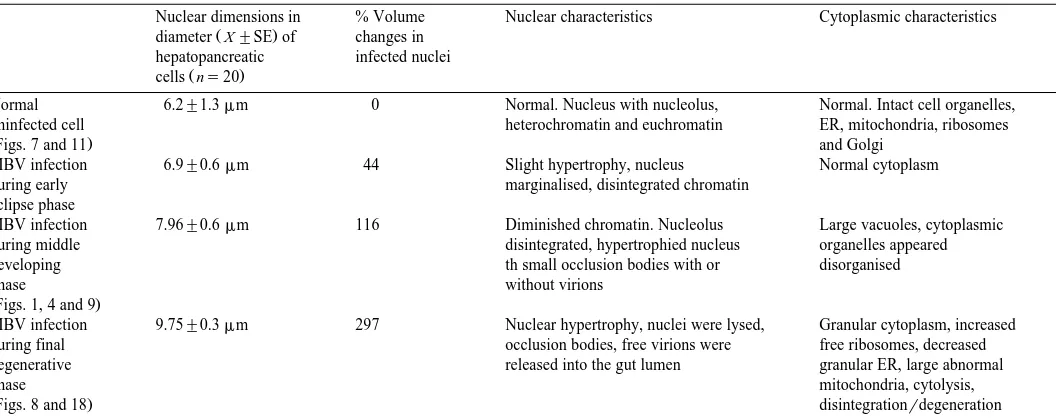
Three distinct phases of MBV infection were recognized, an early eclipse phase, a
Ž .
middle-developing phase and a final degenerative phase Table 2 . In the eclipse phase, no cytopathological changes were observed. The nucleus was slightly hypertrophied
Ž44% with the nucleolus towards the periphery. The chromatin was mostly disintegrated.
but the nucleoplasm appeared normal. Virions and aggregations of polyhedrins, in the form of small occlusion bodies were occasionally observed. Bacterial infections were
Ž .
absent. In the middle-developing phase Figs. 1, 4 and 9 , the chromatin had diminished considerably and small occlusions were apparent in the center of the nucleus. The nucleolus had disintegrated, the nuclear volume had increased by 116%, and occlusion
Fig. 6. Transmission electron micrograph of post-larval P. monodon, a section through an uninfected anterior
Ž . Ž . Ž .
midgut cell showing the cell organelles, nucleus N , microvilli mv , mitochondria M , endoplasmic
Ž . Ž . Ž .
reticulum ER and tight junctions t . Gut lumen L . Scale bars1mm.
Fig. 7. Transmission electron micrograph, post-larval P. monodon, of a section through an uninfected
Ž . Ž . Ž .
hepatopancreatic cell showing the cell organelles, nucleus N , nucleolus n , heterochromatin h , nuclear
Ž .
membrane nm . Scale bars1mm.
Fig. 8. Transmission electron micrograph of a section through MBV infected hepatopancreatic cells of mysis
Ž . Ž .
P. monodon showing a number of type 2 occlusion bodies OB within denatured, electron lucent nuclei N .
Ž . Ž .
Numerous vacuoles V are present within the cells and microvilli mv have been lost from the apical cell
Ž . Ž .
bodies, with or without virions, had developed. Within the cell, the cytoplasm contained large vacuoles and the organelles appeared disorganized. Bacterial infections were
Ž .
the cytoplasm was granular and the cells had undergone cytolysis. Occlusion bodies and free virions, along with the remaining cellular contents were released into the space previously occupied by the MBV-infected cells.
Secondary invaders, such as bacteria, also infected the larvae and post-larvae increasing cytolysis and sloughing-off of the infected cells. In the terminal stages of infection, only the boundaries of the hepatopancreatic and anterior midgut cells were present enclosing occlusion bodies, free or occluded virions, and bacteria which entered
Ž .
the intestinal tract via the hepatopancreatic lumen Figs. 15 and 18 . MBV occlusion bodies and virions were absent in P. monodon eggs and nauplii.
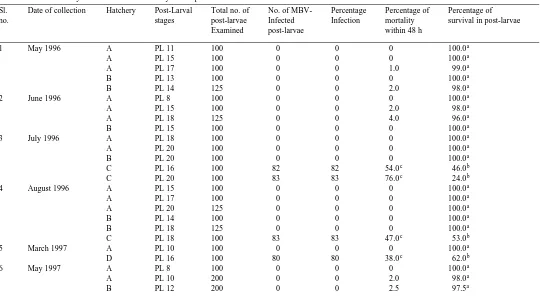
3.3. Field studiesrhatcheries
Histopathological examination of hepatopancreas tissues from P. monodon
post-Ž .
larvae, recovered from four hatcheries A, B, C and D revealed the frequent occurrence of MBV occlusion bodies in post-larvae from hatcheries C and D. Hatcheries A and B
Ž .
were infection-free Table 3 . The percentage occurrence of MBV-infected post-larvae
averaged 84%"2%, with an average mortality rate of 55%"6% and a survival rate of
45%"6%, over a 48-h period. For the uninfected larvae, the mortality rate was less,
Ž .
while the survival rate was 99%"0% Table 3 . The total viable heterotrophic bacterial
Ž 5.
countsrMBV infected post-larva 7.0"0.6=10 were 10 times greater than in
Ž 5.
uninfected larva 0.7"0.1=10 , though the counts were variable. Vibrio counts were
Ž 3. Ž
also greater in MBV infected larvae 5"0.7=10 than uninfected larvae 0.3"0.1=
3. 10 .
Examination of captured P. monodon juveniles and sub-adults collected from the Muttukadu Estuary, and brooders collected from various sites in Tamil Nadu and Andhra Pradesh, showed the widespread occurrence of MBV infected shrimp in India
Žsee Scheme 1 . The brood stocks displayed the highest infection rates, 21%. "0.6%,
while in juveniles and sub-adults, the infection rates were relatively low, 4.4%"0.4%
Ž .
and 5.3%"0.6%, respectively Table 1 .
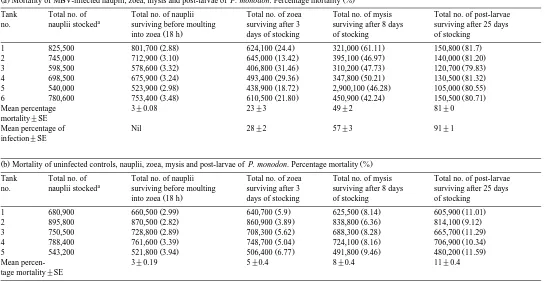
3.4. Experimental trials with nauplii, zoea, mysis and post-larÕae
Ž .
Mortality rates for the larvae nauplii, zoea and mysis and post-larvae of cultured P.
monodon infected with MBV were observed in six separate hatchery tanks with different
stocking densities. MBV infections recorded in the zoea, mysis and post-larvae was 28"2%, 57"3% and 91%"1%, respectively. The highest recorded mortality rates
Fig. 9. Transmission electron micrograph of a section through an hepatopancreatic cell of P. monodon mysis
Ž . Ž .
stage showing crystalline type 1 occlusion bodies OB and hypertrophied nuclei N . Within the nucleus,
Ž .
chromatin is absent and the density of the nucleoplasm is greatly reduced. Nuclear membrane nm ,
Ž .
polyribosomes arrow . Scale bars1mm.
Ž .
Fig. 10. Transmission electron micrograph of post-larval P.monodon showing uninfected UF and an infected
Ž . Ž .
hepatopancreatic cell nuclei N . The infected cell nucleus contains a type 1 MBV occlusion body OB , The
Ž .
occurred in MBV-infected post-larvae, 81%"0%, with lower rates in the zoea and
Ž .
mysis, 23%"3% and 49%"2%, respectively Table 4a . In all cases, the mortality
Ž .
rates in the uninfected larvae and post-larvae were lower Table 4b . The mortality rates
Ž . Ž .
The occurrence of MBV infections in hatchery-reared mysis and post-larvae at, 57%"3% and 91%"1%, respectively, was higher than in the zoea at 28%"2%. In hatchery-reared larval and post-larval shrimp, the prevalence of MBV infections in-creased with increasing age and peaked in the post-larvae.
4. Discussion
The occurrence of MBV has been identified and described in each life cycle stage of the Indian penaeid shrimp, P. monodon. Visible indications of MBV infected P.
monodon included, lethargy with reduced feeding and preening activities. Internally, the
presence of hypertrophied nuclei with intranuclear spherical occlusion bodies confirmed
Ž .
the presence of MBV as described by Lightner and Redman 1981 , Lightner et al.
Ž1983 , Doubrovsky et al. 1988 , Baticados et al. 1991 and Ramasamy et al. 1995 .. Ž . Ž . Ž .
MBV occlusion bodies have been identified only in the nuclei of the hepatopancreatic and anterior midgut epithelial cells of larval and post-larval P. monodon, and only in the hepatopancreas of juveniles, sub-adults and adults. MBV was absent in all other tissues
Ž . Ž .
examined supporting the observations of Lightner et al. 1983 and Vogt 1992 .
Whether these cells have specific surface receptors that bind to the MBV virions and promote rapid invasion, division and proliferation is unknown and requires further investigation, which may help clarify the mode of entry, replication and pathogenesis of MBV in the host cells. MBV-infected cells exhibited necrosis, lysis and sloughing-off of
Ž
cells into the tubule lumen as described in previous studies Lightner and Redman, 1981; .
Baticados et al., 1991 . Infected cells also underwent partial or complete disintegration, releasing free virions and occlusion bodies, and this together with the cannibalistic behaviour of the shrimp greatly enhances transmission of MBV infection.
At the sub-cellular level, MBV-infected hepatopancreatic cells exhibited a reduced cytoplasmic volume, an increased number of vacuoles and free ribosomes with a few dilated ER and Golgi complexes and reduced numbers of mitochondria, as reported in
Ž
previous studies Lightner and Redman, 1981; Lightner et al., 1983; Couch, 1989; .
Halder et al., 1989; Vogt, 1992; Lu et al., 1995, 1996 . The increased numbers of vacuoles may indicate the degree of infection and degeneration or cytolysis of the
Ž .
Fig. 11. Enlarged image of an MBV type 2 crystalline occlusion body OB showing a number of occluded
Ž .
virions unlabeled arrows . The density of the nucleoplasm is reduced compared to uninfected nuclei. Nucleus
Ž .N . Scale bars0.5mm.
Ž .
Fig. 12. Magnified image of a section through a type 1 MBV crystalline occlusion body c showing numerous
Ž .
occluded virions Vi . Scale bars0.2mm.
Ž . Ž .
Fig. 13. Type 1 MBV crystalline occlusion bodies OB containing numerous occluded virions Vi , while some non-occluded virions lie free at the periphery of the occlusion body. Occluded virions cut in longitudinal
Ž .
section LS . Scale bars1mm.
Fig. 14. Magnified image of a cross-sectional view of non-occluded type 2 MBV virions showing the outer
()
P.
Ramasamy
et
al.
r
Aquaculture
184
2000
45
–
66
Table 2
Cytopathological changes in the development of MBV infection in cells of the hepatopancreas in post-larval P. monodon
Nuclear dimensions in % Volume Nuclear characteristics Cytoplasmic characteristics
Ž .
diameter X"SE of changes in hepatopancreatic infected nuclei
Ž .
cells ns20
Normal 6.2"1.3mm 0 Normal. Nucleus with nucleolus, Normal. Intact cell organelles,
uninfected cell heterochromatin and euchromatin ER, mitochondria, ribosomes
ŽFigs. 7 and 11. and Golgi
MBV infection 6.9"0.6mm 44 Slight hypertrophy, nucleus Normal cytoplasm
during early marginalised, disintegrated chromatin
eclipse phase
MBV infection 7.96"0.6mm 116 Diminished chromatin. Nucleolus Large vacuoles, cytoplasmic
during middle disintegrated, hypertrophied nucleus organelles appeared
developing th small occlusion bodies with or disorganised
phase without virions
ŽFigs. 1, 4 and 9.
MBV infection 9.75"0.3mm 297 Nuclear hypertrophy, nuclei were lysed, Granular cytoplasm, increased
during final occlusion bodies, free virions were free ribosomes, decreased
degenerative released into the gut lumen granular ER, large abnormal
phase mitochondria, cytolysis,
infected cells or perhaps the lack of lipid reserves necessary for viral replication. Vogt
Ž1992 showed that three types of hepatopancreatic cells R, F and B and the anterior. Ž .
midgut cells, infected by MBV, exhibited similarities in nuclear alterations namely, nuclear hypertrophy, chromatin marginalisation, the formation of occlusion bodies and virion assembly.
Two types of MBV-infections were identified in P. monodon. Type 1 consisted of polyhedrin sub-units. Type 2 consisted of granulin-like sub-units and is the first known report of this type of occlusion body associated with MBV although, its significance is unknown. Also present were occlusion bodies in which virions were few or absent. These variations may occur because the different occlusion bodies may release different virions, which may represent different species. Further molecular biological characteri-zation studies may help to clarify this situation. P. monodon infected with MBV virions
Ž
with a single-layered envelope have been reported in the Indopacific region Lightner et .
al., 1983 , while those with double-layered envelopes have been reported in Asia
ŽHalder et al., 1989 and Australia along with P. merguiensis Doubrovsky et al., 1988 .. Ž .
However, this is the first record of the presence of rod-shaped MBV virions with both single- and double-layered envelopes in the Indian shrimp, P. monodon.
Polyhedrin is a crystalline protein and a characteristic feature of viruses of the genus
Ž . Ž
Nuclear Polyhedrosis virus NPV belonging to the family Baculoviridae Francki et al., .
1991 . Occlusion bodies appear to stabilize virions and maintain their viability over prolonged adverse conditions. In the shrimp, infection probably occurs through a process
Ž .
similar to that of NPV of insects Rohrmann, 1992 .
Our observations on the morphometry of MBV virions with single- and double-layered envelopes revealed size differences in agreement with earlier reports on MBV virions by
Ž . Ž .
Fegan et al. 1991 and Chen et al. 1995 .
MBV infections in post-larval P. monodon can be divided into three phases. The early eclipse phase is difficult to detect suggesting that major cellular functions had not been altered. In the final degenerative phase, lysis of the nuclei and cytoplasm of the hepatopancreatic cells occurs with occlusion bodies and virions released into inter, or intracellular spaces, or into the lumen of the hepatopancreatic tubules. In adult P.
monodon, this does not occur, either because they are resistant to infection or they are
perhaps able to replace the cells at a faster rate than they are being destroyed, and so,
Ž .
may be considered to act as carriers of MBV. Vogt 1992 also described the MBV infection in R, F and B cells of the hepatopancreas in the anterior midgut cells of P.
monodon but, MBV has not yet been detected in the generative E cells of the
Ž .
hepatopancreas Fegan et al., 1991; Vogt, 1992 .
The fungus Lagenidinum callinectus together with bacterial cells have been found in MBV-infected hepatopancreatic cells. They probably occur as secondary invaders subsequent to a viral infection and may promote degeneration of the tissues leading to
Ž .
rapid death of the host. Similar observations were reported by Baticados et al. 1991 ,
Ž . Ž .
Ramasamy 1995; 1996 and Ramasamy et al. 1995 . Hatchery studies have shown that the MBV infected larvae harboured 10 times more bacteria than uninfected larvae reflecting the role of secondary invaders in the mortality of shrimp larvae.
of factors such as, contaminated rearing tanks, inadequate UV-treatment or chlorination of the source water, or the use of infected brood stocks for spawning. The current study also revealed that from a total of 42 collections of P. monodon post-larvae, MBV
Ž .
infection was present in nine samples 21% . In contrast, the prevalence of MBV infections in hatcheries in Taiwan and Thailand were reported at 98% when females
Ž .
spawned in large numbers up to 40 femalesrtank . However, it was found that rinsing
brood stocks, eggs and nauplii with clean sea water containing iodophor significantly
Ž .
reduced infections Fegan et al., 1991 . In the present study, MBV infections in brood stocks from wild-caught P. monodon were estimated at 20%, however, Fegan et al.
Ž1991 reported a 5.7% MBV infection rate in Thailand. The prevalence of MBV peaked.
in the post-larval stage and decreased with increasing age through to adults. This may be Ž .
due to a number of factors, i brood stocks with light infections may have become Ž .
MBV resistant, ii the virus may exist in a latent form with the host acting as a vector to Ž .
transmit the infection to the larvae, and iii the presence of contaminated faecal matter. Alternatively, MBV may be non-virulent in the juveniles, sub-adults and brood stocks.
Ž .
According to Fegan et al. 1991 , if MBV is carried from the brood stocks to the nauplii, mysis and post-larvae through the oocytes as latent viruses, they would have to be in the
Ž .
form of virions without occlusion bodies, or as free viral DNA. Vogt et al. 1986 and
Ž .
Fegan et al. 1991 reported that MBV infected the hepatopancreatic cells of poorly nourished, weak or stressed P. monodon, however, when the post-larvae are well fed the infection is unable to establish itself and the post-larvae continue to develop. Further research on the role and nature of the nutrients in the diet may help to improve the health and fitness of the larvae.
The mortality rates in the post-larval stage of P. monodon were greater than in either the zoea or mysis, possibly due to the effect of acute MBV infection in the
hepatopan-Ž .
creatic epithelial cells over a period of several days 10–15 days or rapidly rising virion production. However, in the case of the zoea and mysis, the infection is only in the early phase and because the time period associated with this developmental stage is short the mortality rate is low. Though mortalities were observed in nauplii, histopathological and electron microscope studies were unable to confirm the occurrence of MBV virions or occlusion bodies. Mortalities observed in the zoea and mysis might possibly be
Ž .
Fig. 15. Enlarged image of a type 1 crystalline occlusion body OB in which MBV virions are absent.
Ž .
Bacterium b . Scale bars0.5mm.
Fig. 16. Transmission electron micrograph of a type 2 non-crystalline, granulin-like MBV occlusion body
ŽOB within the nucleus of an hepatopancreatic cell of post-larval P. monodon, showing non-occluded virions. Ž .
at the periphery Vi . Scale bars1mm.
Fig. 17. An enlarged image of Fig. 16, showing the details of the type 2 non-crystalline, granulin-like MBV
Ž . Ž . Ž . Ž .
occlusion body OB and the associated virions Vi . Nucleocapsid nc , empty capsule ec . Scale bars0.5 mm.
Fig. 18. Transmission electron micrograph of cytolysed hepatopancreatic cells of post-larval P. monodon
Ž . Ž .
showing a number of type 2 MBV occlusion bodies OB and bacteria b . The boundaries of the cells are
Ž .
()
Prevalence and mortality of MBV-infected hatchery reared post-larval P. monodon
Sl. Date of collection Hatchery Post-Larval Total no. of No. of MBV- Percentage Percentage of Percentage of
no. stages post-larvae Infected Infection mortality survival in post-larvae
()
Mean percentage"SE 99"0
b
84"2 55"6 45"6
a
Percentage of survival in MBV-uninfected larvae. b
Percentage of survival in MBV-infected larvae.
c Ž . Ž .
()
Ž .a Mortality of MBV-infected nauplii, zoea, mysis and post-larvae of P. monodon. Percentage mortality %Ž .
Tank Total no. of Total no. of nauplii Total no. of zoea Total no. of mysis Total no. of post-larvae a
no. nauplii stocked surviving before moulting surviving after 3 surviving after 8 days surviving after 25 days
Ž .
into zoea 18 h days of stocking of stocking of stocking
Ž . Ž . Ž . Ž .
1 825,500 801,700 2.88 624,100 24.4 321,000 61.11 150,800 81.7
Ž . Ž . Ž . Ž .
2 745,000 712,900 3.10 645,000 13.42 395,100 46.97 140,000 81.20
Ž . Ž . Ž . Ž .
3 598,500 578,600 3.32 406,800 31.46 310,200 47.73 120,700 79.83
Ž . Ž . Ž . Ž .
4 698,500 675,900 3.24 493,400 29.36 347,800 50.21 130,500 81.32
Ž . Ž . Ž . Ž .
5 540,000 523,900 2.98 438,900 18.72 2,900,100 46.28 105,000 80.55
Ž . Ž . Ž . Ž .
6 780,600 753,400 3.48 610,500 21.80 450,900 42.24 150,500 80.71
Mean percentage 3"0.08 23"3 49"2 81"0
mortality"SE
Mean percentage of Nil 28"2 57"3 91"1
infection"SE
Ž .b Mortality of uninfected controls, nauplii, zoea, mysis and post-larvae of P. monodon. Percentage mortality %Ž .
Tank Total no. of Total no. of nauplii Total no. of zoea Total no. of mysis Total no. of post-larvae a
no. nauplii stocked surviving before moulting surviving after 3 surviving after 8 days surviving after 25 days
Ž .
into zoea 18 h days of stocking of stocking of stocking
Ž . Ž . Ž . Ž .
1 680,900 660,500 2.99 640,700 5.9 625,500 8.14 605,900 11.01
Ž . Ž . Ž . Ž .
2 895,800 870,500 2.82 860,900 3.89 838,800 6.36 814,100 9.12
Ž . Ž . Ž . Ž .
3 750,500 728,800 2.89 708,300 5.62 688,300 8.28 665,700 11.29
Ž . Ž . Ž . Ž .
4 788,400 761,600 3.39 748,700 5.04 724,100 8.16 706,900 10.34
Ž . Ž . Ž . Ž .
5 543,200 521,800 3.94 506,400 6.77 491,800 9.46 480,200 11.59
Mean percen- 3"0.19 5"0.4 8"0.4 11"0.4
tage mortality"SE a
Initial stock of napulii which metamorphose into zoea, mysis and post larvae, examined in the study. b
associated with stress in combination with a viral infection and, or other unknown factors.
This study has established the occurrence of two types of MBV infections in the Indian penaeid shrimp P. monodon. MBV-infected hepatopancreatic cells exhibited a 300% volume increase in the hypertrophied nuclei. Cytolysis and bacterial invasion was observed. Studies in the hatcheries showed that MBV infected larvae were 10 times more susceptible to bacterial infection.
Acknowledgements
This work was funded by The Department of Biotechnology, Government of India, New Delhi.
References
Alagarswami, K., 1995. Status Report on Shrimp Disease Outbreaks in Coastal Aquafarms on the East Coast of India. CIBA, Madras, India, 27 pp.
Baticados, M.C.L., Pitogo, C.L., Paner, M.G., de la Pena, L.D., Tendencia, E.A., 1991. Occurrence and pathology of Penaeus monodon baculovirus infection in hatcheries and ponds in the Philippines. Isr. J. Aquacult. Bamidgeh 43, 35–41.
Bell, T.A., Lightner, D.V., 1988. A Handbook of Normal Penaeid Shrimp Histology. World Aqua. Soc., Baton Rouge, pp. 1–114.
Ž .
Brock, J.A., Lightner, D.V., Bell, T.A., 1983. A Review of Four Virus BP, BMN, MBV and IHHNV Diseases of Penaeid Shrimp with Particular Reference to Clinical Significance, Diagnosis and Control in Shrimp Aquaculture. Int. Coun. Explor. Sea, Honolulu, HI, pp. 1–18.
Chen, X.F., Wu, D.H., Juang, H., Chi, X.C., Chen, P., 1995. Ultrastructure of Penaeus monodon baculovirus. J. Fish. China 19, 203–209.
Couch, J.A., 1989. The membranous labyrinths in baculovirus infected crustacean cells: possible roles in viral reproduction. Dis. Aquat. Org. 7, 39–53.
Doubrovsky, A., Paynter, J.L., Sambhi, S.K., Atherton, J.G., Lester, R.J.G., 1988. Observations on the ultrastructure of baculovirus in Australian Penaeus monodon and Penaeus merguiensis. Aust. J. Mar. Freshwater Res. 39, 743–749.
Fegan, D.F., Flegel, T.W., Sriurairatana, S., Waiyakratthna, M., 1991. The occurrence, development and histopathology of Monodon baculoÕirus in Penaeus monodon in South Thailand. Aquaculture 96, 205–217.
Ž .
Flegel, T.W., 1997. Major viral diseases of the black tiger prawn Penaeus monodon in Thailand. World J. Microbiol. Biotechnol. 13, 433–442.
Francki, R.I.B., Fauquet, C.M., Kmidson, D.L., Brown, F., 1991. Classification and nomenclature of viruses.
Ž .
Arch. Virol. 2, 1–450, Suppl. .
Fukuda, H., Momoyama, K., Sano, T., 1988. First detection of Monodon baculoÕirus in Japan. Bull. Jpn. Soc. Sci. Fish. 54, 45–48.
Halder, M., Ahne, W., Thomsen, I., 1989. Detection of baculovirus in the tiger prawn Penaeus monodon. J. Vet. Med. B 36, 257.
Ž .
Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T., Williams, S.T. Eds. , 1994. Bergey’s Manual of Determinative Bacteriology, 9th edn. Williams and Wilkins, MD, USA.
Hung, H.H., Perng, T.F., Chang, J.T., Tsung, T.M., Chen, W.L., Roan, M.J., Huang, H.T., Chang, R.R., 1991.
Ž .
Studies of the pathogens causing a high death rate in glass shrimp Penaeus monodon in Pingtung
Johnson, P.T., Lightner, D.V., 1988. Rod-shaped nuclear viruses of crustaceans: gut-infecting species. Dis. Aquat. Org. 5, 123–141.
Ž .
Lightner, D.V., 1983. Diseases of cultured penaeid shrimp. In: McVey, J.P. Ed. , CRC Handbook of Mariculture, Vol. 1, Crustacean Aquaculture. CRC Press, Boca Raton, FL, pp. 289–320.
Ž .
Lightner, D.V., 1993. Diseases of cultured penaeid shrimp. In: McVey, J. Ed. , CRC Handbook of Mariculture, Vol. 1, Crustacean Aquaculture, 2nd edn. CRC Press, Boca Raton, FL, pp. 393–486. Lightner, D.V., Redman, R.M., 1981. A baculovirus-caused disease of the penaeid shrimp, Penaeus monodon.
J. Invert. Pathol. 38, 299–302.
Lightner, D.V., Redman, R.M., Bell, T.A., 1983. Observations on the geographic distribution, pathogenesis and morphology of the baculovirus from Penaeus monodon Fabricius. Aquaculture 32, 209–233. Lightner, D.V., Redman, R.M., Williams, R.R., Mohney, L.L., Clerx, J.P.M., Bell, T.A., Brock, J.A., 1985.
Recent advances in penaeid virus disease investigations. J. World Maricult. Soc. 16, 267.
Lightner, D.V., Bell, T.A., Redman, R.M., 1990. A review of the known hosts, geographic range and current diagnostic procedures for the virus diseases of cultured penaeid shrimp. Advances in Tropical Aquaculture, Tahiti, 20 February–4 March 1989. AQUACOP, IFREMER, Centre deBrest, Plouzane, France. Acter de Colloque, Vol. 9, pp. 113–126.
Lin, C.K., 1989. Prawn culture in Taiwan. What went wrong?. World Aquacult. 20, 19–20.
Ž .
Lu, C.C., Tang, K.F.J., Kou, G.H., Chen, S.N., 1995. Detection of Penaeus monodon-type baculovirus MBV infection in Penaeus monodon Fabricius by in situ hybridization. J. Fish Dis. 18, 337–345.
Lu, C.C., Tang, K.F.J., Chen, S.N., 1996. Morphogenesis of the membranous labyrinth in penaeid shrimp cells
Ž .
infected with Penaeus monodon baculovirus MBV . J. Fish Dis. 19, 357–364.
Ramasamy, P., 1995. Diseases of Shrimp in Aquaculture Systems. Diagnosis and Therapeutic Measures. Vanitha Publications, Madras, India, 99 pp.
Ramasamy, P., 1996. Applications of Electron Microscopy and Cytochemical Techniques in the Diagnosis of Fish Disease. A Laboratory Manual. University of Madras, Madras, India, pp. 1–138.
Ramasamy, P., 1999. The Current Status of Viral Diseases of Prawns in India. Perspectives and Control
Ž .
Measures. Biotech Consortium India BCIL Publications, New Delhi, India, in press.
Ramasamy, P., Brennan, G.P., Jayakumar, R., 1995. A record and prevalence of Monodon baculoÕirus from post-larval Penaeus monodon in Madras, India. Aquaculture 130, 129–135.
Ž
Ramasamy, P., Rajan, P.R., Jayakumar, R., Rani, S., Brennan, G.P., 1996. Lagenidium callinectus Couch,
. Ž .
1942 infections and its control in cultured larval Indian tiger prawn Penaeus monodon Fabricius . J. Fish Dis. 19, 75–82.
Rohrmann, G.F., 1992. Baculovirus structural proteins. J. Gen. Virol. 73, 749–761.
Sindermann, C.J., 1990. Principal Diseases of Marine Fish and Shellfish, Vol. 2, Diseases of Marine Shellfish. Academic Press, New York, NY, pp. 9–40.
Spann, K.M., Lester, R.J.G., 1996. Baculovirus of Metapenaeus bennettae from the Moreton Bay region of Australia. Dis. Aquat. Org. 27, 53–58.
Spann, K.M., Lester, R.J.G., 1997. Viral diseases of penaeid shrimp with particular reference to four viruses recently found in shrimp from Queensland. World J. Microbiol. Biotechnol. 13, 419–426.
Sundararaj, A., Murali Manohar, B.B., Ruby Renchitt Sheela, P., Selvaraj, D., Ravishankar, B., 1996. Occurrence of Monodon baculoÕirus infection in shrimp in Tamil Nadu. Indian J. Fish. 43, 103–105. Tangtongpiroj, J., 1989. A virus disease of black tiger prawns. Fish. News 13, 25–29.
Thikiew, N., 1990. A serious MBV outbreak in black tiger prawn. Livest. Prod. Mag. 7, 62–66.
Vega-Villasante, F., Puente, M.E., 1993. A review of viral diseases of cultured shrimp. Prev. Vet. Med. 17, 271–282.
Ž .
Vogt, G., 1992. Transformation of anterior midgut and hepatopancreas cells by Monodon baculoÕirus MBV in Penaeus monodon post-larvae. Aquaculture 107, 239–248.
Vogt, G., Quinitio, E.T., Pascual, F.P., 1986. Leucaena leucocephola leaves is formulated feed for Penaeus
monodon: a concrete example of the application of histology in nutrition research. Aquaculture 59,