www.elsevier.comrlocateranireprosci
Improved development of DNA-injected bovine
embryos co-cultured with mouse embryonic
fibroblast cells
J.S. Park
a, Y.M. Han
a, C.S. Lee
a, S.J. Kim
a, Y.H. Kim
b,
K.J. Lee
b, K.S. Lee
c, K.K. Lee
a,)a
Korea Research Institute of Bioscience and Biotechnology, Yusong, Taejon 305-333, South Korea
b
Doosan Business Group, Taean-Gun, Chungnam 357-960, South Korea
c
DiÕision of Animal Science and Resources, Chungnam National UniÕersity, Yusong,
Taejon 305-764, South Korea
Received 26 March 1999; received in revised form 14 July 1999; accepted 2 December 1999
Abstract
The in vitro development of DNA-injected bovine zygotes, produced in vitro, was compared
Ž .
when cultured with or without mouse embryonic fibroblasts MEF . The in vivo viability of the embryos produced in these in vitro culture systems was assessed by single or double transfer to recipients taken to term. For these experiments, in vitro fertilized oocytes were not injected
ŽExperiment 1 or were injected with pBL1 gene Experiment 2 and then cultured for 2 days in. Ž .
CR1aa medium supplemented with 3 mgrml BSA at 38.58C in a humidified atmosphere of 5%
CO2 in air. Embryos that developed to the 4- to 8-cell stage at the end of this period were
randomly assigned to the two cultured systems and cultured for a further 5 days in groups of 10 to 15 embryos in 0.75 ml medium. These two culture systems were CR1aa medium alone or
Ž .
co-culture with MEF in CR1aa medium supplemented with 10% fetal bovine serum FBS . Every 48 h, 0.5 ml of the medium was replaced with fresh CR1aa medium and at Day 5 of culture, both
media were supplemented by the addition of 5.56 mM glucose and 1= GMS-X supplement
solutions. Results were assessed as morphological development of the embryos and data were analyzed by Chi-square test or Student’s t-test.
Ž .
The development rate of in vitro fertilization IVF -derived embryos co-cultured with MEF
Ž24.4%, 49r201 was significantly higher than those cultured alone 14.4%, 28. Ž r194; P-0.05 in.
Experiment 1. There was a similar difference between the treatments in the proportions of
Ž
embryos which reached the hatching stage or hatched 10.9%, 22r201 vs. 4.1%, 8r194,
)Corresponding author. Tel.:q82-42-860-4420; fax:q82-42-860-4608.
Ž .
E-mail address: [email protected] K.K. Lee .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
. Ž .
respectively; P-0.05 . DNA-injected embryos co-cultured with MEF 13.7%, 28r205 showed a
Ž
higher developmental rate than that of the embryos cultured without MEF 6.7%, 13r193;
.
P-0.05 in Experiment 2. Following the transfer to recipients of one or two DNA-injected
Ž
blastocysts, the pregnancy rates for two culture systems were similar MEF co-culture 27.4%,
.
23r84; CR1aa culture 24.2%, 16r66 . However, the numbers of calves born alive from these
Ž .
pregnancies were higher on the MEF co-culture group 82.6%, 19r23 than the CR1aa culture
Ž .
group 56.2%, 9r16 . It was concluded that in vitro embryo development to the blastocyst stage and subsequent in vivo development to term of DNA-injected bovine embryos was improved in comparison to culture in CR1aa alone when the last 5 days of in vitro culture were in a MEF co-culture system.q2000 Elsevier Science B.V. All rights reserved.
Ž .
Keywords: Cattle-reproductive technology; Mouse embryonic fibroblasts MEF ; DNA injection; Embryo
co-culture
1. Introduction
In the production of transgenic animals, especially transgenic cattle, it is important to establish an in vitro culture system for mass production of transferrable embryos. Several studies have shown that the development of microinjected, in vitro-produced bovine embryos to the blastocyst stage was considerably lower than that of non-injected
Ž .
control embryos Gagne and Sirard, 1990; Krimpenfort et al., 1991; Peura et al., 1994 . The reduced development of DNA-injected, in vitro-produced bovine zygotes may be due to the pronucleus-injection itself rather than injection-related handling or the overall
Ž . Ž
damage caused by zygote piercing Peura et al., 1994 . In our previous report Han et
.
al., 1996 , the developmental rate of DNA-injected bovine embryos to blastocysts was low, approximately 5% when the embryos were cultured in CR1aa medium alone. Thus, a better culture system needs to be developed in order to improve subsequent develop-ment of the DNA-injected bovine embryos.
Ž .
When bovine embryos derived from in vitro fertilization IVF of in vitro matured follicular oocytes were cultured, they were delayed or arrested at the 8- to 16-cell stage
ŽCamous et al., 1984; Heyman et al., 1987; Eyestone and First, 1989 . A breakthrough.
which overcame this problem was co-culture of the early bovine embryos fertilized in
Ž .
vitro. We have used mouse embryonic fibroblasts MEF for co-culture of bovine
Ž .
embryos. MEF or STO cells irradiated mouse fibroblasts have been generally used for
Ž . Ž .
the development and maintenance of mouse embryonic stem ES cells Joyner, 1993 .
Ž .
Primary MEF can be easily isolated from fetuses in the third trimester 14 to16 days of pregnancy, and 1- and 2-cell ovine embryos subjected to 5 days of co-culture showed
Ž .
significantly better development on STO than EF Rexroad and Powell, 1993 . However, little information is available on the effects of MEF on the in vitro development of IVF-derived, especially DNA-injected, bovine embryos and on the viability after transfer of DNA-injected bovine embryos co-cultured with MEF.
2. Materials and methods
( )
2.1. InÕitro maturation IVM
Ovaries were collected from Holstein cows and heifers at a local slaughterhouse and were transported to the laboratory in 0.9% saline at 22–258C. Immature oocytes were collected from 2 to 5 mm diameter follicles using an 18-gauge needle and a 5-ml syringe. The oocytes were washed three times with 3 ml of 10 mM hepes-buffered
Ž .
TL-HEPES medium Gibco BRL, Life Technologies, Grand Island, NY , based on tyrode-lactate medium and supplemented with 0.3% BSA and 2 mM NaHCO , and once3 with maturation medium, TCM-199 with Eagle’s salts and L-glutamine supplemented
Ž . Ž .
with 10% vrv heat-inactivated fetal bovine serum FBS; Gibco BRL and 25 mM NaHCO . After washing, 20 to 30 oocytes with evenly granulated cytoplasm surrounded3 by compact cumulus cells were transferred into 0.5 ml of maturation medium containing
Ž . Ž
1 ugrml oestradiol Sigma, St Louis, MO and 1 mgrml FSH-Pe Schering-Plough
. Ž .
Animal Health, Kenilworth, NJ in a 4-well multidish Nunclon, Roskilde, Denmark and cultured for 22 to 24 h at 38.58C, 5% CO in air.2
2.2. InÕitro fertilization
Ž
At the end of IVM, oocytes were rinsed twice with TALP Bavister and
Yanagi-.
machi, 1977 and placed in 50ml of fertilization medium, which consisted of modified
Ž .
Tyrode-Lactate medium Fert-TALP; Bavister and Yanagimachi, 1977 . Frozen semen from two Holstein bulls was thawed at 378C in water for 30 s and layered on a 45–90%
Ž .
Percoll Sigma gradient in a 15-ml centrifuge tube. The Percoll gradient was composed of 2 ml of 90% Percoll overlaid with 2 ml of 45% Percoll, both of which were prepared
Ž .
with Sperm TALP SP-TALP; Parrish et al., 1988 . After 20 min of centrifugation at 2000=g, the top layers were removed and the sperm pellet was suspended in
SP-TALP. After 10 min of centrifugation at 700=g, the sperm pellet was resuspended
by the same method. The sperm suspension was introduced into a 50-ml drop of fertilization medium containing 10 oocytes to make the final sperm concentration
Ž 6 .
2=10 rml . When sperm were added to the fertilization drops, 2mgrml heparin, 20
Ž .
mM penicillamine, 10mM hypotaurine and 1mM epinephrine PHE were also added. After 18 h of insemination, the cumulus investment was removed by repeated pipetting in 2 ml TL-hepes medium in a 3-mm petri dish and the oocytes were then placed in CR1aa medium before DNA injection. CR1aa medium was formulated according to
Ž .
Rosenkrans et al. 1993 , and supplemented with 1 mM glutamine and 1= Eagle’s
Ž .
essential amino acids solution Gibco BRL .
2.3. Microinjection of DNA
The DNA used for microinjection was a 5.5-kb fragment of pBL1, which consisted of the bovineb-casein gene promoter, human lactoferrin cDNA and SV40 polyadenylation
Ž .
Ž
similarly to the methods previously described for pig and sheep embryos Hammer et
.
al., 1985 . In order to visualize the pronuclei, cumulus-free oocytes were centrifuged in
Ž
TL-hepes medium at 12,000=g for 7 min in a microcentrifuge Vision Scientific,
.
Korea . The embryos were then microinjected into one pronucleus with DNA solution 21 to 25 h after insemination. Swelling of the pronucleus indicated a successful injection. After DNA injection, surviving zygotes were cultured for 2 days in 50 ml drops of CR1aa medium supplemented with 3 mgrml BSA under light mineral oil
ŽSigma ..
2.4. MEF monolayers
Ž .
MEF monolayers were prepared as described by Joyner 1993 . Briefly, fetuses were collected aseptically at necropsy from Day 14 to 16 pregnant mice and then finely
Ž .
minced using micro-scissors. The minced tissues were washed three times with PBSy
free of Ca2q and Mg2q ions, and trypsinized in 0.1% trypsinr0.05% EDTA solution
Ž
while being stirred. Suspended cells were sieved through a stainless steel mesh Ikemoto,
.
Tokyo, Japan and centrifuged at 700=g for 5 min. The cell pellet was re-suspended in
Ž .
DMEM Gibco BRL containing 10% FBS and the cell suspension was placed in a 100-mm petri dish. The primary fibroblasts were cultured for 2 days until confluent at 378C, 5% CO in air, proliferated through two subsequent passages as described above2 and then frozen at y708C. Freezing medium used for fibroblasts consisted of 10%
Žvrv glycerol and 50% v. Ž rv FBS in PBS. Frozen MEF were thawed in 37. 8C water and then grown in 100 mm petri dishes for 2 days until confluent. MEF were inactivated
Ž .
by treatment with 10 mgrml Mitomycin C Sigma for 2.5 h and then harvested. To co-culture bovine embryos, Mitomycin C-treated cells were rinsed three times with PBS, re-suspended in DMEM containing 10% FBS and then plated at a concentration of 6=105cellsrml placing 0.5 ml of the suspension in each well of a 4-well dish to form monolayers. Approximately 4 h later, all the DMEM was removed and replaced with 0.75 ml of CR1aa medium containing 10% FBS at least 2 h before use.
2.5. InÕitro culture
DNA-injected or IVF-derived bovine zygotes were cultured in 50ml drops of CR1aa medium supplemented with 3 mgrml BSA under light mineral oil. Embryos were selected at approximately 72 h after insemination and used for the culture experiments. At this time, 4- to 6-cell stage embryos and embryos with )6-cells were placed in culture groups denoted as 4- and 8-cell stage embryos, respectively. Approximately half of the cleaved embryos were further co-cultured with MEF and the remainder were cultured without MEF for another 5 days in 0.75 ml of CR1aa medium supplemented with 10% FBS. All in vitro cultures were under oil at 38.58C in a humidified atmosphere of 5% CO . A total of 10 to 15 embryos were subjected to each culture experiment. At2 Day 5 of culture, glucose was added to bring the final concentration to 5.56 mM and
Ž .
well was replaced with fresh CR1aa medium at 48 h intervals. Embryo development was evaluated by morphological appearance under an inverted Nikon light microscope. The day of IVF was designated as Day 0.
2.6. Embryo transfer
Blastocysts that developed from DNA-injected embryos were transferred to recipi-ents. The embryos were transported for about 4 h prior to transfer to recipients in PBS medium supplemented with 15% FBS at 35–378C in a thermos bottle. All recipients were virgin, 14- to 18-month-old Holstein heifers. They were observed twice daily for the onset of standing oestrus and heifers with spontaneous oestrus were used as recipients. Prior to embryo transfer, the recipients were palpated rectally for the presence
Ž . Ž
of a functional corpus lutea CL . A sterile embryo transfer gun 0.25 ml straw; IMV,
. Ž
L’aigle Cedex, France ensheathed in a sanitary sleeve IMV, Chemise Sanitaire,
.
France was used to perform non-surgical embryo transfer. One or two blastocysts, depending on the availability of embryos each day, were transferred to the ipsilateral uterine horn of a recipient heifer on Day 7 or 8 after spontaneous oestrus. Following transfer, the heifers were observed for signs of oestrus, and pregnancy was confirmed by rectal palpation at approximately Day 60 of gestation. The pregnant recipients were normally managed to deliver their calves at term.
2.7. Experimental designs
2.7.1. Experiment 1
To examine the effect of MEF on in vitro development of IVF-derived bovine embryos, immature oocytes were matured, fertilized and cultured in vitro. After 2 days of culture, approximately half of the cleaved embryos were further co-cultured with MEF and the remainder was cultured without MEF for 5 days. These experiments were contemporaneously repeated seven times in each group.
2.7.2. Experiment 2
The development ability of DNA-injected bovine embryos was also compared between two different culture systems. The data for in vitro development of DNA-in-jected embryos were pooled from results of experiments which were performed in our
Ž .
laboratory over approximately one and half years March, 1995 to October, 1996 .
2.7.3. Experiment 3
To investigate whether MEF are effective on in vivo development of DNA-injected bovine embryos, DNA-injected blastocysts that developed from the two different culture systems were transferred to recipients.
2.8. Statistical analysis
Table 1
Comparison of in vitro development of Day 3 IVF-derived bovine embryos co-cultured with or without MEF for 5 days
Group Cell stage No. of embryos No. of embryos Developmental
a
Sub-total 201 1 26 22 24.4
a
No. of total blastocystsrno. of embryos cultured=100.
b
EB: early, MB: mid, EX: expanded, HB: hatched blastocysts.
c,d,e
P-0.05,f,gP-0.05.
SAS. Differences in preimplantation development of DNA-injected embryos between the two culture systems were analyzed by Student’s t-test in SAS.
3. Results
3.1. Experiment 1
To examine if MEF affect the in vitro development of IVF-derived bovine embryos, 663 immature oocytes were matured, fertilized and cultured in vitro. Out of 498 cleaved
Ž .
embryos cleavage rate: 75.1%, 498r663 at Day 3 of culture, only 4- to 8-cell stage
Ž .
embryos were selected and cultured further with or without MEF Table 1 .
Develop-Ž .
mental rate to blastocyst of the embryos co-cultured with MEF 24.4%, 49r201 was
Ž . Ž
significantly higher P-0.05 than that of embryos grown without MEF 14.4%,
.
28r194 . In both culture systems, 8-cell embryos showed a higher developmental rate
Ž .
than 4-cell embryos P-0.05 . Moreover, developmental rate to hatching or hatched
Table 2
Comparison of the in vitro development of DNA-injected embryos co-cultured with or without MEF Group No. of embryos No. of embryos No. of embryos No. of embryos
a b
Ž . Ž .
injected survived % cultured % that developed
c
Ž .
to blasotcysts % U
Ž . Ž . Ž .
CR1aa 2931 2505 85.5 1804 72.0 131 7.2
U
Ž . Ž . Ž .
MEF 3370 2516 74.6 1805 71.7 302 16.7
a
No. of embryos survivedrno. of embryos injected=100.
b
No. of embryos culturedrno. of embryos survived=100.
c
No. of blastocystsrno. of embryos cultured=100. U
Table 3
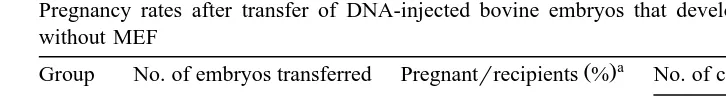
Pregnancy rates after transfer of DNA-injected bovine embryos that developed in CR1aa medium with or without MEF
a
Ž .
Group No. of embryos transferred Pregnantrrecipients % No. of calves that developed to
b
Ž .% means the pregnancy rate: pregnantrrecipients=100.
bŽ .
% : No. of calves live-bornrpregnant recipients=100.
Ž .
blastocysts of IVF-derived embryos was higher P-0.05 in the co-culture group
Ž10.9%, 22r201 than in CR1aa medium alone 4.1%, 8. Ž r194 ..
3.2. Experiment 2
Ž .
Although there was no difference in the survival 85.5% vs. 74.6% and cleavage
Ž .
rates 72.0% vs. 71.7% of DNA-injected zygotes between the two different culture systems, the developmental rate to blastocyst stage of DNA-injected embryos
co-cul-Ž .
tured with MEF 16.7%, 302r1805 was significantly higher than that of the embryos
Ž .
cultured in CR1aa medium alone 7.2%, 131r1804; P-0.01, Table 2 .
3.3. Experiment 3
When DNA-injected bovine blastocysts co-cultured with MEF were transferred to
Ž .
recipients, the pregnancy rate 27.4%, 23r84 was similar to that of the embryos grown
Ž .
in CR1aa medium without MEF 24.2%, 16r66; Table 3 . However, the proportion of
Ž .
live-born calves among was higher in the co-culture group 82.6%, 19r23 than in
Ž .
CR1aa medium alone 56.2%, 9r16 . Among calves developed from co-cultured embryos, one male calf died at 1 day after birth. This calf did not suckle and no further investigations were made into its death. The other calves in both culture groups appeared to be normal at birth and their subsequent growth was normal. We did not carry out any histopathological test on the aborted and still-born calves. Blood and ear tissue samples of the calves were analyzed for the presence of a transgene by Southern blot, but no transgenic animals were identified.
4. Discussion
The effects of MEF on the developmental ability of micromanipulated bovine embryos have not been fully investigated. There has been only one report showing improved development of bovine embryos derived from nuclear transplantation using a
Ž .
MEF co-culture system Takahashi et al., 1993 . The in vitro developmental rate to blastocysts of DNA-injected bovine embryos was very low when culture was in CR1aa
Ž .
the limiting factors in the development of transgenic cattle. The present study demon-strates that MEF are effective on improving the in vitro development of DNA-injected bovine embryos produced in vitro.
There are few reports on the in vitro development of the embryos using co-culture
Ž
system with embryonic fibroblasts in farm animals Rexroad and Powell, 1993;
Taka-.
hashi et al., 1993 . When the developmental ability of early ovine embryos co-cultured
Ž . Ž .
with oviduct cells OM , ovine embryonic fibroblasts EF , or STO cells was investi-gated, the embryos co-cultured with OM or STO cells showed a higher development to
Ž .
blastocyst stage than those of EF Rexroad and Powell, 1993 . STO cell line, a transformed MEF line, sustains lines of undifferentiated cells derived from mouse
Ž .
blastocyst inner cell mass in co-culture Evans and Kaufman, 1981 . It is known that
Ž .
STO cells secrete several cytokines such as leukemia inhibitory factor LIF , Steel factor
Ž
and basic fibroblast growth factor Dolci et al., 1991; Matsui et al., 1991; Cheng et al.,
. Ž .
1994 and potentially could affect embryonic development Schmitt et al., 1991 . However, it remains to be determined if MEF also produce cytokines. Recombinant LIF
Ž .
can maintain the pluripotentiality of ES cells Pease et al., 1990 . Addition of human LIF to synthetic oviduct fluid medium improved embryo growth when added at the
Ž .
8-cell or later stages but not if added earlier Fukui and Matsuyama, 1994 . We also reported that human LIF added at the morula stage contributed to bovine embryonic
Ž .
development through the hatching process Han et al., 1995 .
In the present study, 8-cell stage embryos in both culture systems showed a higher
Ž .
developmental rate to blastocysts than 4-cell stage embryos Table 1 . However, there was a higher rate of development for 8-cell stage embryos to hatching or hatched blastocysts in the co-culture system with MEF compared to culture system of CR1aa medium alone. This suggests that the developmental potential of IVF-derived bovine embryos may be determined at early stage of embryonic development.
The use of MEF for co-culture of mammalian embryos has several advantages over
Ž .
other somatic cells such as bovine oviductal epithelial cells BOEC , granulosa cells and cumulus cells. First, substantial quantities of MEF can be prepared in one procedure. Briefly, primary MEF reach about 1=106 cellsrfetus when confluent in a 100 mm perti dish, and then are proliferated by approximately 2.16=108 cells through three
Ž 6 .
passages 6 plates=10 cellsrpassage . Consequently, the MEF derived from one fetus
Ž 6
will produce hundreds of mololayers for embryo culture 2.4=10 cells are required for
. Ž .
monolayer and a pregnant mouse has generally several 6 to 10 fetuses. Second, co-culture of the embryos with MEF prevents contamination from the cell source because MEF cells are isolated from sterile fetuses unlike co-culture systems using cells
Ž .
of bovine origin Rehman et al., 1994a,b . Third, co-culture cells derived from cattle must be generally harvested from fresh tissue and held in vitro less than one week,
Ž .
which requires a dependable source of tissue Reed et al., 1996 . Whereas, quality-con-trolled stock aliquots of frozen MEF cells could be used for every co-culture experiment. Thus, the MEF co-culture system for bovine embryos is relatively constant and time-saving.
Ž
reported Lu et al., 1987, 1988; Goto et al., 1988; Fukuda et al., 1990; Gordon and Lu,
.
1990 . Transfer of IVF-derived bovine embryos co-cultured with BOEC resulted in a
Ž .
high pregnancy rate of 63% Xu et al., 1992 . Until now, very little has been known about the effects of the MEF co-culture system on in vivo viability after transfer of micromanipulated bovine embryos compared to culture in systems without somatic cells. In this study, the proportion of live-born calves from embryos produced in the MEF co-culture system was higher than from embryos produced in CR1aa medium alone, although there was no difference in the pregnancy rates.
In conclusion, the data suggest that MEF improve the development of IVF-derived, DNA-injected bovine embryos to the blastocyst andror hatching blastocyst stage as well as to term, although they do not have any effect on the pregnancy rate following transfer.
Acknowledgements
Ž .
This study was supported by grants HS2550 and HS2705 from the Ministry of
Ž
Science and Technology, Korea. We thank Y.K. Lee National Livestock Research
.
Institute, Suwon, Korea for statistical assistance.
References
Bavister, B.D., Yanagimachi, R., 1977. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol. Reprod. 16, 228–237.
Camous, S., Heyman, Y., Meziou, W., Menezo, Y., 1984. Cleavage beyond the block stage and survival after transfer of early bovine embryos cultured with trophoblastic vesicles. J. Reprod. Fertil. 72, 479–485. Cheng, L., Gearing, D.P., White, L.S., Compton, D.L., Schooley, K., Donovan, P.J., 1994. Role of leukemia
inhibitory factor and its receptor in mouse primordial germ cell growth. Development 120, 3145–3153. Dolci, S., Williams, D.E., Ernst, M.K., Resnick, J.L., Brannan, C.I., Lock, L.F., Lyman, S.D., Boswell, H.S.,
Donovan, P.J., 1991. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 352, 809–811.
Evans, M.J., Kaufman, M.H., 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156.
Eyestone, W.H., First, N.L., 1989. Co-culture of early cattle embryos to the blastocyst stage with oviductal tissue or in conditioned medium. J. Reprod. Fertil. 85, 715–720.
Fukuda, Y., Ichikawa, M., Naito, K., Toyoda, Y., 1990. Birth of normal calves resulting from bovine oocytes matured, fertilized, and cultured with cumulus cells in vitro up to the blastocyst stage. Biol. Reprod. 40, 114–119.
Fukui, Y., Matsuyama, K., 1994. Development of bovine embryos matured and fertilized in vitro in media containing human leukemia inhibitory factor. Theriogenology 42, 663–673.
Gagne, M., Sirard, M., 1990. Nuclear injection of bovine oocytes after in-vitro maturation. J. Reprod. Fertil.
Ž .
41, 211–212, Suppl. .
Gordon, I., Lu, K.H., 1990. Production of embryos in vitro and its impact on livestock production. Theriogenology 33, 77–87.
Hammer, R.E., Pursel, V.G., Rexroad, C.E., Wall, R.J., Bolt, D.J., Ebert, K.M., Palmiter, R.D., Brinster, R.L., 1985. Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315, 680–683. Han, Y.M., Lee, E.S., Mogoe, T., Lee, K.K., Fukui, Y., 1995. Effect of human inhibitory factor on in vitro
development of IVF-derived bovine morulae and blastocysts. Theriogenology 44, 507–516.
Han, Y.M., Park, J.S., Lee, C.S., Lee, J.H., Kim, S.J., Choi, J.T., Lee, H.T., Chung, B.H., Chung, K.S., Shin, S.T., Kim, Y.H., Lee, K.S., Lee, K.K., 1996. Factors affecting in vivo viability of DNA-injected bovine blastocysts produced in vitro. Therigenology 46, 769–778.
Han, Y.M., Yamashina, H., Koyama, N., Lee, K.K., Fukui, Y., 1994. Effects of quality and developmental stage on the survival of IVF-derived bovine blastocysts cultured in vitro after freezing and thawing. Theriogenology 42, 645–654.
Heyman, Y., Menezo, Y., Chesne, P., Camous, S., Garnier, V., 1987. In vitro cleavage of bovine and ovine early embryos; improved development using co-culture with trophoblastic vesicles. Theriogenology 27, 59–68.
Joyner, A.L., 1993. In: Gene Targeting: A Practical Approach. Oxford Univ. Press, New York, pp. 36–39. Kim, S.J., Cho, Y.Y., Lee, K.W., Yu, D.Y., Lee, C.S., Han, Y.M., Lee, K.K., 1994. Expression of human lactoferrin in milk of transgenic mice using bovine-caseinrhuman lactoferrin cDNA fusion genes. Mol. Cells 4, 355–360.
Krimpenfort, P., Rademakers, A., Eyestone, W., van der Schans, A., van der Broek, S., Kooiman, P., Kootwijk, E., Platenburg, G., Pieper, F., Strijker, R., de Boer, H., 1991. Generation of transgenic dairy cattle using in vitro embryo production. BiorTechnology 9, 844–847.
Lu, K.H., Gordon, I., Chen, H.B., Gallaghes, M., McGoven, H., 1988. Birth of twins after transfer of cattle embryos produced by in vitro techniques. Vet. Rec. 122, 539–540.
Lu, K.H., Gordon, I., Gallaghes, M., McGoven, H., 1987. Pregnancy established in cattle by transfer of embryos derived from in vitro fertilization of follicles oocytes matured in vitro. Vet. Rec. 121, 159–160. Matsui, Y., Toksoz, D., Nishikawa, S., Williams, D., Zsebo, K., Hogan, B.L., 1991. Effect of Steel factor and
leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 353, 750–752.
Parrish, J.J., Susko-Parrish, J., Winer, M.A., First, N.L., 1988. Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171–1180.
Ž .
Pease, S., Braghetta, P., Gearing, D., Grail, D., Williams, R.L., 1990. Isolation of embryonic stem ES cells
Ž .
in media supplemented with recombinant leukemia inhibitory factor LIF . Dev. Biol. 141, 344–352. Peura, T.T., Hyttinen, J.M., Tolvanen, M., Janne, J., 1994. Effects of microinjection-related treatments on the
subsequent development of in vitro-produced bovine oocytes. Theriogenology 42, 433–443.
Reed, W.A., Suh, T.K., Bunch, T.D., White, K.L., 1996. Culture of in vitro fertilized bovine embryos with
Ž .
bovine oviductal epithelial cells, buffalo rat liver BRL cells, or BRL-cell-conditioned medium. Theri-ogenology 45, 439–449.
Rehman, N., Collins, A.R., Suh, T.K., Wright, R.W. Jr., 1994a. Development of in vitro matured and fertilized bovine oocytes co-cultured with buffalo rat liver cells. Theriogenology 41, 1453–1462.
Rehman, N., Collins, A.R., Suh, T.K., Wright, R.W. Jr., 1994b. Development of IVM–IVF produced 8-cell bovine embryos in simple, serum-free media after conditioning or co-culture with buffalo rat liver cells. Mol. Reprod. Dev. 38, 251–255.
Rexroad, C.E. Jr., Powell, A.M., 1993. Development of ovine embryos co-cultured on oviductal cells, embryonic fibroblasts, or STO cell monolayers. Biol. Reprod. 49, 789–793.
Rosenkrans, C.F. Jr., Zeng, G.Q., McNamara, G.T., Schoff, P.K., First, N.L., 1993. Development of bovine embryos in vitro as affected by energy substrates. Biol. Reprod. 49, 459–462.
Schmitt, R.M., Bruyns, E., Snodgrass, H.R., 1991. Hematopoietic development of embryonic stem cells in in vitro: cytokine and receptor gene expression. Genes Dev. 5, 728–740.
Takahashi, S., Kita, M., Imai, H., 1993. Studies on in vitro development of nuclear transplant bovine embryos.
Ž .
Annual Report of Ito Memorial Foundation 11, 6–11 in Japanese .