A hypothetical model of the influence of inorganic
phosphate on the kinetics of pyruvate kinase
Marian Kuczek *
Institute of Biology and En6ironmental Sciences,Uni6ersity of Opole,Kominka4,45-035Opole,Poland
Received 19 February 1999; received in revised form 30 July 1999; accepted 31 August 1999
Abstract
This paper presents a simple solution to the problem of approximating the calculated curve of reaction progress to the measured curve which is usually disturbed by initial oscillation of auxiliary lactate dehydrogenase (LDH) reaction. The experiments leading to the determination of the apparent Km for phosphoenolpyruvate (PEP) and Vm were
performed. For precise estimation of kinetic parameters (KmandVm) of the M1isozyme of pyruvate kinase (PK),
measured by coupling it to LDH reaction, the sequence of Michaelis – Menten for pyruvate kinase and second-order kinetics for lactate dehydrogenase reaction as well as a non-zero initial concentration of lactate was assumed. The functions of apparent Km and Vm of pyruvate kinase with respect to phosphate concentration, computed by an
analysis of the total reaction progress curves, indicate that the reaction mixture contains an uncompetitive inhibitor of pyruvate kinase, and that the phosphate binds this inhibitor. The proposed simple mathematical model of pyruvate kinase Km and Vm increase by inorganic phosphate assumes that the pyridine nucleotides (NAD-derivatives) are
kinase inhibitors. An approximate dissociation constant for pyridine nucleotides – phosphate complex and trueKmof
pyruvate kinase for PEP were estimated. The proposed model fits exactly the entire measured reaction process. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Enzyme kinetics; Complex reaction; Consecutive reaction; Enzyme inhibition
www.elsevier.com/locate/biosystems
1. Introduction
The regulatory mechanism of isozymes of mus-cle pyruvate kinase (PK) has been studied for a long time and has been a source of controversy (Oberfelder et al., 1984; Consler et al., 1992; Farrar and Farrar, 1995; Friesen et al., 1998). Now the mechanism of regulation of PK M gene
transcription is well known. It is also known that the M1type isozyme is non-allosteric (for a review
see, e.g. Yamada and Noguchi 1999). However, the mechanism of the increase of pyruvate kinase reaction rate and its Km for ADP by inorganic
phosphate (Baranowska et al., 1984) still remains obscure.
Often the lactate dehydrogenase (LDH) is used as an auxiliary enzyme to measure the velocity of a pyruvate kinase catalysed reaction. The progress of this reaction can be followed by measuring the
* Fax: +48-77-45-45467.
E-mail address:[email protected] (M. Kuczek)
decrease in the absorbance at 340 nm, that is, at the absorption maximum for NADH. The LDH (auxiliary) reaction is oscillatory and gives rise to certain ‘initial effects’ described in literature (Burgner and Ray, 1984a,b; Goldshtein and Ivanova, 1988). The coupled pyruvate kinase and lactate dehydrogenase reaction is made especially complicated by these LDH initial effects and by the spontaneous NADH decomposition reaction, parallel with the LDH catalysed dehydrogenation. Due to the sequence of reactions and the com-plexity of the LDH reaction, a short time lag or even initial increase in absorbance at 340 nm before its decrease is usually observed. It is clear that these initial effects, being the result of two consecutive reactions, influence the shape of the entire recorded reaction curve. The manner in which the lactate dehydrogenase reaction occurs makes it difficult to estimate the kinetic parame-ters of the pyruvate kinase reaction. In particular, the estimation of the initial velocity of the pyru-vate kinase reaction is distorted by these effects and, in consequence, the estimate of Michaelis constant of pyruvate kinase from initial velocity is imprecise. Estimation of kinetic parameters (Km
and Vm) of primary-pyruvate kinase reaction by
analysis of the entire reaction progress curve (a calculation using integrated velocity equations) requires the solution of a system of differential equations for two consecutive enzymatic and one parallel spontaneous reaction (Fig. 1).
The integration of Michaelis – Menten differen-tial equation yields an implicit form for the sub-strate concentration at any given time, which is known as the time function with respect to sub-strate concentration (Yun and Suelter, 1977). Therefore, the analytical solution of consecutive
enzymatic reaction equations is complicated. Re-cently, numerical and analytical solutions of the system of two differential equations for successive reactions, one: Michaelis – Menten, the other: sec-ond-order, to compute the lactate dehydrogenase
Kmin pyruvate kinase assay conditions have been
reported (Kuczek, 1998). This paper describes an identical approach to finding the solution of pyru-vate kinase and lactate dehydrogenase coupled reaction. This solution allows the fitting of the calculated curve of reaction progress to experi-mental data with the correlation coefficient very close to unity.
2. Materials and methods
Pyruvate free phosphoenolpyruvate (K salt) was prepared from phosphoenolpyruvic acid (We-ichsel et al., 1989) and potassium hydroxide, the product was crystallised twice from water – ethanol. NADH – Na2 and ADP – K were
pur-chased from Sigma, the crystal suspension of bovine heart lactate dehydrogenase (200 U/mg protein) was purchased from Boehringer and bovine brain pyruvate kinase M1 type (160 U/mg
protein) was obtained from Dr G. Terlecki from Wrocl*aw Medical University (Terlecki, 1989). The enzymes were diluted with 0.14 M sodium chlo-ride before use. PEP, ADP and NADH solutions were neutralised with imidazol before use. All other chemicals were obtained from POCh (Poland).
All spectra were recorded with a M-40 spec-trophotometer (Germany) at 25°C.
The initial rate of the pyruvate kinase reaction was estimated spectrophotometrically by deter-mining the decrease of NADH, in a reaction coupled with lactate dehydrogenase according to Baranowska et al. (1984). Enzyme activity was expressed in units (U), as micromoles of NADH dehydrogenated per minute. The molar ab-sorbance change of NADH at 340 nm of 6.22×
103 M−1cm−1was assumed. The standard assay
system: 0.1 M potassium chloride, 15 mM magne-sium chloride, 2.5 mM ADP, 0.13 mM NADH, 0.7 mM PEP and 1 U of LDH in a volume of 2.4 cm3 of 0.1 M imidazole – HCl, 0.1 M. potassium
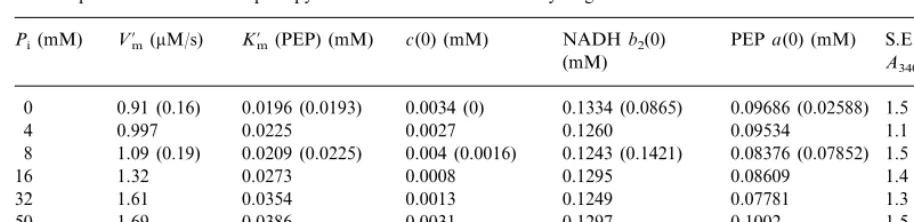
Table 1
Kinetic parameters of the coupled pyruvate kinase and lactate dehydrogenase reactiona K%m(PEP) (mM) c(0) (mM)
Pi(mM) V%m(mM/s) NADHb2(0) PEP a(0) (mM) S.E. (10−4
A340 nm) (mM)
0.0196 (0.0193)
0 0.91 (0.16) 0.0034 (0) 0.1334 (0.0865) 0.09686 (0.02588) 1.5 (2.4)
0.0225 0.0027 0.1260
4 0.997 0.09534 1.1
0.0209 (0.0225) 0.004 (0.0016) 0.1243 (0.1421)
1.09 (0.19) 0.08376 (0.07852)
8 1.5 (2.5)
1.32
16 0.0273 0.0008 0.1295 0.08609 1.4
1.61
32 0.0354 0.0013 0.1249 0.07781 1.3
0.0386 0.0031 0.1297
1.69 0.1002
50 1.5
88 2.01 (0.32) 0.0434 (0.0450) 0.0019 (0.0016) 0.1375 (0.1261) 0.09881 (0.0791) 0.9 (3.2)
aThe results of approximation of Eq. (2) and integrated Eqs. (3) and (4) for the measured absorbance at 340 nm. The parameters
calculated for reaction taking place under reduced activity of kinase are shown in parentheses. The values ofk2:1240 M−1s−1
(0.2 as the rate for absorbance) andk0=0.8 nM/s were adopted. S.E. for calculated and measured absorbance.
phosphate or mixed imidazole – phosphate buffer pH 7.4.
The assay mixture was incubated for 10 min at 25°C and lactate dehydrogenase diluted with 0.14 M NaCl was added to control the contamination with endogenous pyruvate. To record the whole progress of the reaction, 1 U of LDH and
0.1 – 0.15 U of PK (assayed in appropriate buffers) per sample were used. The initial NADH and PEP concentrations are listed in Table 1. Thirty-four to 70 values of absorbance for each progress curve were recorded. Some of the measurements were repeated using approximately one fifth of amount of pyruvate kinase in the sample.
All calculations and numerical solutions of dif-ferential equations were performed using Maple V r. 4 program from Waterloo Maple Inc.
3. Kinetic analysis
To compute the decrease in PEP concentration depending on pyruvate kinase activity I assumed a simple pseudo first-order Michaelis – Menten mechanism of reaction and simple second order reaction for the LDH reaction. This simplification is justified because the ADP concentration ex-ceeds several times theKmof pyruvate kinase for
ADP, and ADP concentration is more than ten times that of PEP; and the reaction of pyruvate with NADH takes place under a large excess of LDH activity in relation to PK activity, i.e. under
low pyruvate concentration. Looking at expres-sions (1) and (2) in Fig. 1 the equilibrium of both reactions is shifted far to the right hand side and the reverse reactions can be disregarded. For the very low rate, independent of the discussed en-zymes, reaction of NADH (with oxygen) a zero-order reaction mechanism has been assumed. The general mechanism studied in this paper is Scheme 1.
The kinetics of the concentration of the reacting substances involved in Scheme 1 are described by Eqs. (1) – (4). In these equations the square brack-ets denoting concentration were omitted.
The concentration of pyruvate in time b1(t) is
equal to the difference between the initial concen-tration of PEP a(0) and the remaining PEP a(t) together with the lactate concentration c1(t).
b1(t)=a(0)−a(t)−c1(t) (1)
The function of NADH concentration in time
b2(t) is equal to its initial concentration b2(0)
minus the formed lactate c1(t) and the
sponta-neously decomposed NADH, which is in turn equal to the product of the zero-order reaction rate k0 and timet:
b2(t)=b2(0)−c1(t)−k0t (2)
The simple Michaelis – Menten equation was as-sumed for the rate of change of PEP:
da(t) dt =
−Vma(t)
Km+a(t) (3)
The rate of increase in lactate concentration takes the form of a kinetic equation for a second-order reaction as shown below:
dc1(t)
dt =k2(a(0)−a(t)−c1(t))(b2(0)−c1(t)−k0t)
(4)
where k2 is the second-order reaction rate
constant.
To find the optimal parameters of the reaction rate equations the least mean square method of fitting the calculated absorbance at 340 nm to measured data was used. To compensate for the delay in recording of absorbency after the addi-tion of pyruvate kinase, and to take into account the initial oscillations of the LDH reaction, a non zero value of c(0) was assumed. The molar ab-sorption coefficient of 6220 M−1 cm−1 for
NADH was used to convert the parameters calcu-lated for absorbance to parameters expressed for concentration.
The possibility that the reaction mixture con-tains an endogenous uncompetitive inhibitor and that the phosphate reversibly binds this inhibitor was also taken into account, because kinetic parameters (MmandKm) increased along with the
increase of inorganic phosphate concentration in the sample, i.e. its effect is opposite to this of uncompetitive inhibitor influence. The expression for the steady-state concentration of the inhibitor can be written in the simplified form as:
i= itK
K+Pi
(5)
where i is the steady-state concentration of in-hibitor, it is the total concentration of the
in-hibitor, K is the dissociation constant for the inhibitor – phosphate complex, and Pi is the
con-centration of the inorganic phosphate. By substi-tuting Eq. (5) into the equation for apparent K%m
(determined in the presence of the uncompetitive
inhibitor) the following expression for K%m can be obtained:
K%m= Km
1+ itK
(K+Pi)Ki
(6)
Similarly the expression for the apparent V%mcan
be rewritten:
V%m= Vm
1+ itK
(K+Pi)Ki
(7)
Inserting Eq. (5) into the rate equation contain-ing parameters for uncompetitive inhibition (Kato and Shimotohno, 1984) gives the differential Eq. (8) which can be used instead of Eq. (3):
da(t)
The simple expressions for substrate and product inhibition as well as for simple reversible substrate(s) interaction with ligand (phosphate) described in literature (Yun and Suelter, 1977; Kato and Shimotohno, 1984; Skalecki et al., 1995) were also taken into account.
4. Results and discussion
The apparent kinetic parameters for seven dif-ferent values of phosphate concentration calcu-lated on the basis of differential Eqs. (3) and (4) and the balance Eq. (2) are presented in Table 1. The parameters of reaction are averages for sev-eral independent experiments with an average variation no greater than 10%. These calculations show that apparent K%m for PEP increases with
respect to phosphate concentration in the buffer as do apparentV%mand the apparentK%mfor ADP
(Baranowska et al., 1984).
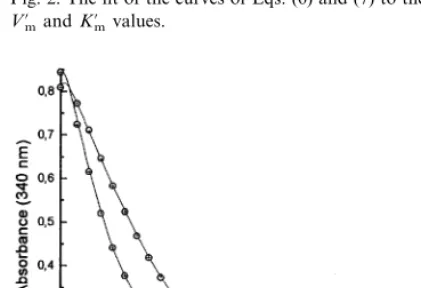
in-Fig. 2. The fit of the curves of Eqs. (6) and (7) to the apparent
V%mandK%mvalues.
lations. Therefore the simplest model of interac-tion of NADH and NAD+ with enzyme –
substrates complex was chosen.
Assuming that the total concentration of in-hibitor is equal to the initial NADH concentra-tion, curves of Eqs. (6) and (7) were fitted to the values of apparent K%m and V%m calculated from Eqs. (3), (4) and (2), to obtain the real Km
for PEP, Ki and K. The fitted curves of K%m and
V%m, with respect to phosphate concentration, and the calculated actual value of Km, Ki,K and
Vm are presented in Fig. 2. Almost the same fit
was obtained when Km=Ki was assumed; the
estimated parameters in this case were: K=2.0 mM, Km andKi=0.061 mM.
The solutions of the system of Eqs. (8), (4) and (2) were fitted to the measured progress curves of absorbance at 340 nm. A large value, greater than 0.9998, of correlation coefficient for experimental and calculated absorbance data was obtained after substituting the above parameters, Km=Ki, K and it=b2(0), into Eq.
(8). The result of fitting the two curves is pre-sented in Fig. 3. This fit shows only a small (2%) discrepancy between the Vm measured in
the phosphate and in imidazole (free of phos-phate) buffer.
According to earlier observations (Kuczek, 1998), a reduction of LDH Km value occurs in
PEP concentration assay conditions. This time a drop in LDH activity was observed in a buffer designed for pyruvate kinase activity assay, i.e. containing PEP, ADP and magnesium chloride. It is a well known fact that some products of NAD+ decomposition are LDH inhibitors, so it
is possible that the PEP, ADP, ATP and mag-nesium ion are also inhibitors. It seems possible that NADH and NAD are able to inhibit the pyruvate kinase. Because of the complex nature of phosphate heteropolyacids and their salts other explanations of the phenomenon of the increase in pyruvate kinase Km and Vm by
inor-ganic phosphate are also possible. Nevertheless, the proposed model is the simplest one and fits perfectly to the experimental data, and it was shown that discrepancies are comparable with measurement errors.
Fig. 3. The fit of the curves of equation system (Eqs. (8), (4) and (2)) to the data of absorbance at 340 nm. The progress of the coupled pyruvate kinase and lactate dehydrogenase reac-tions in imidazole buffer and in phosphate buffer. Circles, experimental data; lines or crosses, calculated data. Parameters of reaction: K=2.0 mM, Km and Ki=0.061 mM, Vm 2.88 mM−1s−1; S.E. 1.7×10−4A340(in imidazole buffer) andVm
2.94mM−1s−1; S.E. 2.3×10−4A340 (in phosphate buffer).
calcu-Acknowledgements
The calculations were performed on computers of the Wrocl*aw Centre of Networking and Super-computing. The author would also like to thank Dr G. Terlecki for providing M1type pyruvate kinase.
References
Baranowska, B., Terlecki, G., Baranowski, T., 1984. The influence of inorganic phosphate and ATP on the kinetics of bovine heart muscle pyruvate kinase. Mol. Cell. Biochem. 64, 45 – 50.
Burgner, J.W., Ray, W.J. Jr, 1984a. The lactate dehydrogenase catalyzed pyruvate adduct reaction: simultaneous general acid – base catalysis involving an enzyme and an external catalyst. Biochemistry 23, 3626 – 3635.
Burgner, J.W., Ray, W.J. Jr, 1984b. On the origin of the lactate dehydrogenase induced rate effect. Biochemistry 23, 3636 – 3648.
Consler, T.G., Jennewein, M.J., Cai, G.Z., Lee, J.C., 1992. Energetics of allosteric regulation in muscle pyruvate ki-nase. Biochemistry 31, 7870 – 7878.
Farrar, G., Farrar, W.W., 1995. Purification and properties of the pyruvate kinase isozyme M1from the pig brain. Int. J.
Biochem. Cell. Biol. 27, 1145 – 1151.
Friesen, R.H.E., Castellani, R.J., Lee, J.C., Braun, W., 1998. Allostery in rabbit pyruvate kinase: development of a
strategy to elucidate the mechanism. Biochemistry 37, 15266 – 15276.
Goldshtein, B.N., Ivanova, A.N., 1988. Simple kinetic models explaining critical phenomena in enzymatic reactions with isomerization of the enzyme and substrate. Mol. Biol. Mosk. 22, 1381 – 1392.
Kato, T., Shimotohno, K., 1984. Estimation of kinetic parameters for substrate and inhibitor in a reaction with an enzyme sample containing different types of inhibitor. Biochim. Biophys. Acta 801, 157 – 162.
Kuczek, M., 1998. Simplified solution of lactate dehydroge-nase catalysed reaction. Cell. Mol. Biol. Lett. 3, 111 – 117. Oberfelder, R.W., Lee, L.L., Lee, J.C., 1984. Thermodynamic linkages in rabbit muscle pyruvate kinase: kinetic, equi-librium, and structural studies. Biochemistry 23, 3813 – 3821.
Terlecki, G., 1989. Purification and properties of pyruvate kinase type M1 from bovine brain. Int. J. Biochem. 21,
1053 – 1089.
Skalecki, K., Mularczyk, W., Dzugaj, A., 1995. Kinetic prop-erties of D-fructose-1,6-bisphosphate 1-phosphohydrolase
isolated from human muscle. Biochem. J. 310, 1029 – 1035. Weichsel, A., Lis, T., Kuczek, M., 1989. The crystal and molecular structure of the phosphoenolpyruvic acid. Car-bohydr. Res. 194, 63 – 70.
Yamada, K., Noguchi, T., 1999. Nutrient and hormonal regu-lation of pyruvate kinase gene expression. Biochem. J. 337, 1 – 11.
Yun, S.L., Suelter, C.H., 1977. A simple method for calculat-ing Km and V from a single enzyme reaction progress
curve. Biochim. Biophys. Acta 480, 1 – 13.
.