www.elsevier.nlrlocateraqua-online
Tolerance of Macrobrachium rosenbergii to white
spot syndrome virus
A.S. Sahul Hameed
a,), M. Xavier Charles
a, M. Anilkumar
ba
Aquaculture DiÕision, Department of Zoology, C. Abdul Hakeem College, MelÕisharam, 632509 Vellore
Dist. Tamil Nadu, India
b
Centre for Biotechnology, Anna UniÕersity, Chennai, 600025, India Accepted 22 August 1999
Abstract
The susceptibility of Macrobrachium idella, M. lamerrae and M. rosenbergii to white spot
Ž .
syndrome virus WSSV was tested by immersion challenge, oral route and intramuscular injection. Their susceptibility to WSSV was compared with that of Penaeus indicus and P.
monodon. The WSSV caused 43.3% and 53.3% mortality in M. lamerrae and M. idella,
respectively, by immersion method and 53.3% and 66.7% mortality in M. lamerrae and M. idella, respectively, by oral route. This virus caused 100% mortality in M. idella, M. lamerrae, P. indicus and P. monodon when the animals were injected WSSV intramuscularly. Moribund animals were screened for the presence of WSSV by western blot or histopathology. The results indicated the susceptibility of marine shrimp and freshwater prawn to this virus except M. rosenbergii. This virus failed to produce mortality with any of the methods of infection applied in M. rosenbergii. The exact mechanism of tolerance of M. rosenbergii to WSSV is not known at present and the possibilities for this tolerance are discussed.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Susceptibility; Macrobrachium; Penaeus; White spot virus
1. Introduction
Ž .
White spot syndrome virus WSSV has been reported to cause severe mortalities of Ž
cultured penaeid shrimp in several parts of Asia including India Takahashi et al., 1994; .
Chen, 1995; Wongteerasupaya et al., 1995; Inouye et al., 1996 . The loss caused by this virus has been estimated to be several million dollars in different parts of India
)Corresponding author. e-mail: [email protected]
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
ŽAnonymous, 1996 . Considerable amount of work has been carried out on this virus in. Ž
recent years Takahashi et al., 1994, 1996; Chou et al., 1995; Wongteerasupaya et al., 1995; Lo et al., 1996; Nunan and Lightner, 1997; Karunasagar et al., 1997; Mohan et al.,
.
1997; Wang et al., 1997; Nunan et al., 1998; Sahul Hameed et al., 1998 . The WSSV has been isolated from Penaeus monodon and its morphology has been studied by Sahul
Ž .
Hameed et al. 1998 . The WSSV was enveloped and elliptical in shape with measure-ment of 266=112 nm, and the nucleocapsid of WSSV was cylindrical in shape Ž420=68 nm with one end flat and other pointed, and having a pattern of opaque and.
Ž transparent striations arranged perpendicular to the long axis of the nucleocapsid Sahul
. Hameed et al., 1998 .
The WSSV has been found to be highly pathogenic to P. indicus and P. monodon ŽSahul Hameed et al., 1998 . The information regarding the pathogenesis of WSSV in. other crustacean species is limited. Experimental infection by WSSV has been carried
Ž .
out in Portunus pelagicus, Scylla serrata and Acetes sp. Supamattaya et al., 1998 . Our current study examines the infectivity and pathogenicity of the WSSV to the juveniles and adults of Macrobrachium idella, M. lamerrae, M. rosenbergii, P. indicus
and P. monodon and relative susceptibility of the freshwater prawn and marine shrimp
to this virus is compared using the mortality data from this study. The ultimate objective of this work was to screen the tolerant crustacean species to this virus in an attempt to lay the basis for a solution for this killer virus through genetic manipulation.
2. Materials and methods
2.1. Preparation ofÕiral inoculum
Ž .
WSSV-infected P. monodon with prominent white spots 200 numbers were col-lected from shrimp farms located near Nellore, India. The hemolymph was drawn directly from the heart of infected shrimp using sterile syringes followed by centrifuga-tion at 3000=g for 20 min at 48C. The supernatant fluid was re-centrifuged at 8000=g
for 30 min at 48C and the final supernatant fluid was filtered through a 0.4 mm filter. The filtrate was then stored at y208C for infectivity studies. The total protein in
Ž .
hemolymph was determined by the method of Lowry et al. 1951 .
2.2. Collection of test animals
Ž . Ž .
Juveniles 1–2 g and adults 5–7 g of M. idella and M. lamerrae were collected from Palar river near Arcot, and the juveniles and adults of M. rosenbergii were respectively collected from a hatchery and farm located near Chennai. The animals were
Ž .
Ground-spring water from a common source was used in all the experiments Ž
conducted on freshwater prawns. The water was filtered through filter paper Sartorius,
. Ž y1.
0.2 mm pore size and exposed to ultraviolet light 30 mW s to sterilize it for the juveniles. For the adults of freshwater prawns, the water was chlorinated and the dechlorinated water was passed through a sand filter and used for the experiments. Natural seawater was used to maintain the marine shrimps for the experiments. It was pumped from the adjacent sea and allowed to sediment to get rid of sand and other particles. The seawater was chlorinated and the dechlorinated water was passed through a sand filter and used for the experiments.
From the experimental animals, five per species were randomly selected and screened for the WSSV by Western blot using polyclonal antibodies raised against the WSSV ŽSahul Hameed et al., 1998 or histological observation. After screening, the healthy. animals were used for infectivity experiments.
2.3. InfectiÕity studies
In the present study, the pathogenicity of WSSV was tested by immersion challenge, oral administration of WSSV through food or intramuscular injection of WSSV.
2.3.1. Experimental infection by bath exposure
For the immersion method of infection, juveniles of M. idella, M. lamerrae or M.
Ž .
rosenbergii 10 per tank were maintained separately in 15-l aquarium tanks at 27–308C. The inoculum of WSSV was introduced to the water at a volume equal to 0.1% of the
Ž .
total rearing medium 1 mlrl . The control groups were exposed to the hemolymph Ž0.1% collected from healthy shrimp. Each trial was conducted in triplicate..
2.3.2. Oral infection
For oral infection, 10 juveniles of M. idella, M. lamerrae, M. rosenbergii, P. indicus or P. monodon were placed separately in a 15-l aquarium tank and starved for 24 h. The animals were fed with WSSV-infected shrimp with prominent white spot at the rate of 5% body weightrday. The shrimp meat divided into three portions and given at intervals of 8 h for 3 days. After the last feeding, the animals were fed commercial feed. In the control group, the animals were fed with uninfected shrimp meat followed by commer-cial feed. Each experiment was conducted in triplicate.
2.3.3. InfectionÕia intramuscular injection
Ž .
Adult freshwater prawns 10 per group and tank were maintained in 100-l aquarium
Ž . Ž
tank at room temperature 27–308C in freshwater whereas marine shrimp 10 per group .
and tank were maintained in tanks at room temperature with salinity ranging between 20 and 25 ppt. The experimental animals were inoculated intramuscularly with
Ž .
In all the experiments, animals were examined twice per day for clinical signs of disease; the number of deaths were recorded and the cumulative percentage mortality was calculated.
2.4. Confirmation of pathogenicity
The specific action of WSSV as a pathogen was confirmed by screening moribund animals for WSSV by above mentioned diagnostic methods. The hemolymph was collected from moribund animals and processed for western blot to detect the WSSV
Ž .
according to the method of Sahul Hameed et al. 1998 . For histological observations, eye stalk, gill or cephalothoracic muscle was cut and preserved in Davidson’s fixative for 48 h and then transferred to 70% alcohol for subsequent histological preparation ŽBell and Lightner, 1998 . Sections of 4–5. mm in thickness were stained with haema-toxylin and eosin.
3. Results and discussions
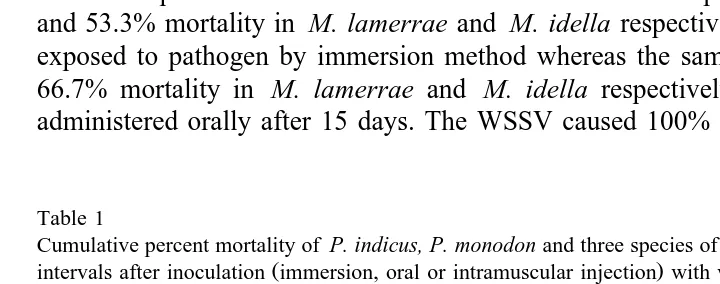
The cumulative percent mortalities for Macrobrachium and Penaeus species are presented in Table 1. The susceptibilities of three species of freshwater prawn and two species of marine shrimp to WSSV were compared and the results indicated that the freshwater prawns were more tolerant than the marine shrimp. The WSSV caused 43.3% and 53.3% mortality in M. lamerrae and M. idella respectively when the animals were exposed to pathogen by immersion method whereas the same virus caused 53.3% and 66.7% mortality in M. lamerrae and M. idella respectively when the pathogen was administered orally after 15 days. The WSSV caused 100% mortality in P. indicus, P.
Table 1
Cumulative percent mortality of P. indicus, P. monodon and three species of Macrobrachium at different time
Ž .
intervals after inoculation immersion, oral or intramuscular injection with white spot virus Time P. indicus P. monodon M. Idella M. lamerrae M. rosenbergii
i.m. Oral i.m. Oral Immersion i.m. Oral Immersion i.m. Oral Immersion i.m. Oral
12 h 0 0 0 0 0 0 0 0 0 0 0 0 0
24 h 19.9 0 33.3 0 0 0 0 0 0 0 0 0 0
36 h 36.0 0 53.3 0 0 30.0 0 0 0 0 0 0 0
48 h 76.6 0 100.0 0 0 46.6 0 0 19.9 0 0 0 0
60 h 93.2 0 0 0 59.9 0 0 26.6 0 0 0 0
72 h 100.0 0 16.7 0 66.7 0 0 40.0 0 0 0 0
96 h 0 43.3 0 73.3 0 0 69.9 0 0 0 0
5 days 26.6 93.2 0 100.0 0 0 76.6 20.0 0 0 0
8 days 100.0 100.0 20.0 30.0 20.0 100.0 33.3 0 0 0
10 days 40.0 46.6 33.3 46.6 0 0 0
15 days 53.3 66.7 43.3 53.3 0 0 0
Immersion — Animals were immersed with 0.1% hemolymph of white spot virus-infected shrimp. Oral — Animals were fed with white spot-infected shrimp.
Ž .
monodon, M. lamerrae and M. idella at the end of 72 h, 48 h, 5 days and 8 days
post-inoculum respectively when the animals were injected with the viral inoculum intramuscularly.
The results obtained in the case of M. rosenbergii were surprising, thus WSSV failed to produce any mortalities in this species by any of the three methods of infection
Ž
applied. We repeated the pathogenicity experiments three times 3–4 replicates in each .
time with M. rosenbergii to confirm these results. From these, 15 animals were selected and WSSV were injected intramuscularly three times at the interval of 1 month and every time the dosage of viral inoculum was increased to double the volume of the previous injection. The animals only response to WSSV was a degree of lethargy apparent for 24 h during the second day.
The clinical signs observed in experimentally infected animals were lethargy and lack of appetite. The uropods, telson, pereiopods and pleopods became reddish in colour. The white spots were observed in the cephalothoracic region of most of the dying animals. In the case of M. lamerrae and M. idella, the white spots were minute. The behavioural pattern included reduced swimming activity, disorientation during swimming and swim-ming on one side. The juveniles of M. rosenbergii did not show any clinical signs of infection whereas the adult M. rosenbergii became lethargic on the second day post-inoculum, but recovered the next day and became normal. In control groups, the animals were normal and healthy, and no mortality was observed.
The Western blot analysis on moribund animals revealed a distinct band of 27 kDa of viral protein, and no background coloration was observed in the control groups. In addition, histological studies of moribund animals revealed degenerated cells, character-ized by basophilic intranuclear inclusion bodies in hypertrophied nuclei of ectodermal and mesodermal cells, whereas no inclusion body was found in the cells of control animals. Basophilic intranuclear inclusions in the hypertrophied nuclei of infected cells
Ž
are the specific diagnostic characteristics of WSSV Wongteerasupaya et al., 1995; .
Lightner, 1996; Wang et al., 1997 . These studies confirm the specific action of WSSV as a pathogen on marine shrimp and freshwater prawns.
The penaeid viruses have been placed in five major families namely Parvoviridae, Ž
Baculoviridae, Reoviridae, Rhabdoviridae and Togaviridae Lu et al., 1991; Lightner et .
al., 1992 . Among these viruses, baculoviruses have been reported to cause a major loss Ž
in penaeid culture systems Boonyaratpalin et al., 1993; Wongteerasupaya et al., 1995; .
Sahul Hameed et al., 1998 . Our current study examined the susceptibility of three species of freshwater prawn and the results showed that M. lamerrae and M. idella were susceptible to WSSV whereas M. rosenbergii was resistant. The results indicate that marine shrimp were more susceptible to WSSV than freshwater prawn. Similar differences in susceptibility to viral, bacterial and fungal infection among penaeid
Ž
shrimp have been reported Lightner et al., 1979; Lu et al., 1994; Sahul Hameed, 1995; .
Sahul Hameed et al., 1998 .
The susceptibilities of many species of decapods to WSSV have been investigated by
Ž .
that the WSSV maintains its infectivity in the freshwater as observed by Chang et al. Ž1998 in P. monodon..
It is worth noting that among the three species of freshwater prawn tested, M.
rosenbergii is considered to be tolerant to the infection caused by WSSV. The
mechanism of resistance to WSSV is not known; resistance in some invertebrates includes the production of bactericidins, lysins and agglutinins. These factors in certain invertebrates following exposure to foreign protein may account in part for increased
Ž .
resistance to certain pathogens Bang, 1967; Mckay and Jenkin, 1969 . This might be the reason for the resistance of M. rosenbergii to WSSV. Further studies need to be carried out to determine the basis for the resistance of M. rosenbergii to WSSV and this will help in the development of disease resistant varieties of Penaeus species.
Acknowledgements
First and second authors thank the Management of C. Abdul Hakeem College for providing the facilities to carry out this work. Authors also thank Dr. Kunthala Jayaraman and Dr. P. Kaliraj, Centre for Biotechnology, Anna University, Chennai for their constant encouragement during this study.
References
Anonymous, 1996. Report of Marine Products Export Development Agency. The Hindu, June 4, pp. 17. Bang, F.B., 1967. Serological responses among invertebrates other than insects. Fed. Proc. 267, 1680–1684. Bell, T.A., Lightner, D.V., 1998. A Hand book of Normal Penaeid Shrimp Histology. World Aquaculture
Society, Baton Rouge, FL, pp. 2–6.
Boonyaratpalin, S., Supamattaya, K., Kasornchandra, J., Direkbusaracom, S., Aekpanithanpong, U., Chantana-chookin, C., 1993. Non-occluded baculo-like virus, the causative agent of yellow-head disease in the black
Ž . Ž .
tiger shrimp Penaeus monodon . Gyobyo Kenkyu 26 3 , 103–109.
Chang, P.S., Chen, L.J., Wang, Y.C., 1998. The effect of ultraviolet irradiation, heat, pH, ozone, salinity and chemical disinfectants on the infectivity of white spot syndrome baculovirus. Aquaculture 166, 1–17.
Ž .
Chen, S.N., 1995. Current status of shrimp aquaculture in Taiwan. In: Browdy, C.L., Hopkins, J.S. Eds. , Swimming Through Troubled Water, Proceedings of the Special Session of Shrimp Farming. Aquaculture’ 95. World Aquaculture Society, Baton Rouge, LA, USA, pp. 29–34.
Chou, H.Y., Huang, C.Y., Wang, C.H., Chiang, H.C., Lo, C.F., 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured Penaeid shrimp in Taiwan. Dis. Aquat. Org. 23, 165–173. Inouye, K., Yamano, K., Ikeda, N., Kimura, T., Nakano, H., Momoyama, K., Kobayashi, J., Miyajima, S.,
Ž . Ž .
1996. The penaeid rod shaped DNA virus PRDV , which causes penaeid acute viremia PAV . Fish Pathol. 31, 39–45.
Karunasagar, I., Otta, S.K., Karunasagar, I., 1997. Histopathological and bacteriologicalstudy of white spot syndrome of Penaeus monodon along the west coast of India. Aquaculture 153, 9–13.
Lightner, D.V., 1996. A handbook of pathology and diagnostic procedures for diseases of penaeid shrimp. Special publication of the World Aquaculture Society, Boca Rouge, LA.
Lightner, D.V., More, D.W., Donald, D.A., 1979. A mycotic disease of cultured penaeid shrimp caused by
Ž .
Lightner, D.V., Poulos, B.T., Bruce, L., Redman, R.M., Mari, J., Bonami, J.R., 1992. New developments in penaeid virology. Application of biotechnology in research and disease diagnosis for shrimp viruses of
Ž .
concern in the Americas. In: Fulks, W., Main, K.L. Eds. , Diseases of cultured penaeid shrimp in Asia and the United States, The Oceanic Institute, Honolulu, pp. 233–256.
Lo, C.F., Leu, J.H., Ho, C.H., Chen, C.H., Peng, S.E., Chen, Y.T., Chou, C.M., Yeh, P.Y., Huang, C.J., Chou, H.Y., Wang, C.H., Kou, G.H., 1996. Detection of baculovirus associated with white spot syndrome virus
ŽWSSV in Penaeid shrimp using polymerase chain reaction. Dis. Aquat. Org. 25, 133–141..
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the follin phenol reagent. J. Biol. Chem. 193, 265–275.
Lu, Y., Nadala, E.C.B. Jr., Brock, J.C., Loh, P.C., 1991. A new virus isolate from infectious hypodermal and
Ž .
hematopoietic necrosis virus IHHNV -infected penaeid shrimps. J. Virol. Methods 31, 189–196. Lu, Y., Tapay, L.M., Loh, P.C., Brock, J.A., 1994. Infection of the yellow-head baculo-like virus in two
species of Penaeid shrimp, Penaeus stylirostris and P.Õannamei. J. Fish Dis. 17, 649–656.
(
Mckay, D., Jenkin, C.R., 1969. Immunity in invertebrates: II. Adaptive immunity in the cray fish Parachaeraps
)
bicarinatus . Immunology 17, 127–137.
Mohan, C.V., Sudha, P.M., Shankar, K.M., Hedge, A., 1997. Vertical transmission of white spot baculovirus in shrimps — a possibility?. Current Science 73, 109–110.
Nunan, L.M., Lightner, D.V., 1997. Development of a non-radioactive gene probe by PCR for detection of
Ž .
white spot syndrome virus WSSV . J. Virol. Methods 63, 193–201.
Ž .
Nunan, L.M., Poulos, B.T., Lightner, D.V., 1998. The detection of white spot syndrome virus WSSV and
Ž .
yellow head virus YHV in imported commodity shrimp. Aquaculture 160, 19–30.
Sahul Hameed, A.S., 1995. Susceptibility of three Penaeus species to a Vibrio campbellii-like bacterium. J. World Aquacult. Soc. 26, 315–319.
Sahul Hameed, A.S., Anilkumar, M., Stephen Raj, M.L., Kunthala Jayaraman, 1998. Studies on the pathogenicity of systemic ectodermal and mesodermal baculovirus and its detection in shrimp by immunological methods. Aquaculture 160, 31–45.
Supamattaya, K., Hoffman, R.W., Boonyaratpalih, S., Kanchanaphum, P., 1998. Experimental transmission of
Ž .
white spot syndrome virus WSSV from black tiger shrimp Penaeus monodon to the sand crab Portunus
Ž .
pelagicus, mud crab Scylla serrata and krill Acetes sp. Dis. Aquat. Org. 32 2 , 79–86.
Takahashi, Y., Itami, T., Kondo, M., Maeda, M., Fujii, R., Tomonaga, S., Supamattaya, K., Boonyaratpalin,
(
S., 1994. Electron microscopic evidence of bacilliform virus infection in kuruma shrimp Penaeus
) Ž .
japonicus . Fish Pathol. 29 2 , 121–125.
Takahashi, Y., Itami, T., Maeda, M., Suzuki, N., Kasornchandra, J., Supamattaya, K., Khongpradit, R., Boonyaratpalin, S., Kondu, M., Kawai, K., Kusuda, R., Hirono, I., Aoki, T., 1996. Polymerase chain
Ž . Ž .
reaction PCR amplification of bacilliform virus RV-PJ DNA in Penaeus iaponicus Bate and systemic
Ž .
ectodermal and mesodermal baculovirus SEMBV DNA in Penaeus monodon Fabricius. J. Fish Dis. 19, 399–403.
Wang, C.S., Tang, K.F.J., Kou, G.H., Chen, S.N., 1997. Light and electron microscopic evidence of white spot
Ž .
disease in the giant tiger shrimp, Penaeus monodon Fabricius and the kuruma shrimp, Penaeus japonicus
ŽBate cultured in Taiwan. J. Fish Dis. 20, 323–331..