16 AGRONOMY JOURNAL, VOL. 93, JANUARY–FEBRUARY 2001
Chou, C.H. 1980. Allopathic researches in the subtropical vegetation Olofsdotter, M., and D. Navarez. 1996. Allelopathic rice for Echinoch-in Taiwan. Comp. Physiol. Ecol. 5:222–234. loa crus-gallicontrol. p. 1175–1181.InProc. Int. Weed Control Chou, C.H., and H.J. Lin. 1976. Autointoxication mechanism ofOryza Conf., 2nd, Slagelse, Denmark. 25-28 June 1996. Dep. of Weed
sativaL.: I. Phytotoxic effects of decomposing rice residues in soil. Control and Pestic. Ecology, Slagelse, Denmark.
J. Chem. Ecol. 2:353–367. Olofsdotter, M., D. Navarez, and K. Moody. 1995. Allelopathic poten-Dilday, R.H., J. Lin, and W. Yan. 1994. Identification of allelopathy tial in rice (Oryza sativaL.) germplasm. Ann. Appl. Biol. 127:
in the USDA-ARS rice germplasm collection. Aust. J. Exp. 543–560.
Agric. 34:901–910. Putnam, A.R., and W.B. Duke. 1974. Biological suppression of weeds: Fay, P.K., and W.B. Duke. 1977. An assessment of allelopathic poten- Evidence for allelopathy in accessions of cucumber. Science 185:
tial inAvenagermplasm. Weed Sci. 25:224–228. 370–372.
Fujii, Y. 1992. The potential biological control of paddy weeds with Rice, E.L. 1984. Allelopathy. 2nd ed. Academic Press, London. allelopathy (allelopathic effect of some rice varieties). p. 305–320. SAS, 1992, SAS/STAT user’s guide. SAS Inst., Cary, NC.
InProc. Int. Symp. Biol. Control and Integrated Management of Smith, R.J. 1988. Weed threshold in southern US riceOryza sativa. Paddy and Aquatic Weeds in Asia, Natl. Agric. Res. Cent., Tsu- Weed Technol. 2:232–241.
kuba, Japan. 19-25 Oct. 1992. Food and Fert. Technol. Cent. for Wilson, R.E., and E.L. Rice. 1984. Allelopathy as expressed by Helian-the Asian and Pacific Region, China. thus annuusand its role in old-field succession. Bull. Torrey Bot. Okuno, K., W. Yan, and R.H. Dilday. 1997. Method for testing allelo- Club 95:432–448.
pathic activity in rice using water-soluble extracts. Breed. Sci. 47:223.
Searching for Rice Allelochemicals: An Example of Bioassay-Guided Isolation
Agnes M. Rimando,* Maria Olofsdotter, Franck E. Dayan, and Stephen O. Duke
ABSTRACT agement strategies for rice, which would be less depen-dent on synthetic herbicides.
A bioactivity-guided isolation method was developed with the
ob-The search for allelochemicals in rice necessitates
jective of isolating the allelochemicals in rice (Oryza sativaL.). Roots
evaluating the activity in a laboratory set-up to
distin-of the allelopathic rice cultivar Taichung Native 1, grown
hydroponi-guish between competition and allelopathy, which
can-cally, were extracted and fractionated, with the activity of the fractions
not be distinctly separated in field studies (Olofsdotter
followed using a 24-well culture plate microbioassay. Some of the
fractions obtained consisted of pure compounds, but none inhibited et al., 1997). Depending on one’s objectives, different the growth of barnyardgrass [Echinochloa crusgalli(L.) Beauv.] at methods could be followed in searching for active con-the lower concentration at which con-they were tested. Identified com- stituents from plants. These include bioassay-guided iso-pounds were azelaic acid;r-coumaric acid; 1H-indole-3-carboxalde- lation, fractionation-driven bioassay, isolate and assay,
hyde; 1H-indole-3-carboxylic acid; 1H-indole-5-carboxylic acid; and and biochemical combinatorial chemistry approaches. 1,2-benzenedicarboxylic acid bis(2-ethylhexyl)ester.r-Coumaric acid,
The advantages and disadvantages of each of these
a known allelochemical, inhibited the germination of lettuce (Lactuca
methods are discussed in more detail by Duke et al. sativaL.) seedlings at 1 mM. However,r-coumaric acid was active
(2000a). We chose bioassay-guided isolation as the best
against barnyardgrass only at concentrations higher than 3 mM. The
way to proceed because the active component is not
two most active fractions obtained from the bioassay-guided isolation
known.
were still a mixture of compounds as analyzed by gas chromatography–
Bioassay-guided isolation integrates the processes of
mass spectrometry (GC-MS). Further fractionation is being done to
isolate and identify the allelochemical(s) in these active fractions. separation of compounds in a mixture, using various
This work has demonstrated the use of bioassay-guided isolation in analytical methods, with results obtained from biologi-identifying allelochemicals in rice and has correlated observed field cal testing. The process begins with testing an extract activity with laboratory experiments. to confirm its activity, followed by crude separation
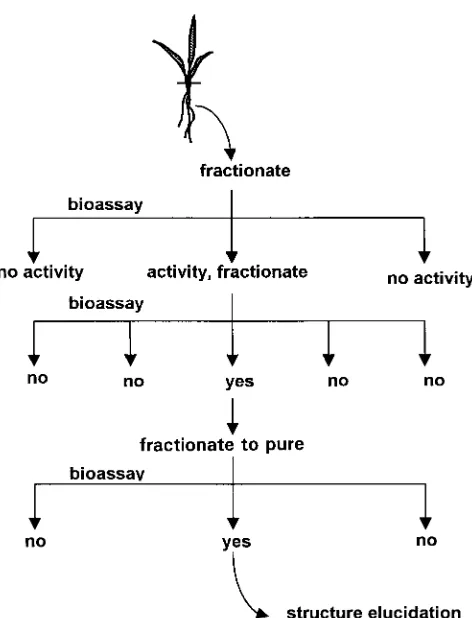
of the compounds in the matrix and testing the crude fractions (Fig. 1). Further fractionation is carried out
O
bservationof apparent allelopathy in rice (Oryza on the fractions that are determined to be active, at asativaL.) has recently drawn great attention (Olofs- certain concentration threshold, whereas the inactive dotter, 1998), and there is much interest in identification fractions are set aside or discarded. The process of frac-of the allelochemical(s). Identification frac-of the phytotoxic tionation and biological testing is repeated until pure compound(s) responsible for allelopathy will allow effi- compound(s) are obtained. Structural identification of cient generation of more allelopathic cultivars through the pure compound then follows. This methodology pre-traditional breeding or biotechnology-based genetic al- cludes overlooking novel compounds that are often terations. Such cultivars could become important tools missed in studies that only identify those compounds with which the investigator is familiar. Moreover, the in the development of advanced integrated weed
man-possibility of discovering an unknown molecular site of action is maximized (Duke et al., 2000b).
A.M. Rimando, F.E. Dayan, and S.O. Duke, USDA-ARS-NPURU, In carrying out bioassay-guided isolations of allelo-National Center for Natural Products Research, P.O. Box 8048, Uni- chemicals from rice, there are several important factors versity, MS 38677; M. Olofsdotter, Weed Science, IRRI-KVL,
Thor-that must be considered. First, the rice cultivar to be valdsens vej 40, 1871 Fredericksberg C, Denmark. Received 30 Nov.
1999. *Corresponding author ([email protected]).
Fig. 2. Bioassay-guided fractionation of the root extract of rice culti-var TN1.
Fig. 1. General scheme for bioassay-guided isolation.
Cultivar TN1 was grown hydroponically. The seeds were extracted has to be chosen with care to make sure that surface-sterilized with NaOCl for 30 min, soaked in distilled allelopathy is a likely explanation of the effects on weeds water, and germinated in 9-cm petri dishes (20 seeds dish21)
seen in the field and laboratory. Second, an appropriate under room temperature. Twelve days after soaking, uniform bioassay needs to be chosen that eliminates nonactive seedlings were placed in holes in a styrofoam float that was fractions without giving too many false positive results. placed in a 24-L pail. The float allowed the roots to be sub-And third, the target weed to test in the bioassay is an merged in hydroponic solution. There were five seedlings and 20 L of hydroponic solution per pail. The five rice seedlings important factor to make sure that laboratory screening
were planted in holes, 10 cm apart, in a Styrofoam float. The actually targets weeds of interest from a field
perspec-pail was wrapped with aluminum foil to inhibit algae growth tive. The use of miniaturized whole organism bioassay
and placed in a cooling bench (258C). The hydroponic culture is most desirable in a bioassay-guided isolation from an
solution (Yoshida et al., 1976, p. 61–67) was changed every economic standpoint.
2.5 d and pH was maintained at 5.5 throughout the experiment. Although there have been attempts to identify allelo- After 1 mo of growth, the roots were separated from the chemicals from rice (Kim and Shin, 1998; Mattice et al., shoots, and roots were dried. The dried roots were powdered 1998) to date, no laboratory has attempted to conduct and extracted with MeOH/H2O (50:50), and the extract dried bioassay-directed isolation of rice-generated allelo- under vacuum. The dried extract was subjected to column chemicals. This paper describes the general strategy and chromatographic separations as outlined in Fig. 2.
methodology used in the bioassay-guided isolation of
allelochemicals as applied in the isolation of phytotoxic Phytotoxicity Assay
compounds from the rice cultivar Taichung Native 1 The activity of TN1 root extract and its column fractions (TN1), an accession reported to be allelopathic (Dilday were monitored using a 24-well plate assay previously reported et al., 1998). (Rimando et al., 1998). Briefly described, barnyardgrass [Echi-nochloa crusgalli(L.) Beauv.] seeds were placed on filter paper
MATERIALS AND METHODS set at the bottom of the well (5 seeds well21). Samples were
dissolved in acetone and tested at determined concentrations
Plant Material (1 g L21 for extracts and less pure fractions, 0.5 g L21, for
purer factions; 3.0 mMfor pure compounds) in a final volume The rice cultivar TN1 was selected as the plant material to
be extracted after several experiments, both in the laboratory of 200mL (10% acetone in H2O) in the wells. Sample was
added in duplicate wells. Each plate had duplicate H2O only
and the field, indicated that this cultivar was allelopathic
against several weed species in the rice ecosystem (Dilday et and 10% acetone in H2O control wells. The plate was sealed
with parafilm and placed in a growth chamber (258C with a al., 1998; Olofsdotter and Navarez, 1996). Roots were chosen
as the most likely plant source of allelochemicals in the 16-h photoperiod at 400mmol m22s21photosynthetically
18 AGRONOMY JOURNAL, VOL. 93, JANUARY–FEBRUARY 2001
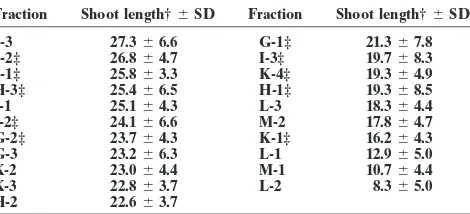
Table 2. Effect of rice fractions on barnyardgrass roots. root lengths were measured. Barnyardgrass was used as the
test species, as this is an important weed in the rice ecosystem. Fraction Root length†6SD Fraction Root length†6SD As a parallel check, phytotoxicity of the extracts and fractions
J-3 46.8619.9 M-2 27.565.4
against lettuce (Lactuca sativaL.) seedlings was also
moni-H-3‡ 46.067.9 H-1‡ 27.3615.8
tored. Effects on growth of lettuce seedlings were rated visu- G-3 41.7615.2 K-3 25.764.4 ally on a scale of 0 (no effect) to 5 (complete inhibition of I-1 38.366.7 I-2‡ 25.2612.1
J-1‡ 37.066.9 K-1‡ 24.766.1
growth). Phytotoxicity of r-coumaric acid against
barn-G-2‡ 35.063.6 L-1 23.969.5
yardgrass and lettuce was tested a concentrations as shown
J-2‡ 32.0614.1 I-3‡ 23.5611.6
in Table 5. Analysis of variance using the general linear model H-2 29.5610.7 K-4‡ 19.069.4 (GLM) procedure (SAS Inst., 1998) was carried out on the G-1‡ 29.5616.4 M-1 11.665.8
L-3 27.865.8 L-2 9.167.1
data (barnyardgrass root and shoot length), and the means
K-2 27.6610.7
were separate by LSD at theP50.05 level.
† Mean root length (cm), ctrl is 34.4;n59 or 10; LSD (0.05)58.94. ‡ Tested at 3 mM; all other fractions at 0.5 g L21.
Chemical Identification
Thin layer chromatography was done on Silica gel plates dwarfism, which is present in modern rice varieties, and (Macherey-Nagel, Alugram Sil G/UV254, 0.25-mm layer) using which may explain why many modern rice varieties have
a combination of CH2Cl2and MeOH as developing solvent. allelopathic potential (Olofsdotter et al., 1997).
Column chromatography was performed on Silica gel (CH2Cl2
Production of plant material was done in hydroponics, to MeOH gradient elution) and C18 sorbents (H2O to MeOH
which makes it very easy to separate plant parts from gradient elution). All organic solvents used were purchased
each other. Furthermore, the plant material is clean from Fisher Scientific (Norcross, GA). Identification of pure
and without pollutants from soil or other solid growth compounds was carried out using nuclear magnetic resonance
(NMR) (Bruker Avance DPX 300) and mass spectroscopy medium. The rice was grown for 1 mo, which is a short (Hewlett Packard 5989A). Fractions were also analyzed by production time for plant material.
gas chromography–mass spectrometry (GC-MS) (Hewlett Because no studies have ever been done as far as Packard 5890 Series II gas chromatograph coupled to Hewlett isolation of the allelochemicals in TN1 is concerned, it Packard 5989A MS engine). Gas chromography–mass spec- was decided to do a bioassay-directed isolation in order trometry conditions are as follows: DB-5MS 30-m length by
not to miss any of the active compounds/allelochemicals. 0.25 mm i.d. by 0.25-mm film capillary column (J&W Scientific,
The simplicity, economy (small amount of sample re-Folsom, CA); injector temp. 2508C, oven temperature
pro-quired for testing), and the fast turn-around time at grammed at 1208C initial temperature held for 1 min, then
which assay results are obtained (4 d) made the 24-increased 128C min21to 3208C and held at this temperature
well plate assay an ideal and convenient assay for the for 5 min. The carrier gas was helium at a flow rate of 1.9 mL
min21. Mass spectrometry zone temperature was: transfer line purposes of this study. Furthermore, this assay allowed
at 2808C, source at 2508C, quadruple at 1008C. Ionization volt- the observation of gross physiological effects in the
age was at 70 eV. whole plant, and most importantly, results from this
assay correlated with the activity of a known allelopathic rice cultivar TN1 (Table 1).
RESULTS AND DISCUSSION
The dried, powdered roots of TN1 were initially ex-Several rice cultivars have demonstrated allelopathic tracted with MeOH/H
2O (50:50) and tested for
phyto-property against barnyardgrass or other weeds. Among toxic activity. The extract was active, and it was then the rice cultivars evaluated from prior screening proce- partitioned between EtOAc and H
2O. The EtOAc
frac-dures to distinguish between competition and allelopa- tion was active, whereas the H
2O fraction was not.
Fol-thy, field experiments, and greenhouse studies, TN1 had lowing a bioassay-guided fractionation of the organic shown allelopathic effect against barnyardgrass, horse- portion, seven fractions (G-M, Fig. 2) were obtained, purslane (Trianthema portulacastrum L.), ducksalad which had activity against barnyardgrass at assay con-(Heteranthera limosa), and Ammannia sp. (Dilday et centration of 1 g L21. Preparative layer chromatographic
al., 1998; Olofsdotter et al., 1997). Cultivar TN1 was work on these fractions yielded further fractions and chosen for extraction and isolation of allelochemicals, also some pure compounds. These fractions were ana-not only for being the “parent” allelopathic cultivar lyzed by GC-MS to determine the identity of the pure but also because of results obtained from preliminary
studies. In a blinded study, root extracts from three rice Table 3. Effect of rice fractions on barnyardgrass shoots. samples (V69, V216, and VO1) were tested for activity Fraction Shoot length†6SD Fraction Shoot length†6SD against lettuce and barnyardgrass using 24-well culture
J-3 27.366.6 G-1‡ 21.367.8
plate assay. Sample V01, which was an extract of TN1, J-2‡ 26.864.7 I-3‡ 19.768.3 inhibited the growth of barnyardgrass significantly (Ta- J-1‡ 25.863.3 K-4‡ 19.364.9
H-3‡ 25.466.5 H-1‡ 19.368.5
ble 1). The TN1 cultivar also carries the gene for
semi-I-1 25.164.3 L-3 18.364.4
I-2‡ 24.166.6 M-2 17.864.7
Table 1. Effect of rice root extracts on lettuce and barnyardgrass. G-2‡ 23.764.3 K-1‡ 16.264.3
Fig. 3. Chromatogram of TN1 active fractions (A) L-2 and (B) M-1. Peaks at 4.9 and 6.2 min appear to be common between these two fractions.
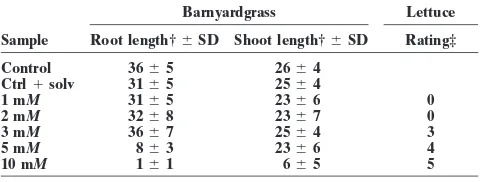
compounds and in the case of a mixture, to identify the r-coumaric acid inhibited the growth of barnyardgrass roots only at higher concentrations (10 and 5 mM), but components of the mixture. Fractions comprised of only
one peak were analyzed further by1H-NMR for identity was inactive at 3 mM (Table 5). At 5 mM, it did not
inhibit growth of the shoots.r-Coumaric acid was phyto-verification. Pure compounds were tested at 3 mM,
while fractions were tested at a lower concentration toxic to lettuce at 10, 5, and 3 mM.
A bioassay-guided isolation procedure to identify the (0.5 g L21) than was used for routine isolation work,
to determine where the activity resides among these allelochemicals in rice was shown to be advantageous in this study. It enabled the correlation of field activity samples. Results showed fractions M-1 and L-2 to have
the highest activity, inhibiting growth of barnyardgrass of allelopathic rice on barnyardgrass with laboratory experiments. Results were obtained which showed roots (Table 2) and shoots (Table 3) significantly.
The GC-MS analysis of M-1 and L-2 showed that r-coumaric acid not likely to be the allelochemical in TN1, but rather other fractions that do not contain this these two most active fractions were still a mixture of
compounds (Fig. 3). Two peaks appear to be in common reportedly phytotoxic compound showing better activ-ity. Within a relatively short period of time, the ground-between these two fractions, i.e., retention times 4.9 and
6.2 min. Conclusions cannot be made at this point as to work for more detailed isolation and identification of the allelochemicals in rice was established. Although whether either one or both of these compounds is/are
the allelochemical(s). Further bioassay-directed frac- phytotoxicity was used as the indication of biological activity, final isolation of the phytotoxic compound(s) tionation is being carried out to isolate the
allelochemi-cal(s) from these fractions. will lead to further work in demonstrating its activity in the field to prove allelopathy. Future work also in-None of the pure compounds isolated (G-1, G-2, H-1,
H-3, I-2, I-3, J-1, J-2, K-1, K-4) strongly inhibited barn- cludes the identification of the phytotoxin(s) isolated from the roots in the hydroponic culture solution. yardgrass. These compounds were identified, where
possible, by matching GC-MS data with mass spectral Identification of the allelochemical(s) will also allow the chemical fingerprinting of other rice cultivars. Isola-library and by 1H-NMR (Table 4). One of the
com-pounds was identified asp-coumaric acid (fraction G- tion and identification of the allelochemical provides a basis for studies to determine its biosynthesis, identifica-2). r-Coumaric acid has been reported to be weakly
phytotoxic (Lydon and Duke, 1989; Reynolds, 1978) tion of the enzymes and the genes encoding the en-zymes, and applying genetic engineering techniques to and an allelochemical (Chou, 1992; Einhellig, 1987;
Koch and Wilson, 1977). It was also identified by GC- enhance the production of the allelochemical. The ulti-mate goal of these studies is to produce highly allelo-MS as one of the compounds present in rice samples in
a study on the effect of allelopathic rice varieties on pathic rice variety in order for the crop to have its own defense against associated weeds.
ducksalad (Mattice et al., 1998). In our studies, however,
Table 4. Compounds isolated from root extract of Taichung Na- Table 5. Effect ofr-coumaric acid on barnyardgrass and lettuce.
tive 1 rice.
Barnyardgrass Lettuce
Fraction Compound
Sample Root length†6SD Shoot length†6SD Rating‡
G-1 MW 176
Control 3665 2664
G-2 r-Coumaric acid
Ctrl1solv 3165 2564
H-1 1H-Indole-3-carboxylic acid
1 mM 3165 2366 0
H-3 Azelaic acid
2 mM 3268 2367 0
I-2 1H-Indole-5-carboxylic acid
3 mM 3667 2564 3
I-3 MW 317 (TMS derivative)
5 mM 863 2366 4
J-1 MW 255
10 mM 161 665 5
J-2 1H-Indole-3-carboxaldehyde
K-1 MW 208 † Means in cm;n59 or 10.
20 AGRONOMY JOURNAL, VOL. 93, JANUARY–FEBRUARY 2001
M.R. Tellez, et al. 2000b. Strategies for the discovery of bioactive
SUMMARY
phytochemicals. p. 1–20.InW.R. Bidlack et al. (ed.) Phytochemi-cals as bioactive agents. Technomic Publ. Co., Lancaster, PA. This study has shown the utility of bioassay-guided
Einhellig, F.A. 1987. Interactions among allelochemicals and other search for the allelochemicals in Taichung Native 1 rice.
stress factors of the plant environment. Am. Chem. Soc. Symp. A known phytotoxin and an allelochemical,r-coumaric Ser. 330:343–357.
acid was shown not to inhibit the growth of barn- Kim, K.U., and D.H. Shin. 1998. Rice allelopathy research in Korea. yardgrass following activity-guided isolation. None of p. 39–44.InM. Olofsdotter (ed.) Allelopathy in rice. IRRI,
Ma-nila, Philippines. the other identified compounds isolated showed activity
Koch, S.J., and R.H. Wilson. 1977. Effects of phenolics acids on hypo-against barnyardgrass when tested using a 24-well plate cotyl growth and mitrochondrial respiration in mung bean ( Phaseo-microbioassay. The bioassay-guided isolation work di- lus aureus). Ann. Bot. 41:1091–1092.
rected the activity to two fractions that have two peaks Lydon, J., and S.O. Duke. 1989. The potential of pesticides from plants. p. 1–41.InL.E. Craker and J.E. Simon (ed.) Herbs, spices, in common as determined from a GC-MS analysis. Work
and medicinal plants: Recent advances in botany, horticulture and is continuing on the isolation and identification of the al- pharmacology. Vol. 4. Oryx Press, Phoenix, AZ.
lelochemicals. Mattice, J., T. Lavy, B. Skulman, and R. Dilday. 1998. Searching for allelochemicals in rice that control ducksalad. p. 81–98.InM. Olofsdotter (ed.) Allelopathy in rice. IRRI, Manila, Philippines. ACKNOWLEDGMENTS Olofsdotter, M. (ed.). 1998. Allelopathy in rice. IRRI, Manila,
Phil-ippines. We thank Stacy Allen, Amber Hale, and Artemio Madrid
Olofsdotter, M., and D. Navarez. 1996. Allelopathic rice for Echinoch-for their invaluable technical assistance.
loa crus-gallicontrol. p. 1175–1181.InH. Brown et al. (ed.) Proc. of the 2nd Int. Weed Control Congress. Vol. 4, Copenhagen, Den-mark. 25–28 June 1996. Dep. of Weed Control and Pesticide
Ecol-REFERENCES
ogy, Slagelse, Denmark.
Chou, C.H. 1992. Allelopathy in relation to agricultural productivity Olofsdotter, M., D. Navarez, and M. Rebulanan. 1997. Rice allelopa-in Taiwan: Problems and prospects. p. 179–203.InS.J.H. Rizvi and thy: Where are we and how far can we get? p. 99–104.InThe 1997 V. Rizvi (ed.) Contributions to plant ecology. Vol. 1. Chapman & Brighton Crop Protection Conf., Brighton, UK. 17–20 Nov. 1997. Hall, London, UK. Weeds. Vol. 1. The British Crop Protection Council, Brighton, UK. Dilday, R.H., W.G. Yan, K.A.K. Moldenhauer, and K.A. Gravois. Reynolds, T. 1978. Comparative effects of aromatic compounds on
1998. Allelopathic activity in rice for controlling major aquatic inhibition of lettuce fruit germination. Ann. Bot. 42:419–422. weeds. p. 7–26.InM. Olofsdotter (ed.) Allelopathy in rice. IRRI, Rimando, A.M., F.E. Dayan, M.A. Czarnota, L.A. Weston, and S.O. Manila, Philippines. Duke. 1998. A new photosystem: II. Electron transfer inhibitor Duke, S.O., F.E. Dayan, J.G. Romagni, and A.M. Rimando. 2000a. fromSorghum bicolor. J. Nat. Prod. 61:927–930.
Natural products as sources of herbicides: Current status and future SAS Institute. 1982. Software version 7.00. SAS Inst., Cary, NC. trends. p. 99–111.InWeed research. Vol. 40. Blackwell Science Yoshida, S., D.A. Forno, J.H. Cock, and K.A. Gomez. 1976. Labora-Ltd., Oxford, UK. tory manual for physiological studies of rice. 3rd ed. IRRI,