www.elsevier.nlrlocateraqua-online
The use of larval fatty acids as an index of growth
in Mytilus edulis L. larvae
Nikos Leonardos
), Ian A.N. Lucas
1 School of Ocean Sciences, Menai Bridge LL59 5EY, N. Wales, UKAccepted 16 September 1999
Abstract
Continuous cultures of Chaetoceros muelleri, Skeletonema costatum and Rhinomonas
reticu-lata of controlled biochemical compositions were fed to Mytilus edulis larvae over a 2-week
period and the larval growth was assessed. The eggs as well as the larvae which subsequently developed were analyzed for their relative fatty acid profile and content; 27 fatty acids were identified by means of GCrMS. Larval 15:0 and saturated fatty acids, in general, were found to be negatively correlated with larval growth, while polyunsaturated and ny3 fatty acids were positively correlated with growth. It is therefore proposed that the proportions of certain fatty acids and groups of fatty acids may be used as indices of growth and condition in M. edulis larvae.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Fatty acids; Growth — larvae; Mytilus edulis; Skeletonema costatum; Rhinomonas reticulata
1. Introduction
In various bivalve larvae nutritional studies, larvae have been used to bioassay the
Ž
nutritional value of algal, bacteria, yeast or artificial diets Laing, 1987; Brown et al.,
.
1996; Thompson et al., 1996 . However, different larval batches may have had inher-ently different survival or growth characteristics depending on broodstock origin.
Ž .
Variation in genetic composition Del Rio-Portilla, 1996 and broodstock nutrition,
)Corresponding author. Vochtingstr 1-7, D-72076 Tubingen, Germany. E-mail:¨ ¨ [email protected]
1
Also corresponding author. Tel.: q44-1248-382871; fax: q44-1248-382871; e-mail: [email protected].
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
conditioning and stage of the gonadal cycle may thus make comparisons of algal nutritional value assayed in this manner difficult to interpret.
Growth and survival of bivalve larvae is strongly influenced by broodstock condition-ing in a number of ways. Seasonal variation in environmental conditions, with resultcondition-ing variation in the stage of gonadal development, will in turn influence the subsequent
Ž .
gamete viability Lannan et al., 1980 . The correlation of broodstock conditioning and
Ž .
larval survival and growth was shown by Gallager and Mann 1986 to be characterized by the lipid content of the eggs. They found that variations in the broodstock condition-ing protocol could produce large fluctuations in the lipid content of the eggs, thus providing the link between broodstock conditioning and larval survival and growth.
Ž .
Wilson et al. 1996 suggested that broodstock nutrition was also important for the
Ž .
viability of the larvae of Ostrea chilensis, while Berntsson et al. 1997 have demon-strated the effect of broodstock diet on fatty acid composition, survival and growth rates in larvae of O. edulis. They found that the larval growth rate was significantly correlated
Ž .
with the content of the total polyunsaturated fatty acids PUFAs and especially the 22:6 ny3 representative. It has been suggested that lipid can be used as an index of
Ž .
growth and viability in the larvae of three species of bivalve Gallager et al., 1986 and has been proposed more widely as a condition index for fish and crustacean larvae
ŽFraser, 1989 . Despite these reports, there is little conclusive evidence to suggest a.
correlation between larval fatty acids and growth.
In the present report, three microalgal species were grown under controlled condi-tions to produce cells of known biochemical composition. These diets were then supplied to Mytilus edulis larvae for a 14-day feeding period. The fatty acid content of the larvae was assessed, as was their final length. These data were examined to investigate if groups of larval fatty acids could be correlated with growth in M. edulis larvae. To insure that the resulting fatty acid variation was a result of the different feeding diets and not due to the inherent biochemical variability of the larval batches, the corresponding egg batches were also analyzed for their fatty acid content. The larval fatty acid results from the various trials were pooled for statistical analysis, provided there was agreement in the composition of the original eggs.
2. Materials and methods
Ž .
Chaetoceros muelleri Lemmermann 1898 CCAP 1010r3 , Rhinomonas reticulata
ŽLucas , Novarino CCAP 995. Ž r2 and Skeletonema costatum Greville Cleve were. Ž . Ž
used as food for M. edulis larvae. A control diet of PaÕloÕa lutheri Droop CCAP
.
931r1 and R. reticulata in a 4:1 ratio was used. The test algae were grown using a continuous culture regime under a light–dark cycle of 14–10 h, at 20"18C. Two light
Ž y1 y2
intensities were employed high light, HLs890–950 Kphotons s m and low light,
y1 y2.
LLs275–337 Kphotons s m , and three nutrient conditions were used in each
Ž . Ž . Ž .
system and Conway medium. Algal culturing methods and conditions are described in
Ž .
detail in Leonardos and Lucas 2000a .
Larval cultures were started at a concentration of 30,000 larvae ly1. These were transferred into sufficient plastic 1-l beakers to give three replicates for each treatment. To each beaker, the respective species and quantities of algae were added and then filled to 1 l and left at 14"18C. Changes of the 0.2-mm filtered UV-radiated, seawater and feeding of the larvae was carried out every second day. The respective algal food was
Table 1
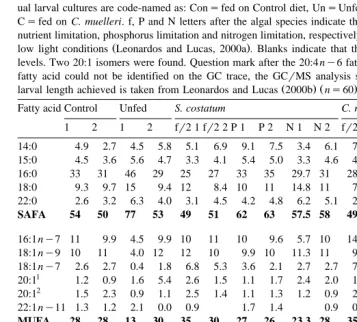
Fatty acid profile of various batches of M. edulis eggs, sampled prior to fertilization
Values indicate percentage of total identifiable fatty acids. HLsHigh Light, LLsLow Light algal growth conditions. Blank spaces indicate that the fatty acid was below detection levels. Mixt. P.U.sMixture of polyunsaturated fatty acids identified with additional traces from the GCrMS traces. The table contains two unidentified isomers of the 22:2 fatty acid. SAFAsSaturated fatty acid, MUFAsMonounsaturated fatty acid, PUFAsPolyunsaturated fatty acid.
Fatty acid S. costatum – C. muelleri S. costatum – C. muelleri R. reticulata
added to the beaker at a density to bring the final concentration to 50 cellsmly1. Three individual replicate larval cultures were grown, two of which were analyzed for their fatty acid content after the 14-day feeding trial, and their respective mean final length was also recorded. Detailed descriptions of the mussel larval rearing techniques are
Ž .
described in Leonardos and Lucas 2000b .
The eggs and larvae samples for fatty acid analysis were obtained by filtering a known number of eggs or larvae onto a Whatman No. 4 filter which had been
Table 2
Fatty acid content of M. edulis larvae obtained after 2 weeks of feeding on S. costatum and C. muelleri
Ž .
Individual fatty acid is expressed as percentage of total identifiable fatty acids percentage TIFA . SAFAs Saturated fatty acid, MUFAsMonounsaturated fatty acid, PUFAsPolyunsaturated fatty acid. Two individ-ual larval cultures are code-named as: Consfed on Control diet, UnsUnfed larvae, Ssfed on S. costatum, Csfed on C. muelleri. f, P and N letters after the algal species indicate that the alga was cultured under no nutrient limitation, phosphorus limitation and nitrogen limitation, respectively. The two diatoms were grown at
Ž .
low light conditions Leonardos and Lucas, 2000a . Blanks indicate that the fatty acid was below detection levels. Two 20:1 isomers were found. Question mark after the 20:4 ny6 fatty acid indicates that although the fatty acid could not be identified on the GC trace, the GCrMS analysis suggested an identification. Mean
Ž . Ž .
larval length achieved is taken from Leonardos and Lucas 2000b ns60 .
Fatty acid Control Unfed S. costatum C. muelleri
1 2 1 2 fr2 1 fr2 2 P 1 P 2 N 1 N 2 fr2 1 fr2 2 P 1 P 2 N 1 N 2
Larval 147 148 115 116 155 158 124 128 165 173 165 163 151 156 163 158 length in
microns
pre-washed in methanol, and then the filter was stored in a clean glass vial with
Ž .
chloroformrmethanol qbutyl hydroxyl toluene 2:1 solution. The vials were stored at
y408C.
All the glassware used were pre-washed with chloroform to avoid lipid contamina-tion. The lipids were extracted using a modification of the method of Bligh and Dyer
Ž1959 ; fatty acid methyl esters were obtained by methylation with 12% BF. 3 in
Ž .
methanol Metcalfe et al., 1966 . A methanolic solution of C23:0 was used as an external
Table 3
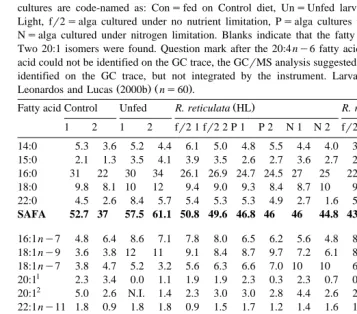
Fatty acid content of M. edulis larvae obtained after 2 weeks of feeding on R. reticulata
Ž .
Individual FA are expressed as percentage of total identifiable fatty acids percentage TIFA . SAFAsSaturated fatty acid, MUFAsMonounsaturated fatty acid, PUFAsPolyunsaturated fatty acid. Two individual larval cultures are code-named as: Consfed on Control diet, UnsUnfed larvae, HLsHigh Light, LLsLow Light, fr2salga cultured under no nutrient limitation, Psalga cultures under phosphorus limitations and Nsalga cultured under nitrogen limitation. Blanks indicate that the fatty acid was below detection levels. Two 20:1 isomers were found. Question mark after the 20:4 ny6 fatty acid indicates that although the fatty acid could not be identified on the GC trace, the GCrMS analysis suggested an identification. N.I.sfatty acid identified on the GC trace, but not integrated by the instrument. Larval length achieved is taken from
Ž . Ž .
Leonardos and Lucas 2000b ns60 .
Ž . Ž .
Fatty acid Control Unfed R. reticulata HL R. reticulata LL
1 2 1 2 fr2 1 fr2 2 P 1 P 2 N 1 N 2 fr2 1 fr2 2 P 1 P 2 N 1 N 2
Larval 199 192 138 137 162 172 168 180 198 184 205 181 161 163 178 172 length in
microns
Ž .
standard. Equipment and instrumentation of gas chromatography GC and gas
chro-Ž . Ž .
matography–mass spectroscopy GCrMS are as in Leonardos and Lucas 2000a . Identification was carried out by comparison with known cod liver oil traces. To determine the quality of fatty acid, Mass Lab software, supplied with the instrument, was used.
All statistical analyses were carried out with the aid of the Minitabw
. To investigate the use of larval fatty acids as an index of growth, correlation analysis was used. This type of analysis was used rather than regression analysis because the assumptions of model I regression analysis do not hold true with this type of data. For a more detailed discussion and useful examination of various aspects of regression and correlation
Ž .
analysis, the reader is referred to Sokal and Rohlf 1987 .
3. Results
The fatty acid profiles of the different batches of eggs used in the trials were similar
Ž .
in qualitative terms Table 1 . However, there was a closer agreement in relative percentage terms between the fatty acid profiles of those eggs used in the low light S.
costatum–C. muelleri and the high and low light R. reticulata experiments than those
used in the high light S. costatum–C. muelleri experiment; therefore, the resultant larvae from the latter feeding experiment were not included in the subsequent statistical analysis. The fatty acid profiles of the larvae after the 14-day feeding trial are shown in Table 2 for the S. costatum–C. muelleri and in Table 3 for the R. reticulata experi-ments. The final mean larval length of the two larval cultures fed diets shown in these
Ž .
tables was taken from Leonardos and Lucas 2000b . These two larval batches exhibited
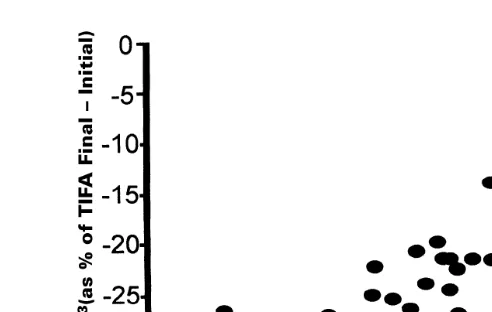
Fig. 2. Relationship between larval SAFA expressed as final minus initial percentage of total identified fatty acids and corresponding length of the larvae achieved after the 14-day feeding trial.
differences in the larval fatty acid content as a response to the different diet quality rather than the fatty acid content of the original eggs. This was seen when the proportion
Žexpressed as percentage of TIFA of any given fatty acid or fatty acid group was.
plotted against the length of the corresponding larvae. Of all the fatty acids identified, there was a significant correlation of larval growth with the 15:0, SAFA, PUFA and
ny3 FAs. Representative plots of the data from Table 3 of the final percentage less the initial percentage of these fatty acids against the length of the corresponding larvae are shown in Figs. 1–4 with their corresponding correlation values ofy0.72,y0.74, 0.70
Ž .
Fig. 4. Relationship between larval ny3 fatty acids ny3 FA expressed as final minus initial percentage of total identified fatty acids and corresponding length of the larvae achieved after the 14-day feeding trial.
and 0.76 for 15:0, SAFA, PUFA and ny3 FAs correspondingly. These correlation values suggest strong positive correlation of larval length with the PUFA and ny3 FAs and also indicate a negative correlation between the 15:0 and SAFA.
4. Discussion
Ž .
It has been demonstrated Lannan et al., 1980 that broodstock management strongly influences subsequent larval viability. The influential mechanism was not known by the authors at that time, but it was thought to be related to the initial triacylglycerol content furnished to each egg by the parent during the conditioning period and was also
Ž .
season-dependent Gallager and Mann, 1986 . Additionally, it has been shown that broodstock nutrition is important for the viability of the larvae and spat of many marine
Ž .
bivalves Wilson et al., 1996; Berntsson et al., 1997 . But it is not only larval growth that is influenced by egg quality; in some cases, settlement, spat growth and juvenile
Ž
survival are also influenced by egg quality, particularly of the egg lipid content Bayne,
.
1972; Bayne et al., 1975, 1978; Helm et al., 1973; Kraeuter et al., 1982 . The results from the analyses of egg fatty acid composition, in the present work, confirm that there is variation in these components, which was furnished by their parents. It can be seen
ŽTable 1 that the eggs used for the low light experiment involving S. costatum and C.. muelleri have a much closer fatty acid profile to those used for the R. reticulata
Ž .
for almost all the fatty acids identified. Therefore, the differences in these two batches of eggs probably contributed little to the overall variation of the larvae obtained at the end of the feeding experiments.
Ž .
Lipid, in general, has previously been used as a growth index Gallager et al., 1986 .
Ž .
Fraser 1989 reviewing the relative research tried to establish a condition index of environmentally stressed larvae, of fish, bivalves and crustaceans, with lipid content.
Ž .
Recently, Berntsson et al. 1997 showed a significant relationship between larval growth rate and the 22:6 ny3 content of the broodstock in O. edulis. Although this particular fatty acid was not found to act as an index of larval growth in this study, the
ny3 FAs as a group, were positively correlated with larval growth. The fatty acid analysis of these M. edulis larvae clearly indicated that several fatty acids and fatty acid groups can be used as an index of growth of the larvae.
It has been assumed that increased SAFA content would indicate a non-optimal
Ž .
physiological state Helm et al., 1991; Frolov and Pankov, 1992 as borne out by the negative correlation found here. It is interesting to note the negative correlation of 15:0
ŽFig. 1 with larval growth in the light of this compound’s supposed bacterial origin. ŽVolkman et al., 1989 . If this negative correlation is correct, it would then suggest that.
the microbial load inevitably associated with the larval cultures may hinder larval
Ž .
growth Lewis et al., 1988 . Fatty acids profiles could thus provide a quick
semi-quanti-Ž
tative indication of the extent of the bacterial load in mass cultures Volkman et al.,
.
1989 . This is in accordance with traditional culturing practices which aim to reduce the
Ž .
microbial load of the larval cultures Loosanoff and Davis, 1963 , as well as with
Ž
various reports on particle size selection for M. edulis Jørgensen, 1996; Defossez and
.
Hawkins, 1997 which seem to indicate that the larvae are not utilizing bacteria, at least in a way that effects their growth rate. This contrasts with the reported beneficial role of
Ž
some bacterial strains in axenic Crassostrea gigas cultures Douillet and Langdon,
.
1993, 1994 . Some bacterial strains, when added with microalgae in the larval rearing vessel, have been shown to promote greater larval growth more than when the alga is
Ž .
fed alone Yasuda and Taga, 1980; Douillet, 1987; Intriago and Jones, 1993 . The role of the bacterial fauna present in the rearing vessels is a matter of ongoing debate. But, it should be emphasized that the risk of introducing potentially harmful bacteria in the
Ž
culture can have devastating effects, and thus outweighs the potential benefit Volkman
.
et al., 1989 .
The exact physiological role of the SAFAs is not understood in marine larvae. Some SAFA, like 16:0 and 18:0 are not thought to be essential, but may accumulate in larvae,
Ž .
for example, Patinopecten yessoensis Whyte et al., 1989 . In this current work, the negative correlation with larval growth also suggests that although they constitute an energy source, they do not, either collectively or individually, represent an absolute requirement for larval development.
Ž
PUFAs have been shown to be both nutritionally important e.g., Helm et al., 1973;
.
Webb and Chu, 1983; De Pauw et al., 1984; Kanazawa, 1985; Enright et al., 1986a,b
Ž
and to be indicative of the physiological state of the larvae Langdon and Waldock,
.
1981 . The role of PUFAs may be important in many different developmental stages of the larvae, not least because many marine animals appear to have a limited ability to
Ž
. Ž .
et al., 1986a,b . The strong positive correlation Fig. 4 found in this study between PUFAs and growth clearly supports this theory.
Similar arguments can be raised for the ny3 group of fatty acids. This family of fatty acids is considered to be nutritionally important to adult Cra. Õirginica and
Ž .
juvenile Cra. gigas Langdon and Waldock, 1981 and has been regarded as
nutrition-Ž
ally essential for vigorous growth Joseph, 1982; Watanabe et al., 1983; Levine and
.
Sulkin, 1984; Waldock and Holland, 1984 . The dietary role of this group has also been demonstrated by the poor nutritional value of algal species with a small percentage of
Ž .
this fatty acid family De Pauw et al., 1984; Helm and Laing, 1987; Whyte et al., 1990 . The present analysis also supports the use of this group as a direct index of growth for
M. edulis larvae.
It must be emphasized, however, especially with the findings of Leonardos and Lucas
Ž2000b , that even though there is a clear correlation between certain larval fatty acids.
and larval growth, this cannot be extrapolated directly to similar relationships between dietary fatty acids and larval growth. In fact, for the same fatty acids that were found to be a positive growth index for M. edulis larvae, there is direct evidence that they were negatively correlated with larval growth when examined from a dietary perspective. Many researchers in aquaculture, due to this strong correlation of the larval ny3 PUFA with larval growth, and the known limited ability of many marine larvae to synthesize
Ž .
several ny3 FAs Langdon and Waldock, 1981 use this in a ‘‘a rule of thumb’’
Ž .
ranking of algal diets Watanabe et al., 1983; Brown et al., 1996 . In sharp contrast with
Ž . Ž .
these suggestions, Dickey-Collas and Geffen 1992 , Thompson et al. 1993; 1994 and
Ž .
Leonardos and Lucas 2000b report a negative correlation between dietary PUFA and
ny3 with larval growth and its positive correlation with dietary SAFA, especially the 16:0 fatty acid. It is clear that, unless there is direct experimental evidence, results should not be extrapolated beyond their context.
Although the exact significance of most single fatty acids or fatty acid families is still
Ž .
unclear Volkman et al., 1989 , they can nevertheless be used to describe the physio-logical state of M. edulis larvae. But it is obvious that more studies are required to identify the role of most of the mainly PUFAs in larval biology.
Acknowledgements
The first author would like to thank Andy Beaumont for his help and helpful comments throughout this work and J. East for his invaluable help with the fatty acid analyses. The present work is part of a PhD research thesis funded by the Greek State
Ž .
Scholarship Foundation I.K.Y. whose help is acknowledged.
References
Bayne, B.L., 1972. Some effects of stress in the adult on the larval development of Mytilus edulis. Nature 237, 459.
Bayne, B.L., Holland, D.L., Moore, M.N., Lowe, D.L., Widdows, J., 1978. Further studies on the effects of stress in the adult on the eggs of Mytilus edulis. J. Mar. Biol. Assoc. U. K. 58, 825–841.
Berntsson, K.M., Jonsson, P.R., Wangberg, S.A., Carlsson, A.S., 1997. Effects of broodstock diets on fatty acid composition, survival and growth rates in larvae of the European flat oyster, Ostrea edulis. Aquaculture 154, 139–153.
Bligh, E.G., Dyer, W.J., 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917.
Brown, M.R., Barrett, S.M., Volkman, J.K., Nearhos, S.P., Nell, J.A., Allan, G.L., 1996. Biochemical composition of new yeasts and bacteria evaluated as food for bivalve aquaculture. Aquaculture 143, 341–360.
Defossez, J.M., Hawkins, A.J.S., 1997. Selective feeding in shellfish: size-dependent rejection of large particles within pseudofaeces from Mytilus edulis, Ruditapes philippinarum and Tapes decussatus. Mar. Biol. 129, 139–147.
Del Rio-Portilla, M.A., 1996. Genetic aspects of Mytilus edulis in relation to spawning and hatchery culture. PhD Thesis, University of Wales, Bangor.
De Pauw, N., Morales, J., Persoone, G., 1984. Mass cultures of microalgae in aquaculture systems: progress and constraints. Hydrobiologia 116r117, 121–134.
Dickey-Collas, M., Geffen, A.J., 1992. Importance of the fatty acids 20:5v3 and 22:6v3 in the diet of plaice
ŽPleuronectes platessa larvae. Mar. Biol. 113, 463–468..
Douillet, P., 1987. Effect of bacteria on the nutrition of the brine shrimp Artemia fed on dried diets. In:
Ž .
Sorgeloos, P., Benston, D.A., Decleir, W., Jasper, E. Eds. , Artemia Research and its Applications. Ecology, Culturing, Use in Aquaculture, Vol. 3. Universa Press, Weteren, Belgium, pp. 295–308. Douillet, P.A., Langdon, C.J., 1993. Effects of marine bacteria on the culture of axenic oyster Crassostrea
gigas Thunberg. Biol. Bull. 184, 36–51.
Douillet, P.A., Langdon, C.J., 1994. Use of a probiotic for the culture of larvae of the Pacific oyster
ŽCrassostrea gigas Thunberg . Aquaculture 119, 25–40..
Enright, C.T., Newkirk, G.F., Craigie, J.S., Castell, J.D., 1986a. Evaluation of phytoplankton as diets for juvenile Ostrea edulis L. J. Exp. Mar. Biol. Ecol. 96, 1–13.
Enright, C.T., Newkirk, G.F., Craigie, J.S., Castell, J.D., 1986b. Growth of juvenile Ostrea edulis L. fed Chaetoceros gracilis Schutt of varied chemical composition. J. Exp. Mar. Biol. Ecol. 96, 15–26. Fraser, A.J., 1989. Triacylglycerol content as a condition index for fish, bivalve and crustacean larvae. Can. J.
Fish. Aquat. Sci. 46, 1868–1873.
Frolov, A.V., Pankov, S.L., 1992. The reproduction strategy of oyster Ostrea edulis L. from the biochemical point of view. Comp. Biochem. Physiol. 103B, 161–182.
Gallager, S.M., Mann, R., 1986. Growth and survival of larvae Mercenaria mercenaria L. and Crassostrea
Õirginica Gmelin relative to broodstock conditioning and lipid content of eggs. Aquaculture 56, 105–121.
Gallager, S.M., Mann, R., Sasaki, G.C., 1986. Lipid as an index of growth and viability of three species of bivalve larvae. Aquaculture 56, 81–103.
Helm, M.M., Laing, I., 1987. Preliminary observations on the nutritional value of ‘‘Tahiti Isochrysis’’ to bivalve larvae. Aquaculture 62, 281–288.
Helm, M.M., Holland, D.L., Stephenson, R.R., 1973. The effect of supplementary algal feeding of a hatchery breeding stock of Ostrea edulis L. on larval vigour. J. Mar. Biol. Assoc. U. K. 53, 673–684.
Helm, M.M., Holland, D.L., Utting, S.D., East, J., 1991. Fatty acid composition of early non-feeding larvae of the European flat oyster Ostrea edulis. J. Mar. Biol. Assoc. U. K. 71, 675–691.
Intriago, P., Jones, D.A., 1993. Bacteria as food for Artemia. Aquaculture 113, 115–127. Jørgensen, C.B., 1996. Bivalve filter feeding revisited. Mar. Ecol.: Prog. Ser. 142, 287–302.
Joseph, J.D., 1982. Lipid composition of marine estuarine invertebrates: Part II. Mollusca. Prog. Lipid Res. 21, 109–153.
Kanazawa, A., 1985. Nutrition of penaeid prawns and shrimps. In: Taki, Y., Llobrera, J.L., Primavera, J.H.
ŽEds. , Proc. 1st Int. Conf. Cult. Penaeid PrawnsrShrimps. South East Asian Fisheries Development.
Center, Iloilo, Philippines, pp. 123–130.
Kraeuter, J.N., Castagna, M., Van Dessel, R.R., 1982. Egg size and larval survival of Mercenaria mercenaria L. and Agropecten irradians Lamarck. J. Exp. Mar. Biol. Ecol. 56, 3–8.
Langdon, C.J., Waldock, M.J., 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. U. K. 61, 431–448.
Lannan, J.E., Robinson, A., Breeze, W.P., 1980. Broodstock management of Crassostrea gigas: II. Brood-stock conditioning to maximize larval survival. Aquaculture 21, 337–345.
Leonardos, N., Lucas, I.A.N., 2000a. Effects of environmental parameters in the growth and biochemical composition, with emphasis in fatty acid content, of four microalgae. J. Appl. Phycol., submitted. Leonardos, N., Lucas, I.A.N., 2000b. The nutritional value of algae grown under different culture conditions,
for Mytilus edulis L. larvae. Aquaculture, in press.
Levine, D.M., Sulkin, S.D., 1984. Nutritional significance of long-chain polyunsaturated fatty acids to the
Ž .
zoeal development of the brachyuran crab, Euryanopeus depressus Smith . J. Exp. Mar. Biol. Ecol. 81, 211–223.
Lewis, T.E., Garland, C.D., O’Brien, T.D., Fraser, M.I., Tong, P.A., Ward, C., Dix, T.G., McMeekin, T.A., 1988. The use of 0.2mm membrane-filtered seawater for improved control of bacteria levels in microalgal
Ž .
cultures fed to larval Pacific oysters Crassostrea gigas . Aquaculture 69, 241–251. Loosanoff, V.L., Davis, H.C., 1963. Rearing of bivalve molluscs. Adv. Mar. Biol. 1, 1–136.
Metcalfe, L.D., Schimtz, A.A., Pelka, J.R., 1966. Rapid preparation of fatty acid esters from lipids for gas chromatography. Anal. Chem. 38, 514–515.
Sokal, R.R., Rohlf, F.J., 1987. Introduction to Biostatistics, 2nd edn. W.H. Freeman and Co., New York. Thompson, P.A., Guo, M.-X., Harrison, P.J., 1993. The influence of irradiance on the biochemical
composi-Ž
tion of three phytoplankton species and their nutritional value for larvae of the Pacific oyster Crassostrea
.
gigas . Mar. Biol. 117, 259–268.
Thompson, P.A., Guo, M.-X., Harrison, P.J., 1994. Influence of irradiance on the nutritional value of two
Ž .
phytoplankton species fed to larval Japanese scallops Patinopecten yessoensis . Mar. Biol. 119, 89–97. Thompson, P.A., Guo, M.-X., Harrison, P.J., 1996. Nutritional value of diets that vary in fatty acid
Ž .
composition for larval Pacific oysters Crassostrea gigas . Aquaculture 143, 379–391.
Volkman, J.K., Jeffrey, S.W., Nichols, P.D., Rogers, G.I., Garland, C.D., 1989. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 128, 219–240. Waldock, M.J., Holland, D.L., 1984. Fatty acid metabolism in young oysters, Crassostrea gigas:
polyunsatu-rated fatty acids. Lipids 19, 332–336.
Watanabe, T., Kitajima, C., Fujita, S., 1983. Nutritional values of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34, 115–134.
Webb, K.L., Chu, F.L.E., 1983. Phytoplankton as a food source for bivalve larvae. In: Pruder, G.D., Langdon,
Ž .
C., Conklin, D. Eds. , Proceedings of the 2nd International Conference on Aquaculture Nutrition. Biochemical and Physiological Approaches to Shellfish Nutrition. World Mariculture Society Special Publication 2, pp. 272–291.
Whyte, J.N.C., Bourne, N., Hodgson, C.A., 1989. Influence of algal diets on biochemical composition and
Ž .
energy reserves in Patinopecten yessoensis Jay larvae. Aquaculture 78, 333–347.
Whyte, J.N.C., Bourne, N., Hodgson, C.A., 1990. Nutritional condition of the rock scallop, Crassadoma gigantea Grey, larvae fed mixed algal diets. Aquaculture 86, 25–40.
Wilson, J.A., Chaparro, O.R., Thompson, R.J., 1996. The importance of broodstock nutrition on the viability of larvae and spat in the Chilean oyster Ostrea chilensis. Aquaculture 139, 63–75.
Ž
Yasuda, L., Taga, N., 1980. A mass culture method for Artemia salina using bacteria as food. La Mer Bull.
.