www.elsevier.nlrlocateraqua-online
Influence of dietary recombinant microbial lipase
on performance and quality characteristics of
rainbow trout, Oncorhynchus mykiss
Troels Samuelsen
a, Mai Isaksen
b, Ewen McLean
a,)a
Aalborg UniÕersity, Aquaculture Section, SohngaardsholmsÕej 57, DK-9000 Aalborg, Denmark
b
Danisco Cultor, Edwin Rahrs Vej 38, DK-8220 Brabrand, Denmark
Received 26 April 2000; received in revised form 11 September 2000; accepted 11 September 2000
Abstract
In order to assess whether supplementary lipase affected growth and body composition of trout,
Ž . Ž y1. Ž . Ž
four diets were produced, consisting of A feed containing high 2083 mg kg , B low 208.3
y1. Ž . Ž . Ž y1. Ž .
mg kg concentrations of lipase, C heat-treated inactivated lipase 2083 mg kg , and D a
Ž
basal control diet. Rainbow trout ns40rtank; initial wt. 23.22"4.81 g; length 124.7"6.35
.
mm were fed, according to commercial feed tables, 6 daysrweek for 202 days. Retained activity
Ž .
of supplemental lipase was verified by monitoring free fatty acid appearance FAA , which was
Ž . Ž .
significantly higher P-0.05 in treated feed and oil. Lipase addition had no effect P)0.05 on growth, fillet proximate composition, hepatosomatic, cardiac, or gut indices, and carcass percent-age. However, lipase supplementation influenced the mono-unsaturated fatty acid profiles of the
Ž .
fillet P-0.05 .q2001 Elsevier Science B.V. All rights reserved.
Keywords: Diet; Fillet composition; Lipase; Trout; Quality
1. Introduction
Ž .
Contemporary diets, augmented with hydrolytic exo enzymes assist digestion and absorption of low-grade ingredients, permit earlier weaning, and enhance growth and
)Corresponding author. Present address: Department of Fisheries Science and Technology, College of Agriculture, Sultan Qaboos University, P.O. Box 34, Al-Khod 123, Sultanate of Oman. Tel.:q968-51-51-91; fax:q968-51-34-18.
Ž .
E-mail address: [email protected] E. McLean .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved.
Ž .
Ž . Ž .
feed conversion ratios FCR Conneely, 1992; Lyons, 1992; Ritz et al., 1995 . Addition
Ž .
of phytase to monogastric feeds has led to improvements in FCR and phosphorus P
Ž
utilization and, hence, to a reduction in environmental pollution Cantor and Perney,
.
1992; Walsh et al., 1993 . Since similar production and environmental concerns are associated with aquaculture, a natural progression in the field of dietary enzyme supplementation will likely follow with aquafeeds. A number of studies have already been undertaken in this field. Two areas of particular interest to aquaculture include the use of exoenzymes in larval feeds to assist weaning, and addition of phytase to plant protein-based grower diets in efforts to enhance P availability and reduce environmental loading.
Several other enzymes have been used experimentally as additives in aquafeeds.
Ž .
Although without success, Rodger et al. 1995 incorporated mammalian pancreatic
Ž .
enzymes 4 grkg into Atlantic salmon diets in an attempt to treat fish for pancreas
Ž
disease. Addition of a-amylase to salmon diets did not improve performance Carter et
.
al., 1992 , but mixed proteasercarbohydrase preparations, when added to a soybean
Ž .
meal-based diet, increased appetite and growth and enhanced FCR Carter et al., 1994 . Enzymatic pre-treatment of feeds has also been evaluated as a strategy for enhancing FCR and growth. Thus, exposure of soybean residue to papain was reported to improve
Ž .
growth of common carp Wong et al., 1996 , while increased growth and whole body protein levels were recorded in fingerlings fed diets treated with a mixture of enzymes
Žamylase, protease, b-glucanase, b-glucosidase and cellulase: 1.5 grkg; Bogut et al.,
.
1994 .
Acyl hydrolases or lipases are present in various fish tissues, including the gut
ŽSmith, 1989 . In rainbow trout, lipases express fatty acid specificity, hydrolysing. Ž
triacylglycerols containing C18:1 ny9 in preference to C16:0 Henderson and Tocher,
.
1987 . In fish, no correlation or adaptive response to lipase activity appears to exist with
Ž .
feed lipid content or increased lipid intake Kuz’mina and Gelman, 1997 . This latter observation is of relevance since lipid levels in currently available aquafeeds, especially those for salmonids, have increased dramatically over the last five years. Commonly, lipids represent 30% of salmonid diets, with approximately 80% in the form of
Ž .
triglycerides, with 1–2% being free fatty acids Rasmussen et al., 2000 . Even though these so-called ‘high energy’ aquafeeds are associated with deterioration in flesh quality
ŽAndersen et al., 1997; Bell et al., 1998; Eckhoff et al., 1998 their use will likely.
increase due to the benefits they provide, including enhanced FCR and decreased P
Ž .
loading Alsted, 1991 . Methods that might neutralize the negative aspects of high-en-ergy feeds, or enhance their utility are, thus, deserved of attention. Only one study has
Ž .
2. Materials and methods
2.1. Animals and husbandry
Ž .
Rainbow trout, Onchorhynchus mykiss 23.22"4.81 g; 124.7"6.35 mm , were
2 Ž .
randomly distributed into eight 600-l, 1-m surface area fibreglass tanks ns40rtank . Tanks were supplied with aerated ground water at a flow rate of 3 l miny1. Water temperature and oxygen levels were monitored continuously throughout using Oxygaurdw and MqS datalogging systems. Over the period of study, water temperature ranged between 6.08C and 11.28C. Oxygen concentrations at the water outlet were maintained above 7.0 mg O ly1. Photoperiod was controlled to provide a 12:12 h dark–light cycle.
2
Prior to trial start, animals were acclimated to experimental conditions for 10 days and
Ž .
individually identified using passive integrated transponders Fish Eagle, UK . Fish were fed according to commercial feeding tables 6 daysrweek, using a band feeder system
ŽBioMar, Brande, Denmark ..
2.2. Diet manufacture
Ž . Ž y1.
Four diets were produced, consisting of A feed containing high 2083 mg kg ,
Ž .B low 208.3 mg kgŽ y1. concentrations of lipase GrindamylŽ e Exel 16, Danisco
. Ž . Ž . Ž y1.
Cultor, Brabrand, Denmark , C heat-treated inactivated lipase 2083 mg kg , and
Ž .D the basal control diet. Heat treatment of lipase diet C was by autoclave for 30 minŽ .
at 1208C followed by oven heating at 1208C for 1 h. Feeds were formulated in accordance to commercial standards for high-energy diets, to contain 25% lipid. A non-extruded 3-mm pellet containing 8.29% lipid was used for the preparation of feeds.
Ž .
These pellets were extruded using an experimental extruder BioMar and coated with
Ž .
heated 30–358C fish oil under vacuum. For lipase-supplemented diets, a fungal lipase complex, originating from Thermomyces, but produced by fermentation in Aspergillus
Ž .
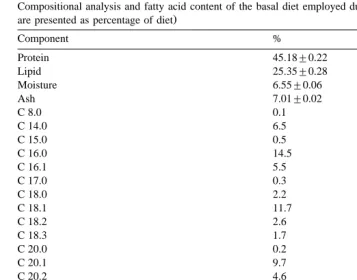
oryzae, was employed. The lipase was in powder form and comprised protein lipase and a starch carrier. The appropriate amount of enzyme for each feed type was mixed in fish oil by gentle stirring over 10 min and then added to feeds as described above. Dietary proximate composition and FFA profile is presented in Table 1.
2.3. Analytical procedures
2.3.1. Growth performance
Individual weight and length measurements were taken for all animals at monthly intervals. Prior to handling, animals were fasted for 2 days. Animals were anaesthetised
Ž0.0002% benzocaine; Sigma during manipulations. FCR was calculated using the.
formula:
amount of feed ingested g
Ž .
FCRs .
Table 1
Ž
Compositional analysis and fatty acid content of the basal diet employed during the present study all values
.
are presented as percentage of diet
Component %
Condition factor CF and weight and length specific growth rates SGR%rday were
Ž . Ž .
computed as described in McLean et al. 1997 and Bassompierre et al. 1998 ,
Ž
respectively. Somatic indices were recorded for liver, heart and gut ns35 per
. Ž .
treatment , using the methods described in Christensen and McLean 1998 , while fillet yield and carcass percentage were evaluated according to the methods in Ostenfeld et al.
Ž1995 ..
2.3.2. Fatty acid analysis
Fatty acid composition of feeds and fillets were determined using extracted oil
Ž .
samples using a modified Bligh and Dyer 1959 method. Oil samples were
Ž . Ž .
esterifiedrinterestified with methanol anhydrous by means of an acid catalyst HCl . The resultant methylesters were extracted with MTBE and determined by means of
Ž .
gas–liquid chromatography Perkin-Elmer 8320 Capillary Gas Chromatograph . The carrier gas used was helium, and the column used was a Cabowax 20. The response factors for each component relative to C-18 fatty acid methyl ester was calculated from
Ž .
2.3.3. Proximate composition
Compositional analyses were undertaken on fillets from eight animals per treatment; ns4 per duplicate tank per treatment. Dry matter and moisture fractions were deter-mined by heating homogenized samples at 1058C for 24 h. The homogenized sample’s inflammable components were removed by incineration at 5508C for 22–24 h for ash
Ž .
determination. Calculation of protein Kjeldahl-N was accomplished according to
Ž .
AOAC 1984 . Lipid presence was quantified using the method described by Bligh and
Ž .
Dyer 1959 .
2.3.4. Quantification of lipase actiÕity
Lipase activity, following addition to fish oil and feed was quantified indirectly by following the appearance of free fatty acids. Feed samples were examined at 0, 60, 120 and 240 min post-lipase addition whereas for oil, control and high lipase-treated samples
Ž
were examined at 0 and 240 min post-enzyme addition. Enzymatic reversibility i.e., the
.
possibility of fatty acids reverting back to triglycerides was evaluated 24 h after enzyme addition. The lipase reaction was stopped with the addition of 37% HCl. Titrations were
Ž .
performed after lipid extraction Bligh and Dyer, 1959 . Lipase activity was examined for all experimental feed types.
2.4. Experimental model and tests
Each treatment was undertaken in duplicate with tanks being randomly assigned. Since each tank was given one treatment, tanks were nested within treatments. The experiment was performed as a two-factor nested design, where the factor treatment was considered as a fixed factor and fish tanks as a random factor. The two-factor nested design model applied to the ANOVA was:
yijksmqAiqT A j i
Ž . Ž .
q´Ž .
ij k ,Ž .
where A was the treatment, T A the tank effect with fixed treatment, and ´ the residual. By performing an analysis of variance upon recorded data, the effects of the
Ž .
treatment and tank were estimated as a probability value P . Data from the evaluation of lipase activity were examined through a two-factor model:
yijsmqAiqTjq
Ž
AT ij.
q´ij.Factors were treatment and time. An analysis of variance was performed upon recorded data. The effects of the treatment and time were estimated as a probability
Ž .
value P . The data from investigation of FFA in the fillet were investigated using a one-factor model:
yijsmqAiq´ij
Ž .
with the factor being a combination of treatment and time i.e., A end . An analysis of variance was performed upon recorded data. The effects of the combined treatmentrtime
Ž .
factor were estimated as a probability value P . All statistical analyses employed
Ž .
SigmaStat software packages Jandel Scientific and results are presented as "95%
Ž .
3. Results
3.1. Lipase actiÕity
Retention of enzymatic activity in feed oils was examined 240 min post-addition of lipase. At time 0, percent FFA presence in the oil was 5.32"0.74%. Similar values
Ž5.26"1.52; P)0.05 were recorded for control samples 240 min later, but high lipase.
Ž .
supplementation increased percent FFA presence significantly P-0.05; 6.90"1.49 . Following a reaction period of 1440 min, lipase addition resulted in a 29% increase in
Ž . Ž
FFA P-0.05 when compared to control oil 5.97"1.46% vs. 4.64"0.64%,
respec-. Ž .
tively . Differences P-0.05 in FFA presence were also discernible between the high
Ž . Ž .
lipase supplemented 6.63"0.47% and control 6.05"0.46% and lipase inactivated
Ž6.07"0.46% feeds 60 min post-enzyme addition. Each feed type returned identical.
FFA profiles.
3.2. Growth and fillet composition
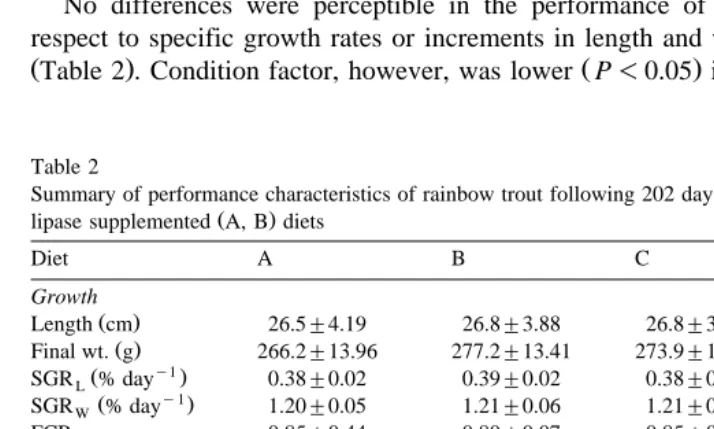
No differences were perceptible in the performance of the different groups with respect to specific growth rates or increments in length and weight throughout the trial
ŽTable 2 . Condition factor, however, was lower P. Ž -0.05 in fish fed the control diet..
Table 2
Ž .
Summary of performance characteristics of rainbow trout following 202 days feeding with control C, D and
Ž .
Final wt. g 266.2"13.96 277.2"13.41 273.9"13.38 277.2"13.60
y1
Condition factor 1.41"0.02 1.42"0.03 1.40"0.02 1.37"0.03
Mass
Ž .
Fillet yield % 53.37"1.75 53.15"1.03 52.72"1.47 53.85"1.45
Ž .
Carcass % 40.80"1.99 41.19"1.03 41.17"1.47 40.62"1.45 Cardiac index 0.15"0.007 0.15"0.009 0.16"0.008 0.15"0.007
1 a a b a
HSI 1.31"0.04 1.28"0.05 1.45"0.06 1.29"0.05
a,b b a a,b
Gut index 9.34"0.20 8.94"0.27 9.44"0.32 9.13"0.26
Fillet composition
Moisture 72.95"0.77 73.05"0.93 72.77"0.90 73.37"0.67 Lipid 7.68"0.84 7.28"0.82 7.31"0.47 7.68"0.57 Protein 18.33"0.16 18.40"0.16 18.48"0.19 18.35"0.27 Ash 1.33"0.04 1.36"0.05 1.34"0.05 1.34"0.04
Ž .
Compositional analyses were performed on fillets ns8 per treatment . All other data sets represent means of
Ž .
35 animals per treatment"95% confidence limits Walpole and Myers, 1993 . Different superscripts indicate
Ž .
significant differences row-wise P-0.05 .
1
Identical yields were obtained for fillet percentage and carcass. Differences were seen for hepatosomatic index with fish given the inactivated lipase diets returning relatively
Ž .
larger livers P-0.05 than all other groups. The same fish also contrasted with respect
Ž .
to their gut index, which was higher P-0.05 when compared against trout fed low
Ž .
lipase diets Table 2 . No differences were recorded in FCRs between groups throughout
Ž .
the trial P)0.05; Table 2
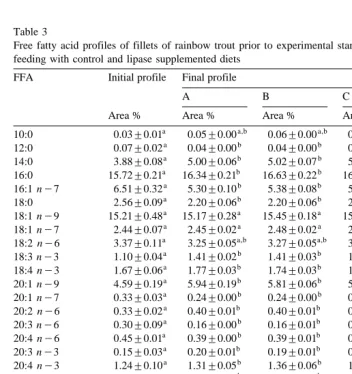
Table 3
Ž .
Free fatty acid profiles of fillets of rainbow trout prior to experimental start day 0 and following 202 days feeding with control and lipase supplemented diets
FFA Initial profile Final profile
16:0 15.72"0.21 16.34"0.21 16.63"0.22 16.61"0.24 16.28"0.17
a b b b b
16:1 ny7 6.51"0.32 5.30"0.10 5.38"0.08 5.36"0.12 5.37"0.11
a b b b a,b
18:0 2.56"0.09 2.20"0.06 2.20"0.06 2.25"0.08 2.28"0.07
a a a a a
18:1 ny9 15.21"0.48 15.17"0.28 15.45"0.18 15.29"0.22 15.40"0.28
a a a a a
22:1n11q13 5.81"0.25 7.87"0.18 7.72"0.14 7.73"0.20 7.67"0.12
a a,b a,b b a,b
22:6 ny3 17.59"0.86 15.48"0.46 15.10"0.31 15.22"0.23 15.66"0.24
a b b b b
Saturated 22.27"0.25 23.63"0.27 23.94"0.30 24.07"0.33 23.57"0.16
a b b b a
Mono-unsaturated 35.85"0.87 37.62"0.50 37.73"0.21 37.62"0.29 37.43"0.32
a b b b b
PUFA 33.59"0.88 30.43"0.57 29.99"0.47 29.96"0.34 30.67"0.35
a b b b b
Different superscripts indicate significant differences row-wise P-0.05 . Data are presented as "95%
Ž .
Proximate analysis revealed similar values across treatments for all parameters
Ž . Ž .
examined P)0.05; Table 2 . Significant P-0.05 differences in fillet FFA profiles
Ž .
were apparent between trial start and termination Table 3 . The overall trend in the total amount of saturated and monounsaturated fatty acids present in the fillets were towards increased levels, irrespective of diet fed. In contrast, the trend for fillet polyunsaturated
Ž . Ž .
fatty acids PUFA was towards significant P-0.05 declines, with a concomitant
Ž . Ž .
decline P-0.05 in the PUFA to saturated fatty acid ratio Table 3 .
4. Discussion
The present study sought to determine whether supplementation of trout feed with a commercially available recombinant microbial lipase, enhanced digestibility andror
Ž .
altered fillet free fatty acid FFA profiles of treated fish. An important aspect of the study, therefore, was to establish that added lipase remained active. Examining the appearance of FFA in fish oil and feeds treated with the enzyme authenticated this. The 29% increase in oil FFA provided confidence in the retained bioactivity of the lipase, a fact further confirmed by the increased presence of FFA in enzyme-treated feeds. Moreover, since enzyme activity was measurable, even following 24-h incubation in fish oil, the stability of the preparation was also ascertained. Nevertheless, the assay employed must be considered as an indirect indication of the enzyme’s potency, since lipase itself was not directly quantified.
Lipases have been isolated from the teleost stomach, pyloric caeca, liver, intestine,
Ž . Ž .
pancreas and hepatopancreas. Thus, Babkin and Bowie 1928 and Mackay 1929 provided an early demonstration of lipase presence in the intestinal mucosa of teleosts,
Ž .
while more recent work e.g., Ribeiro et al., 1999 has mapped the development of lipase along the gut of other species. Extracts of caecal contents from rainbow trout have been shown to retain triacylglycerol lipase activity with pH optima of 8 at 308C, with
Ž
significantly increased activity in the presence of bile salts Henderson and Tocher,
.
1987 . Like the trout enzyme, the microbial lipase employed in the present experiment expressed pH optima of 8.0 at 308C and maintained activity at pH 6.5 at 88C. Thus, the acid environment of the fish stomach might be expected to inhibit lipase activity. This likelihood, although not tested directly during the current trial and potentially limiting, would not impact the usefulness of dietary lipase supplementation since it is evident that the enzyme was active in the feed. Moreover, if supplemental lipase were able to avoid gastric phase inhibition, then it is possible that proteases present in the digestive tract would degrade or further restrain the action of the enzyme.
In vertebrates, lipids, and especially dietary triacylglycerols, are absorbed by the intestinal and, in fish, caecal epithelia, probably in the form of fatty acids, but mostly as
Ž .
mono-, and diglycerides or as droplets Sargent et al., 1989 . Recent studies have
Ž
demonstrated differential absorption of FFA across isolated enterocytes Perez et al.,
.
composition. The response of treatment groups to feeds incorporating lipase however, was identical to control groups. Thus, growth rates, in terms of weight and length gain, were unaffected. Nevertheless, the overall performance of all groups was comparable to
Ž
other growth studies with the same species under similar conditions of husbandry e.g.,
.
Gregory and Wood, 1998 .
Carcass percentage and fillet proximate composition remained unaltered by dietary lipase supplementation. Nonetheless, a more detailed examination of FFA profiles indicated that treatment impacted the FFA profile of the fillet; specifically the profile of monounsaturated FFAs. However, whether this would impact the perceived quality of the product requires further investigation. It is noteworthy that there were only slight differences in fatty acid composition of the edible tissue when compared with the fatty acid composition of the diets. Similar findings have been reported by Luzzana et al.
Ž1994 . The results relating to changes in FFA profiles from initial to end values serve to.
confirm that the development in fish fillet FFA profile is manipulable by, and adjusts to, that of the free fatty acid profile in the feed. The addition of lipase to aquafeeds,
Ž .
therefore, might be more suited to diets that contain poorer quality i.e., non-marine oils. Such could be the case where vegetable oils are employed on economic or availability grounds. The only other study to have employed dietary addition of lipase,
Ž .
that of Koven et al. 1993 , was performed on gilthead seabream larvae, and focused upon oleic acid incorporation into tissue lipids. In that study, a 3.42-times increase in incorporation was observed in 45-day-old larvae lipid tissues, suggesting that lipase addition may be beneficial at least for larval stages of fish. Supplementary or exo-lipase nevertheless clearly was without effect in rainbow trout. It is interesting to note that
Ž .
Kuz’mina and Gelman 1997 reported that an increase in fat content in fish food would not result in an increased lipase activity. These results thereby suggest that fish may have problems adapting to higher levels of fat than naturally encountered. Moreover, high levels of dietary lipid may depress the digestibility of other nutrients, such as amino acids, by restricting the action of digestive enzymes, an effect that may be reduced with lipase supplementation.
Future studies should be undertaken to determine whether supplementary dietary enzymes retain activity in the gut and if the agastric condition may effect their action. It would also be of interest to determine the lipid content and composition of digesta from the stomach, foregut, hindgut and rectum of trout fed commercial diets supplemented with lipase. This might provide information with which to examine the process of lipid digestion in this species and indicate whether a higher concentration of lipase would return a more favorable effect. It is also possible that the well-developed digestive system of rainbow trout makes it an unsuitable animal for dietary lipase supplementa-tion.
References
Alsted, N.S., 1991. Studies on the reduction of discharges from fish farms by modification of the diet. In:
Ž .
Andersen, U.B., Thomassen, M.S., Bencze Røra, A.M., 1997. Texture properties of farmed rainbow trout˚ ŽOncorhynchus mykiss : effects of diet, muscle fat content and time of storage on ice. J. Sci. Food Agric..
74, 347–353.
AOAC, 1984. Official Methods of Analysis of the Association of Official Analytic Chemists. 14th edn. Association of Official Analytic Chemists, Washington, DC.
Ž
Babkin, B.P., Bowie, D.J., 1928. The digestive system and its function in Fundulus heteroclitus North
.
American Killifish . Biol. Bull. 54, 254–278.
Bassompierre, M., Ostenfeld, T.H., McLean, E., Torrissen, K., 1998. Growth and in vitro protein digestion of Atlantic salmon with genetically different trypsin isozymes. Aquacult. Int. 6, 47–56.
Bell, J.G., McEvoy, J., Sargent, J.R., 1998. Flesh lipid and carotenoid composition of Scottish farmed Atlantic
Ž .
salmon Salmo salar . J. Agric. Food Chem. 46, 119–127.
Bligh, E.G., Dyer, W.J., 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917.
Bogut, I., Opacak, A., Stevic, I., Bogdanic, C., 1994. Influence of polyzymes added to the food on the growth
Ž .
of carp fingerlings Cyprinus carpio . Ribarstvo 52, 65–74.
Cantor, A.H., Perney, K.M., 1992. Phytase: can phytase reduce the expense and environmental threat of excess phosphorus in animal feeds? Experience with poultry diets and implications for the future. In: Lyons, T.P.
ŽEd. , Biotechnology in the Feed Industry. Alltech Technical Publications, KY, USA, pp. 293–301.. Ž
Carter, C.G., Houlihan, D.F., McCarthy, I.D., 1992. Feed utilization efficiencies of Atlantic salmon Salmo
.
salar L. parr: effect of a single supplementary enzyme. Comp. Biochem. Physiol. 101A, 369–374.
Carter, C.G., Houlihan, D.F., Buchanan, B., Mitchell, A.I., 1994. Growth and feed utilization efficiencies of seawater Atlantic salmon, Salmo salar L., fed a diet containing supplementary enzymes. Aquacult. Fish. Manage. 25, 37–46.
Ž
Christensen, S.M., McLean, E., 1998. Compensatory growth in Mozambique tilapia Oreochromis
mossambi-.
cus , fed a sub-optimal diet. Ribarstvo 56, 3–19.
Conneely, O.M., 1992. From DNA to feed conversion: using biotechnology to improve enzyme yields and
Ž .
livestock performance. In: Lyons, T.P. Ed. , Biotechnology in the Feed Industry. Alltech Technical Publications, KY, USA, pp. 57–66.
Ž
Eckhoff, K.M., Aidos, I., Hemre, G.-I., Lie, O., 1998. Collagen content in farmed Atlantic salmon Salmo
.
salar, L. and subsequent changes in solubility during storage on ice. Food Chem. 62, 197–200.
Gregory, T.R., Wood, C.M., 1998. Individual variation and interrelationships between swimming performance,
Ž .
growth rate, and feeding in juvenile rainbow trout Oncorhynchus mykiss . Can. J. Fish. Aquat. Sci. 55, 1583–1590.
Henderson, R.J., Tocher, D.R., 1987. The lipid composition and biochemistry of freshwater fish. Lipid Res. 26, 281–347.
Koven, W.M., Kolkovski, S., Tandler, A., Kissil, G.W., Sklan, D., 1993. The effect of dietary lecithin and lipase, as a function of age, on ny9 fatty acid incorporation in the tissue lipids of Sparus aurata larvae. Fish Physiol. Biochem. 10, 357–364.
Kuz’mina, V.V., Gelman, A.G., 1997. Membrane-linked digestion in fish. Rev. Fish. Sci. 5, 99–129. Luzzana, U., Serrini, G., Moretti, V.M., Gianesini, C., Valfre, F., 1994. Effect of expanded feed with high fish
oil content on growth and fatty acid composition of rainbow trout. Aquacult. Int. 2, 239–248.
Ž .
Lyons, T.P., 1992. Strategy for the future: the role of biotechnology in the feed industry. In: Lyons, T.P. Ed. , Biotechnology in the Feed Industry. Alltech Technical Publications, KY, USA, pp. 1–22.
Ž .
MacKay, M.E., 1929. The digestive system of the eel-pout Zoarces onguitharis . Biol. Bull. 56, 8–23. McLean, E., Devlin, R.H., Byatt, J.C., Clarke, W.C., Donaldson, E.M., 1997. Evaluation of a controlled
release formulation of recombinant bovine growth hormone on growth and seawater adaptation in coho
ŽOncorhynchus kisutch and chinook O. tshawytscha salmon. Aquaculture 156, 113–128.. Ž .
Ostenfeld, T., Thomsen, S., Ingolfsdottir, S., Ronsholdt, B., McLean, E., 1995. Evaluation of the effect of live
Ž .
haulage on metabolites and fillet texture of rainbow trout Oncorhynchus mykiss . Water Sci. Technol. 31, 233–237.
Perez, J.A., Rodriguez, C., Henderson, R.J., 1999. The uptake and esterification of radiolabelled fatty acids by
Ž .
enterocytes isolated from rainbow trout Oncorhynchus mykiss . Fish Physiol. Biochem. 20, 125–134. Rasmussen, R.S., Ostenfeld, T.H., Ronsholdt, B., McLean, E., 2000. Manipulation of end product quality in
Ribeiro, L., Sarasquete, C., Dinis, M.T., 1999. Histological and histochemical development of the digestive
Ž .
system of Solea senegalensis Kaup, 1858 larvae. Aquaculture 171, 293–308.
Ritz, C.W., Hulet, R.M., Self, B.B., Denbow, D.M., 1995. Growth and intestinal morphology of male turkeys as influenced by dietary supplementation of amylase and xylanase. Poult. Sci. 74, 1329–1334.
Rodger, H.D., Murphy, K., Drinan, E.M., Kennedy, G., 1995. Apparent lack of response of salmon affected by pancreas disease to pancreatic enzyme replacement therapy. Vet. Rec. 136, 489–491.
Ž .
Sargent, J., Henderson, R.J., Tocher, D.R., 1989. The lipids. In: Halver, J.E. Ed. , Fish Nutrition. Academic Press, Inc., San Diego, pp. 153–218.
Ž .
Smith, L.S., 1989. Digestive functions in teleost fishes. In: Halver, J.E. Ed. , Fish Nutrition. 2nd edn. Academic Press, San Diego, CA, pp. 331–421.
Walpole, R.E., Myers, R.H., 1993. Probability and Statistics for Engineers and Scientists. 5th edn. Prentice-Hall, Englewood Cliffs, NJ 07632.
Walsh, G.A., Power, R.F., Headon, D.R., 1993. Enzymes in the animal-feed industry. TIBTECH 11, 424–430. Wong, M.H., Tang, L.Y., Kwok, F.S., 1996. The use of enzyme-digested soybean residue for feeding common