www.elsevier.nlrlocateraqua-online
Minimum inhibitory concentrations of
chloramphenicol, florfenicol,
trimethoprim
r
sulfadiazine and flumequine in
seawater of bacteria associated with scallops
ž
Pecten maximus larvae
/
Lise Torkildsen
a,), Ole B. Samuelsen
a, Bjørn T. Lunestad
b,
Øivind Bergh
aa
Institute of Marine Research, Department of Aquaculture, P.O. Box 1870 Nordnes, N-5817 Bergen, Norway
b
Directorate of Fisheries, P.O. Box 185, N-5804 Bergen, Norway
Accepted 14 October 1999
Abstract
Ž .
The purpose of this study was to find the minimum inhibitory concentrations MIC for the following antibacterial agents: chloramphenicol, florfenicol, flumequine and the combination
Ž .
trimethoprimrsulfadiazine to bacteria associated with scallop Pecten maximus larvae. To evaluate possible effects of components in seawater to the antimicrobial activity of these agents, MIC values were established on Mueller Hinton agar dissolved in either distilled water added 2% NaCl or 25‰ seawater. For flumequine and trimethoprimrsulfadiazine, the MIC values increased significantly using 25‰ seawater compared to 2% NaCl. Chloramphenicol and florfenicol did not show any significant increase in MIC values using 25‰ seawater compared to 2% NaCl. A significant increase in MIC values was found for chloramphenicol in the second egg group, using 25‰ seawater compared to 2% NaCl. It is concluded that flumequine, trimethoprimrsulfadiazine and chloramphenicol are, to a varying degree, antagonised by components in seawater.q2000
Elsevier Science B.V. All rights reserved.
Keywords: Scallop larvae; MIC-test; Antibacterial agents antagonised by seawater
)Corresponding author. Tel.:q47-55-23-63-61; fax:q47-55-23-63-79.
Ž .
E-mail address: [email protected] L. Torkildsen .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
Ž .
Aquaculture of scallop Pecten maximus larvae is often associated with high
Ž .
mortality, suspected to have a bacterial etiology Nicolas et al., 1996 . Although only
Ž .
recently, a pathogen was described Lambert et al., 1998 , the bacterial etiology can be demonstrated by the prophylactic use of antibacterial agents, which increases overall
Ž .
survival of the cultures Robert et al., 1996 . So far, the agent routinely used in
Ž .
European hatcheries is chloramphenicol Robert et al., 1996 . As the application of chloramphenicol to animals intended for human consumption has now been banned in
Ž
the countries of the European Union and the European Economic Area Anonymous,
.
1990, 1994 , there is a need to evaluate alternative therapeutic drugs.
In recent years, the antibacterial agents most used in Norwegian aquaculture have been oxytetracycline, oxolinic acid, flumequine, florfenicol and trimethoprimr
sulfadia-Ž . Ž .
zine Tribrissenq Markestad and Grave, 1997 . The total consumption of antimicrobial Ž
agents in Norwegian aquaculture in 1997 was 555.7 kg, divided in oxolinic acid 445.5
. Ž . Ž . Ž .
kg , flumequine 71.4 kg , florfenicol 26.5 kg , oxytetracycline 11.8 kg and
ben-Ž .
zylpenicillinrdihydrostreptomycine 0.5 kg , measured as active component. These statistics are generated by The Department of Quality Control, Directorate of Fisheries, Norway, on the basis of copies of veterinary prescriptions for fish. The consumption of antimicrobial agents in Norwegian aquaculture in 1987 was almost 50 000 kg, measured as active component. The dramatic reduction observed during recent years, is due to an optimisation of the rearing conditions and development of effective vaccines.
The purpose of the present study was to establish minimum inhibitory concentration
ŽMIC values of chloramphenicol, florfenicol, flumequine and the combination trimetho-.
primrsulfadiazine for bacterial strains isolated from scallop larvae in aquaculture. Based on these MIC values, optimal therapeutic procedures for the treatment of scallop larvae with antibacterial agents will be evaluated. Since the therapeutic procedures will be used in a marine environment, the antagonising effect of seawater on antibacterial agents was measured.
2. Materials and methods
2.1. Source of scallop eggs and larÕae
All egg groups were collected from a commercial scallop hatchery, Scalpro, Rong, near Bergen, Norway. The broodstock originated from Hordaland County, and was conditioned in the hatchery. The two egg groups were collected in March and April 1997. The spawnings were induced by thermal shock, and eggs were fertilised by the
Ž .
method described by Gruffyd and Beaumont 1970 . After fertilisation, the embryos
Ž .
were distributed in two tanks 800 l and kept in stagnant seawater at 188C"1. The seawater was obtained from the nearby fjord at 60 m and filtered using a 1mm bag filter
ŽGaf filters, Belgium . The seawater in the tanks was renewed three times a week.. Ž
Larvae in one of the tanks were treated prophylactically with chloramphenicol 10 mg
y1.
Ž . Ž .
galbana Parke Tahitian strain, PaÕloÕa lutheri Droop and Chaetoceros calcitrans
Ž . y1
Takano 1:1:2 at a total concentration of 50 cells ml .
2.2. Isolation of bacteria
Bacterial samples were taken in triplicate from one untreated tank and from one tank treated with chloramphenicol. The total number of larvae was counted under a dissection microscope. Each sample consisted of approximately 100 000 eggs, and between 50 000 and 100 000 larvae. The eggs and larvae were washed three times in 25‰ sterile
Ž . Ž .
seawater SSW WTW, LF 196, Weilheim, Germany prior to homogenisation in 10 ml 25‰ SSW. Dilution series were made using 25‰ SSW plated out on Petri dishes with
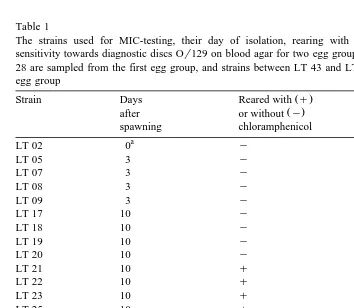
Table 1
The strains used for MIC-testing, their day of isolation, rearing with or without chloramphenicol and sensitivity towards diagnostic discs Or129 on blood agar for two egg groups. Strains between LT 02 and LT 28 are sampled from the first egg group, and strains between LT 43 and LT 70 are sampled from the second egg group
Ž . Ž .
Strain Days Reared with q Or129
Ž .
The samples were taken after fertilisation. b
Ž . Ž .
Difco 2216 Marine Agar MA Difco, Detroit, MI, USA , Tryptone Citrate Bile
Ž . Ž . Ž .
Sucrose agar TCBS Oxoid, Basingstoke, England and Tryptone Soy Agar TSA
ŽOxoid, Basingstoke, England . Following aerobic incubation for 3 days at 18. 8C, Colony
Ž .
Forming Units CFU were counted, and the number of colonies per larvae calculated. Representative colonies were selected for further characterisation and 14 strains from
Ž .
egg group 1 and 13 strains from egg group 2 were tested for MIC values Table 1 . All
Ž
strains used in the MIC-testing were tested for Gram staining Merck, Darmstadt,
.
Germany . The strains from both egg groups were tested with the diagnostic disc Or129
Ž .
150mg, Oxoid, Basingstoke, England . Biochemical tests were done with the
commer-Ž .
cial kit API 20E BioMerieux, France , modified by the incorporation of NaCl,
´
Ž .
according to MacDonell et al. 1982 . The kit includes the following tests:b -galacto-sidase, arginine dihydrolase lysine decarboxylase, ornithine decarboxylase, Simmon’s
Ž .
citrate, production of H S, urease Ferguson , tryptophane desaminase, indole, acetoine,2
Ž .
protolysis of gelatin, glucose oxidative and fermentative , acidification of mannitol,
Ž .
inositol, sorbitol, rhamnose, saccarose, melibiose, amygdaline and Lqarabinose, cy-tochrome oxidase, production of nitrites, production of nitrogen, and production of gas from glucose and catalase. In addition, some of the strains were tested for growth in
ŽŽ .
different salt concentrations TGY Tryptone Glucose Yeast, Oxoid, Baisingstoke,
. Ž .
England , and some strains were checked for the presence of flagella Leifson, 1961 . Based on the results of these characterisation tests, the isolates were classified to genus
Ž .
level according to the scheme presented by Muroga et al. 1987 .
2.3. Determination of MIC
Determination of MIC values was performed using an agar dilution method
ŽWashington, 1985; Samuelsen and Lunestad, 1996 . The system included strains with.
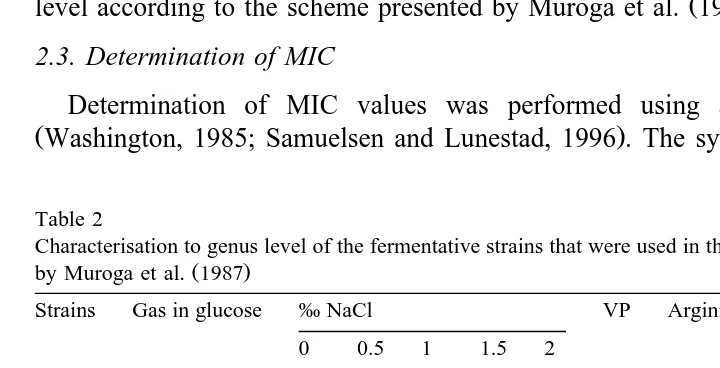
Table 2
Characterisation to genus level of the fermentative strains that were used in this study, according to the scheme
Ž .
by Muroga et al. 1987
Strains Gas in glucose ‰ NaCl VP Arginine Lysine Genus
Table 3
Characterisation of the non-fermentative strains that were used in this study, according to the scheme by
Ž .
Muroga et al. 1987
Strain Polar Peritrichous Genus
flagellation flagella
LT 07 q y Pseudomonas
LT 09 q y Pseudomonas
LT 18 q y Pseudomonas
LT 21 y q Agrobacterium
a a
LT 25
LT 54 q y Pseudomonas
LT 62 q y Pseudomonas
a
Not measured.
known MIC values. For chloramphenicol and florfenicol, the reference strain was
Escherichia coli ATCC 25922, whereas for flumequine and trimethoprimrsulfadiazine,
the Aeromonas salmonicida strains 7136 and 7137, respectively, from The National Veterinary Institute, Oslo, Norway, were used.
Strains from both egg groups were tested on Mueller Hinton agarrbroth supple-mented with 2% NaCl and Mueller Hinton agarrbroth made with 25‰ seawater. The strains were transferred to 10 ml Mueller Hinton broth and incubated for 48 h at 208C, giving a final cell density of approximately 5=108 mly1. Bacteria were transferred to
Mueller Hinton agar using an inoculation loop of 10ml. The Mueller Hinton agar dishes contained increasing concentrations of the antibacterial agents: chloramphenicol,
flor-Ž . y1
fenicol, and trimethoprimrsulfadiazine 1:5 from 0.13 to 16 mg ml in two-fold dilution. The dishes with flumequine ranged from 0.013 to 8 mg mly1 in two-fold
Ž
dilution. The antibacterial agents were obtained from Norsk Medisinaldepot Bergen,
.
Norway . The agar dishes were incubated for 72 h at 208C. The lowest concentration of chloramphenicol, florfenicol and flumequine where complete inhibition occurred, and the lowest concentration of trimethoprimrsulfadiazine at which marked inhibition
Ž . Ž .
Fig. 1. Numbers of CFU in scallops cultures reared with filled symbols and without open symbols
Ž . Ž .
chloramphenicol. The measurements of CFU were performed on TCBS triangle , TSA circle and MBA
Žsquare agar dishes. a First egg group. b Second egg group. For the TCBS plates on D0 with and without. Ž . Ž .
Table 4
Ž y1.
Summarised MIC values mg ml for the four antibacterial agents with Mueller Hinton agar made with 2%
Ž . Ž .
NaCl NaCl or 25‰ seawater SW , for bacterial strains from both egg groups
Antibacterial agent Medium First egg group Second egg group
Range Minimum Range Minimum
average value average value
Chloramphenicol NaCl 0.5–16 3.2 0.13–16 2.48
SW 0.13–16 3.6 0.5–)16 )9.4
Florfenicol NaCl 0.5–8 2.8 0.13–4 1.9
SW 1–16 3.6 0.25–)16 )7
Fluemequine NaCl 0.03–1 0.49 0.03–1 0.47
SW 1–)8 )6.6 2–)8 )6.5
Trimethoprimr NaCl 0.5–4 1.9 0.13–4 2.01
sulfadiazine SW 1–16 4.8 0.5–16 6.2
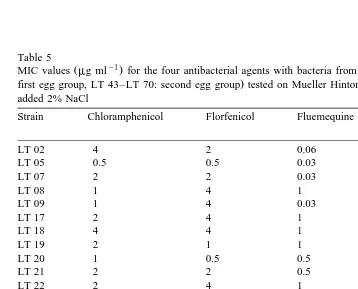
Table 5
Ž y1. Ž
MIC values mg ml for the four antibacterial agents with bacteria from both egg groups LT 02–LT 28:
.
occurred was recorded as the MIC value. The MIC testing was performed in duplicate. The results of the MIC-test were tested for statistical significance by the Mann–Whitney
U-test. P-0.05 was considered significant.
Ž
The pH was measured in agar plates using a pH-meter Sentron 2001, Sentron, The
.
Netherlands supplied with a probe specially designed to measure pH in semisolid samples.
3. Results
All bacteria isolated were Gram-negative and all strains were rod-shaped, except LT 02. All strains were oxidase-positive, except LT 63. A total of 15 strains were
Ž . Ž .
fermentative Table 2 and 12 strains were non-fermentative Table 3 .
Mortality was 100% in the untreated tanks. No larvae were left in the untreated tanks 13 and 17 days after spawning for the first spawning group and for the second spawning
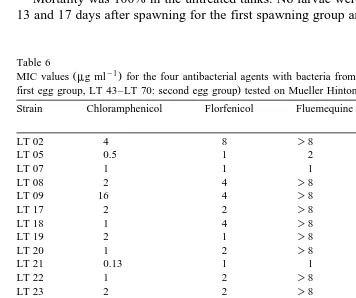
Table 6
Ž y1. Ž
MIC values mg ml for the four antibacterial agents with bacteria from both egg groups LT 02–LT 28:
.
first egg group, LT 43–LT 70: second egg group tested on Mueller Hinton agar with 25‰ seawater
group, respectively, whereas survival of the larvae in the tank with chloramphenicol was approximately 30% at time of settling. The settling took place between 21 and 28 days after spawning.
In general, the numbers of CFU were lower for larvae treated with chloramphenicol
Ž .
than for untreated larvae Fig. 1 . A larger difference in CFU between treated and untreated larvae was found on TCBS agar than on MBA and TSA media. The difference in the number of CFU between treated and untreated cultures was larger in the second
Ž .
egg group than in the first Fig. 1 . In the first egg group, the ratio of CFU between treated and untreated larvae on TCBS agar was 41 on day 13 after fertilisation. The corresponding ratio was 413 for the second egg group. The same pattern was found for TSA agar where the ratios were estimated to be 2 for the first egg group and 23 for the second. For MBA dishes, the ratios were 3 and 5 for the first and second egg group, respectively.
Differences in MIC values were found between strains grown on Mueller Hinton agar
Ž .
dissolved in 2% NaCl and Mueller Hinton agar dissolved in 25‰ seawater Table 4 . In
Ž . Ž .
the cases of fluemequine Ps0.000 and for trimethoprimrsulfadiazine Ps0.013 , the MIC values were significantly higher for both egg groups with 25‰ seawater than
Ž . Ž .
with 2% NaCl. For chloramphenicol Ps0.16 and florfenicol Ps0.49 , the differ-ences between 2% NaCl and 25‰ seawater were not significant. However, a
signifi-Ž .
cantly higher MIC value was found for chloramphenicol Ps0.021 using 25‰ seawater compared to 2% NaCl in the second egg group.
The pH was measured to be 7.4 in agar plates made with 2% NaCl and 7.2 in agar plates made with 25‰ seawater. The MIC values for each of the individual strains are listed in Tables 5 and 6.
4. Discussion
The 30% survival at the settling stage found in the treated tanks can be considered a
Ž .
good survival rate in scallop larval cultivation Robert et al., 1996 . The higher mortality observed in the untreated tanks indicates a bacterial cause, a hypothesis that is further supported by our finding of a lower number of CFU in treated larvae compared to untreated larvae. The viable counts are still low compared to the results of Nicolas et al.
Ž1996 , who found CFU values approximately 1.5–2 times higher than the CFU values.
found in this investigation. This suggests that a further investigation of the species composition is needed since the causative agent of the mortality of the larvae has yet to be identified. All antibacterial agents were shown to have an effect against the isolates. However, in vivo trials would be necessary to determine whether some of the agents could be suitable as replacements for chloramphenicol.
florfenicol, these ratios would possibly be even larger had the range of concentrations been extended.
Seawater contains a wide array of dissolved components, where sodium, potassium,
Ž .
calcium and magnesium are the dominant cations Pytkowicz and Kester, 1971 . The components found in seawater may influence bacterial susceptibility to antimicrobial agents in several ways. The observed changes in MIC values due to addition of seawater may be explained either by effects on the physiology of the bacterium, by effects on components in the medium other than the antimicrobial agent, or by chemical interac-tions with the antimicrobial substance itself.
The antibacterial agents examined in this study, target cell components inside the bacterial cell and must therefore pass the cell envelope to have an effect. The permeability of the cell envelope has important implications for antimicrobial suscepti-bility, as decreasing permeability leads to increasing resistance and increased permeabil-ity gives higher susceptibilpermeabil-ity. Seawater components may influence on the physiological properties of the bacterial cells, causing changes in susceptibility. As an example, the
Ž .
strongly negatively charged molecules of the lipopolysaccharide layer LPS in the outer membrane of Gram-negative bacteria may be neutralised by cations from seawater, giving reduced permeability of positively charged molecules of antibacterial agents
ŽHancock, 1997 . Several authors Davis et al., 1971; Gilbert et al., 1971; Brenner and. Ž .
Sherris, 1972 have reported that variations in the media used may effect the results of
Ž .
susceptibility tests. Gilbert et al. 1971 reported that the variations in the concentration of Ca2qand Mg2qbetween various media and within different lots of the same medium
significantly affected the susceptibility of Pseudomonas aeruginosa to gentamicin. In
Ž . 2q
the study by Davis et al. 1971 , Ca was shown to reduce the antimicrobial activity of both polymyxin E and B against P. aeruginosa.
Several authors have addressed the effect of cations like Ca2q and Mg2q on the Ž
activity of antibacterial agents used in aquaculture Lunestad and Goksøyr, 1990; Barnes
. Ž .
et al., 1995; Pursell et al., 1995 . Pursell et al. 1995 reported a 20–40-fold reduction in flumequine activity against strains of Aeromonas salmonicida, using TSA diluted with 2.5% NaCl and added 54 mmol Mg2q compared to TSA diluted only with 2.5% NaCl.
A similar reduction in antibacterial activity due to the addition of Mg2q to the medium
Ž .
was found by Barnes et al. 1995 for flumequine and oxolinic acid. Therefore, our observation that the addition of 25‰ seawater to the test medium resulted in a significant increase in MIC-values for the tested organism against flumequine, compared to a medium with 2% NaCl, was hardly surprising. More surprising, however, is our
Ž .
observation, at least when compared to the findings of Barnes et al. 1995 , that the antibacterial effect of the combination trimethoprimrsulfadiazine was affected by the addition of seawater to the medium. In our investigation, we found a significant increase in MIC-values for the tested strains from both egg groups against trimethoprimr
sulfa-Ž .
diazine using 25‰ seawater in the medium compared to 2% NaCl Tables 2 and 3 .
2q Ž .
Using IsoSensitest agar with and without 50 mmol Mg , Barnes et al. 1995 concluded that no difference in MIC values for strains of A. salmonicida could be observed when testing ormethoprim and sulfadimethoxine individually. However, all the
Ž . y1 Ž
strains tested by Barnes et al. 1995 had MIC values of 16mg ml and higher )128
y1. 2q
increases in MIC values, as those observed in our investigation, would therefore be difficult to detect. None of the papers that have reported an inhibition of antibacterial agents by divalent cations or seawater hold data on changes in bacterial susceptibility by adding seawater to media containing chloramphenicol or florfenicol.
Tetracycline molecules have several potential binding sites and the ability of various tetracyclines to form stable complexes with di- and trivalent cations is well-known
ŽClive, 1968; Berthon, 1988; Berthon et al., 1983 . This complex formation has been. Ž
shown to result in a major reduction in antibacterial activity Lunestad and Goksøyr,
. Ž . Ž .
1990 . Based on the results of Ratcliffe and Smith 1983 , Smith and Lewin 1988 and
Ž . 2q
Palmer et al. 1992 that Mg has a greater effect on the activity of flumequine at the
Ž .
pH range of seawater 7.5–8.5 than at lower pH, it is tempting to suggest that complexation between the negatively charged flumequine and Mg2qis a major
contribu-tor to the inhibicontribu-tory effect.
The fact that all strains tested with respect to flumequine in this investigation showed much higher MIC values on media containing seawater support the suggestions of a general, non-specific inhibitory mechanism initiated by the cations. For the trimetho-primrsulfadiazine combination, chloramphenicol and florfenicol, on the other hand, only some of the strains increased their MIC values when NaCl was replaced with
Ž .
seawater in the media Tables 4 and 7 . This suggests a more strain-specific resistance mechanism initiated by the seawater cations and a simple complex formation between the drugs and the cations is therefore less likely.
Trimethoprim and sulfadiazine are weak electrolytes with p K values of 6.6 and 6.5,a
Ž .
respectively Meyerson, 1970; Windholz, 1983 . A difference in pH of the media used will therefore influence the ratio of ionisedrnon-ionised forms of the antibacterial, and hence, the lipophilic properties of the drugs. Increasing the medium pH from 7.2 to 7.4 led to a decrease in the non-ionised form of sulfadimethoxine of 6%, whereas a 6% increase in the non-ionised form was calculated for trimethoprim. Whether this change will have any detectable effect on the MIC values is, however, impossible to say without further research.
Ž .
Oxolinic acid is a weak acid with p Ka value of 6.9 Samuelsen et al., 1992 . Although some effect on the antibacterial activity may be expected by changing the medium pH, the dominating effect on the in vitro activity of flumequine is, as shown by
Ž . 2q 2q
Pursell et al. 1995 , initiated by the addition of Ca and Mg to the media. Fukui et
Ž .
al. 1987 showed that changing the pH of the medium from 6 to 8 had only minor influence on the antibacterial activity of florfenicol and chloramphenicol against various fish pathogens.
Components in seawater may also interact directly with constituents of the medium. If such interactions cause changes to the growth rate of the bacterium under examina-tion, changes in MIC value may be observed. One example is the interaction between Mg2q and phosphate. Autoclaving phosphate in media with high concentrations of
Mg2qadded as natural or synthetic seawater results in a variable degree of precipitation,
Ž . Ž .
probably as Mg PO3 4 2 Smith, 1998 . The effect has been reported to give a significant
Ž .
decrease in MIC values recorded for oxolinic acid Smith, 1998 .
per se or other components in the medium, as well as effects on the physiological state of the bacterium, including permeability of the bacterial envelope. The relative impor-tance of each of these factors for different bacteria and antimicrobial components are not readily established.
Acknowledgements
We thank Dr. Thorolf Magnesen, Scalpro, Rong, Norway, for collaboration and discussions. We also thank Dr. Steinar Høie, National Veterinary Institute, Oslo, Norway, and Dr. Asbjørn Digranes, Haukeland Sykehus, Bergen, Norway, for supplying standard strains for the MIC tests. The technical assistance by Kari Andersen and Heidi Kongshaug is highly appreciated. This study was financially suppported by The Norwe-gian Research Council, grants 120099r122 and 129406r122.
References
Ž . Ž .
Anonymous, 1990. Council regulation EEC No.2377r90 1990 . The European Union.
Ž . Ž .
Anonymous, 1994. Commission regulation EC No. 2701r94 1994 . The European Union.
Barnes, A.C., Hastings, T.S., Amyes, S.G.B., 1995. Aquaculture antibacterials are antagonized by seawater cations. J. Fish Dis. 18, 463–465.
Berthon, G., 1988. Metal ion–tetracycline interactions in biological fluids: Part 8. Potentiometric and
Ž . Ž .
spectroscopic studies on the formation of Ca II and Mg II complexes with 4-dedimethylamino-tetra-cycline and 6-desoxy-6-demethyl-tetra4-dedimethylamino-tetra-cycline. J. Inorg. Biochem. 33, 193–210.
Berthon, G., Brion, M., Lamps, L., 1983. Metal ions–tetracycline interactions in biological fluids: 2. Potensiometric study of magnesium complexes with tetracycline, oxytetracycline, doxycycline and minocy-cline and discussion of their possible influence on the bioavailability of these antibiotics in blood plasma. J. Inorg. Biochem. 19, 1–18.
Brenner, V.C., Sherris, J.C., 1972. Influence of different media and bloods on the results of diffusion antibiotic
Ž .
susceptibility tests. Antimicrob. Agents Chemother. 1 2 , 116–122.
Clive, D.L.J., 1968. Chemistry of tetracyclines. Q. Rev. Chem. Soc. 22, 435–456.
Davis, S.D., Iannetta, A., Wedgwood, R.J., 1971. Activity of colistin against Pseudomonas aeruginosa:
Ž .
inhibition by calcium. J. Infect. Dis. 124 6 , 610–612.
Fukui, H., Fujihara, Y., Kano, T., 1987. In vitro and in vivo antibacterial activity of florfenicol, a new florinated analog of thiamphenicol, against fish pathogens. Fish Pathol. 22, 201–207.
Gilbert, D.N., Kutscher, E., Ireland, P., Barnett, J.A., Sanford, J.P., 1971. Effect of the concentration of magnesium and calcium on the in vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J. Infect. Dis. 124, 37–45.
Gruffyd, Ll.D., Beaumont, A.R., 1970. Determination of the optimum concentration of eggs and spermatozoa for the production of normal larvae in Pecten maximus. Helgolander Wiss. Meeresunters. 20, 486–497.¨
Ž .
Hancock, R.E.W., 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5 1 , 37–42. Lambert, C., Nicolas, J.L., Cilia, V., Corre, S., 1998. Vibrio pectenicida sp. nov., a pathogen of scallop
ŽPecten maximus larvae. Int. J. Syst. Bacteriol. 48, 481–487..
Leifson, E., 1961. The effect of formaldehyde on the shape of bacterial flagella. J. Gen. Microbiol. 25, 131–133.
Lunestad, B.T., Goksøyr, J., 1990. Reduction in the antibacterial effect of oxytetracycline in seawater by complex formation magnesium and calcium. Dis. Aquat. Org. 9, 67–72.
Markestad, A., Grave, K., 1997. Reduction of antibacterial drug usage in Norwegian fish farming due to
Ž .
vaccination. In: Gudding, R., Midtlyng, A., Brown, F. Eds. , Fish Vaccinology. Dev. Biol. Stand. 90 Karger, Basel, pp. 365–369.
Meyerson, B., 1970. Sulfonamider, Farmakologisk Oversikt. In: Meyerson, B., Nordbring, F., Winberg, J.
ŽEds. , Socialstyrelsens kommitte for lakemedelsinformasjon. Sulfonamider. Farmakologo. Klinik, pp..
1–20, In Swedish.
Muroga, M., Higashi, M., Keitoku, H., 1987. The isolation of intestinal microflora of farmed Red Seabream
ŽPagrus major and Black Seabream Acanthopagrus schlegeli at larval and juvenile stages. Aquaculture. Ž .
65, 79–88.
Nicolas, J.L., Corre, S., Gauthier, G., Robert, R., Ansquer, D., 1996. Bacterial problems associated with scallop Pecten maximus larval culture. Dis. Aquat. Org. 27, 67–76.
Palmer, R., Kawai, K., Kusuda, R., 1992. In vitro activity of quinolone antibacterials against selected fish pathogens. Gyobyo Kenkyu 27, 131–142.
Pursell, L., Samuelsen, O.B., Smith, P., 1995. Reduction in the in-vitro activity of flumequine against
Aeromonas salmonicida in the presence of the concentrations of Mg2q and Ca2q ions found in seawater.
Aquaculture 135, 245–255.
Pytkowicz, R.M., Kester, D.R., 1971. The physical chemistry of seawater. Annu. Rev. Oceanogr. Mar. Biol., 11–60.
Ratcliffe, N.T., Smith, J.T., 1983. Effects of magnesium on the activity of 4 quinolone antibacterial agents. J. Pharm. Pharmacol. 35, 59.
Robert, R., Miner, P., Nicolas, J.L., 1996. Mortality control of scallop larvae in the hatchery. Aquacult. Int. 4, 305–313.
Samuelsen, O.B., Lunestad, B.T., 1996. Bath treatment, an alternative method for the administration of the quinolones fluemequin and oxolinic acid to halibut Hippoglossus hippoglossus, and in vitro antibacterial activity of the drugs against some Vibrio sp. Dis. Aquat. Org. 27, 13–18.
Samuelsen, O.B., Lunestad, B.T., Husevag, B., Hølleland, T., Ervik, A., 1992. Residues of oxolinic acid in˚
wild fauna following medication in fish farms. Dis. Aquat. Org. 12, 111–119.
Smith, J.T., Lewin, C.S., 1988. Chemistry and mechanisms of action of the quinolone antimicrobials. In:
Ž .
Andriole, V.T. Ed. , The Quinolones. Academic Press, London, pp. 23–82.
Smith, P., 1998. Towards the establishment of a breakpoint concentration for the determination of resistance to oxolinic acid in the marine microflora. Aquaculture 166, 229–239.
Washington, J.A. II, 1985. Susceptibility test: agar dilution. In: Lennette, E.H., Balows, A., Haausler, W.J. Jr.,
Ž .
Shadomy, H.J. Eds. , Manual of Clinical Microbiology, pp. 967–971, Washington, DC.