Additional phosphate stabilises uninterrupted growth of a
Dioscorea deltoidea
cell culture
Oleg Kandarakov
a, Christine Titel
b, Ludmila Volkova
a, Alexander Nosov
a,
Rudolf Ehwald
b,*
aTimiryaze
6 Institute of Plant Physiology,Russian Academy of Science,Botanicheskaya ul. 35,Moscow,127276, Russia bHumboldt Uni
6ersity Berlin,Institute of Biology,In6alidenstraße 42,D-10115 Berlin, Germany
Received 28 November 1999; accepted 28 April 2000
Abstract
Suspension cells ofDioscorea deltoideaWall (strain D-1) were maintained in a semicontinuous culture (SCC) in shake flasks at a high growth rate. It was shown that continuous propagation growth of this culture is unstable on Murashige’s and Skoog’s (MS) medium due to P starvation. On a P-enriched MS-medium the SCC was stable even at mean specific growth rates \0.3 day−1.
Highest volumetric concentrations of furostanol glycosides were obtained, when a P-enriched SCC was not further subcultivated but fed with sucrose. The investigated culture is able to control phosphate uptake and to prevent toxicity on media with excess P. High concentrations of cellular Pidid not effect the ratio of furostanol to starch. © 2000 Elsevier Science Ireland Ltd. All rights
reserved.
Keywords:Dioscorea deltoidea; Furostanol glycosides; Phosphate limitation; Plant cell culture; Secondary metabolism; Semicontinuous culture www.elsevier.com/locate/plantsci
1. Introduction
Uninterrupted plant cell propagation at contin-uous or discontincontin-uous dilution with fresh medium has found useful applications [1 – 8]. The semicon-tinuous culture (SCC) is a repeated batch culture with relatively short culture periods between the dilution steps. By an appropriate choice of the length of these periods and adjusting the dilution rate (d) to a value slightly below the maximum specific growth rate of the culture, a steady state of uninterrupted growth is reached, whereby mean value of the specific growth rate (m) equalsd. For special biotechnological purposes and also physio-logical studies [9,10] it can be of great value that the fraction of resting or differentiating cells is small or even negligible in a SCC at sufficient high values of d.
The transfer of a batch culture to a continuous dilution regime is not successful in every case. Kandarakov et al. [11] tried to establish chemostat cultures of Dioscorea deltoidea Wall. In the fer-mentor, the cell density increased with a high specific growth rate before the chemostat regime was established. At continuous dilution of the culture by fresh medium, the growth rate could be adapted to the dilution rate for certain periods only, whereafter the growth rate and the percent-age of viable cells decreased rapidly. This break-down phenomenon occurred at dilution rates which were significantly lower than the maximum specific growth rate.
The relatively low phosphate concentration of the applied medium (defined by Murashige and Skoog [12]) was found to be suboptimal for the growth of a Chenopodium album L. suspension culture at high cell density [13]. We investigated in this study whether P deficiency is the reason of the reported break-down of the D. deltoidea cell cul-* Corresponding author. Fax: +49-30-20938635.
E-mail address:[email protected] (R. Ehwald).
ture in the regime of uninterrupted growth and whether an increased P concentration effects the production of furostanol glycosides by this culture.
2. Materials and methods
2.1. Plant cell culture
The investigated strain D-1 of a D. deltoidea suspension had been initiated from a rhizome [14]. The suspension cultures (200 or 150 ml in each culture vessel) were grown in 500 ml flasks on a rotary shaker (radius 2.5 cm) at 200 rpm and 27°C in dim light. The mineral medium described by Murashige and Skoog [12] was applied in combi-nation with 40 g l−1 sucrose, 0.25 mg l−1
2,4-dichlorophenoxyacetic acid and 0.1 mg l−1kinetin
(N6-furfurylaminopurine). The phosphate
concen-tration was either 0.17 g KH2PO4 l−1 standard
MS-medium) or 0.4 g KH2PO4 l−1 (P-enriched
MS-medium). Uninterruptedly growing semicon-tinuous cultures were established by periodically replacing a certain portion (a) of the suspension broth by fresh medium. The mean dilution rate (d
=1/Dt lnf ) was obtained from the dilution factor (f=1/1 –a) and the culture period (Dt). Cells previously grown on SCC were transferred to the stationary phase by repeated addition of 10 ml of concentrated sucrose solution (300 g l−1) to the
suspension (150 ml). The sucrose solution was added when the refractometric index of the medium was lower than 2%. Stock suspensions were kept on standard MS-medium with a culture period of 14 days and a dilution factor of 6. Samples for analysis were obtained either at the end of the subculture period in the SCC, when defined volumes of the suspension were removed and replaced by fresh medium, or by withdrawal of 10 ml suspension from batch cultures.
2.2. Parameter determinations
Inorganic phosphate (Pi) in the medium and in
the cells (after extraction with 7% trichloroacetic acid) was measured by the molybdenum blue-method. Viability was estimated as the percentage of unstained cells after mixing one volume of cell suspension with one volume of 0.1% phenosafranin or Evan’s blue. Sucrose
consump-tion was calculated from the decrease in refracto-metric index. Yield coefficients (Y) of phosphate and sucrose were calculated from data obtained with suspensions growing in SCC at a relatively constant dry wt-level in subsequent culture periods [15]:
Y=Ct−C0
S0−St
(d=m),
whereCtandC0are the cell dry wt-concentrations
(g l−1), and S
0 and St the concentrations of the
respective substrate (mmol l−1 or g l−1) in the
medium after (index 0) and prior (index t) to dilution. The cell number was counted in a haemo-cytometer (Fuchs – Rosenthal) after maceration of cell aggregates in 20% chromic acid for 30 min at 60°C and passing twice through a fine needle (0.8 mm in diameter, 40 mm in length). Cell cluster-size distribution was analysed with a laser particle analyzer. The mitotic index and the ploidy spec-trum were investigated after fixation of the cells with ethanol – acetic acid, 3:1 (v/v), and staining with aceto-orcein. At least 100 metaphase plates were analyzed on squashed preparations. Furostanol glycosides were determined colorimet-rically [16]. The standard of furostanol glycosides was obtained from Professor V. Paseshnichenko, Bach Institute of Biochemistry (Moscow).
The cell wall cut-off, a measure of wall pore size, was analyzed by a method described previ-ously [13,17]. It represents the Stokes’ radius of a dextran fraction, which reaches 50% of the inner space of ethanol-denatured cells (diffusion time 30 min).
3. Results
3.1. Uninterrupted propagation of cells on standard and P-enriched MS-medium
sud-denly and the cultures diluted out (Table 1A). The semicontinuous culture (SCC) was then estab-lished on a MS-medium with enhanced concentra-tion of KH2PO4. Here the dilution rate could be
raised to more than 0.3 day−1. The culture was
able to keep the cell dry weight (dry wt.)-concen-tration at a relatively high level (10 – 15 g −1
before dilution). No break-down was observed (Table 1B).
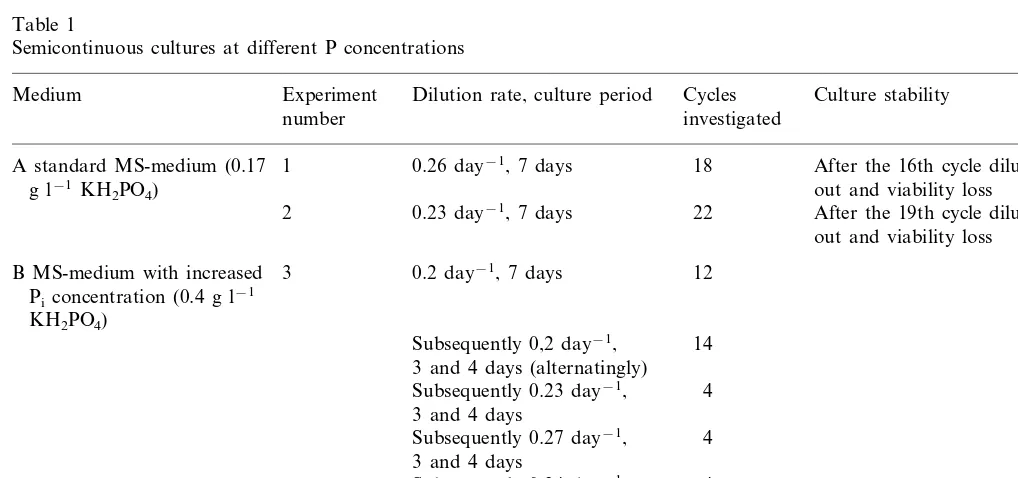
To confirm that P limitation is responsible for the break-down phenomenon, a SCC was estab-lished on the P-enriched medium, and repeatedly, parts of this culture were subcultivated on stan-dard medium. Fig. 1 shows that uninterrupted growth was stable on P-enriched and unstable on standard medium.
To compare the cultures growing with different phosphate levels, parallel SCCs (d=0.23 day−1, Dt=72 h) were established on both standard and P-enriched MS-medium (Table 2). The data ob-tained for the mitotic index and the polyploidy pattern (not shown here) were not suitable for a quantitative comparison, as these values showed strong fluctuations at both P-levels. Obviously, the SCCs were not in an ideal asynchronous state. After a transition period of about 12 days a relatively constant final cell dry wt. concentration was obtained in 15 subsequent cycles at both P levels. Pi was completely absorbed from the
P-en-riched medium during the second day. Yield
co-efficients (Y) of P were higher (5.36 g dry wt. per mmol P) on the standard MS-medium than on the P-enriched medium (3.88 g dry wt. per mmol P). This means that at the end of one subculture period the cells harvested from the P-enriched medium had a higher total P concentration (sum of free and esterified) in their dry wt. (258 mmol per g dry wt.) than the cells harvested from stan-dard medium (187 mmol per g dry wt.). On the standard MS-medium the Picontent in the cells at
the end of the subculture period was 0.6 – 1.0 mmol per g dry wt. (ca 0.5% of the total cellular P). During break-down of cell growth on the standard medium, when cell concentration decreased and more phosphate was available per cell (Fig. 1), cellular Piincreased to 5 – 6mmol per g dry wt. On
the P-enriched medium, cellular Pi was 3.7 – 5.1 mmol g−1and thus remained small in comparison
to total P (ca. 2%).
On a fresh (fr. wt.) and dry wt.-basis, the con-tents of furostanol glycosides were similar in both variants. However, due to the higher dry wt.-con-centration, the volumetric productivity of furostanol glycoside synthesis was markedly im-proved in the P-enriched suspension. The ratio of fr. wt. to dry wt. was independent of the P-level, whereas the cell cluster size was slightly reduced at higher P (Table 2). The cell wall cut off [13] of the uninterruptedly growing cells (3.3 nm) was rela-tively high in comparison to the value obtained in
Table 1
Semicontinuous cultures at different P concentrations
Experiment
Medium Dilution rate, culture period Cycles Culture stability
number investigated
A standard MS-medium (0.17 1 0.26 day−1, 7 days 18 After the 16th cycle diluting out and viability loss g l−1KH
2PO4)
2 0.23 day−1, 7 days 22 After the 19th cycle diluting
out and viability loss B MS-medium with increased 3 0.2 day−1, 7 days 12
Piconcentration (0.4 g l−1
KH2PO4)
Subsequently 0,2 day−1, 14
3 and 4 days (alternatingly) Subsequently 0.23 day−1, 4
3 and 4 days
4 Subsequently 0.27 day−1,
3 and 4 days
Subsequently 0.34 day−1, 4
3 and 4 days
Subsequently 0.31 day−1, 18 No diluting out until the end
Fig. 1. Semicontinuous cultures on P-enriched and standard MS-medium. Cells of a batch culture (Dt=14 days,f=5) growing on the P-enriched medium were adapted to a semicontinuous regime (Dt=3 or 4 days, alternating, mean d=0.2 day−1) on
standard MS-medium () and P-enriched medium (). At day 78,dwas fixed to 0.23 day−1(Dt=3 days). At the times indicated
by the arrows, a part of the suspension grown on the P-enriched medium was further subcultivated on the standard MS-medium. Cell dry wt.-concentration refers to the end of the subculture period. Each variant is represented by values of two suspensions cultured in parallel. Mean furostanol content (% of fr. wt.) was 0.8290.08 on standard medium and 0.8390,10 on P-enriched medium.
Table 2
Parameters obtained for uninterrupted growth on standard and P-enriched mediuma
P-enriched medium
Parameter Standard medium n
11.491.10
Cell dry wt. concentration (g l−1) 6.7090.90 14b
Volumetric biomass production (g dry wt. l−1day−1) 1.1190.62 1.9390.41 14c
Ratio fr. wt. to dry wt. 8.0291.07 8.3990.63 14b
4.6090.70
2.2090.80 14c
Sucrose consumption (g l−1day−1)
0.4190.07 d
Yield coefficient for sucrose (g dry wt. g−1) 0.5190.20
3.8890.53
5.3691.02 d
Yield coefficient for phosphate (g dry wt. mmol−1)
4.1790.96
Furostanol glycosides (% dry wt.) 4.8790.78 14b
519.80976.70 14b
Furostanol glycosides (mg l−1) 261.30962.60
81.10921.80
38.50918.40 14c
Volumetric production of furostanol glycosides (mg l−1day−1)
138.2594.99
Cell cluster size (mm) 125.3095.49 14b
aSampling was started at day 12 of the semicontinuous culture with a 72 h cycle. The figures represent the mean values9S.D.
ofnsubsequent determinations, each was obtained at the end of a cycle in the steady state phase.
bValues obtained at the end of a subcultivation cycle.
cValues obtained from the difference between the start and final values in a subcultivation cycle. dCalculated on the base of experimental mean values.
the stationary phase (2.5 nm) but did not signifi-cantly depend on the P-level.
3.2. Growth, cellular Pi, starch and furostanol in
undiluted cultures
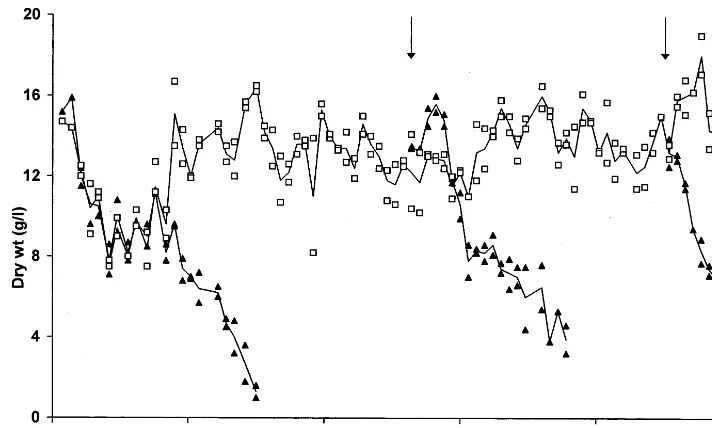
Volume fractions of SCC growing on P-enriched medium were not further diluted with fresh
medium but instead a concentrated sucrose solu-tion was added, when the refractometric index of the medium indicated sugar depletion. In the high-density cultures thus obtained (final cell dry wt.-concentration about 40 g l−1), viability was
Fig. 2. Increase in cell dry weight and furostanol accumula-tion in high-density cultures grown on P-enriched MS-medium. Cells of a culture which had been growing uninterruptedly on P-enriched MS-medium (Dt=3 days,d=
0.23 day-1, compare to Fig. 1) were not further diluted but
sucrose depletion was prevented. Two experiments of differ-ent duration are shown (a) and (b). Data for dry weight (open symbols) and furostanol content (full symbols) represent mean values of two suspensions cultured in parallel.
wt.-concentration reached, high volumetric furostanol concentrations (1.4 g per l of suspen-sion) were obtained (Fig. 2).
Cells of the suspension growing on the P-en-riched medium were transferred to an undiluted batch regime at different P concentrations (2 – 20 mM). The cellular Pi levels in the obtained
high-density cultures were very low at total P concen-trations of 2 and 4 mM (Table 3). As no detectable Piremained in the medium and the dry
wt.-concentrations at harvest time were similar in these variants, the content of esterified P in the final dry wt. increased strongly (by nearly 100%) when the total P was raised from 2 to 4 mM. Increased cellular Pi levels at higher total P
con-centrations (8 and 16 mM) indicate saturation of the cells’ P-assimilation capacity. There was no further increase in cellular Pi, when the total P
concentration in the suspension was raised from 16 to 20 mM.
A considerable part of the dry wt. in sucrose-supplemented high density cultures is starch. High Pi in the medium did not reduce the accumulation
of starch markedly (Table 3). There was no signifi-cant relation between the starch and furostanol contents (data not shown).
4. Discussion
In the batch regime for the maintenance of cell suspension cultures, all detectable Pi of the
medium is absorbed rapidly by the cells in the
Table 3
High density cultures on media with different P concentration
Experiment number Total P concentration in the suspension (mmol l−1)
2 4 8 16 20
1 3.4 18.6 150.8 270.0 285.0 Cellular Pi(mmol per g dry wt.)a
2 2.6 21.4 152.8 276.2 274.0
1 177.6 175.7
Fr. wt. (g l−1) 176.4 177.0 172.0
2 206.4 209.3 202.0 220.7 284.4
1 22.5
Dry wt. (g l−1)a 24.2 23.8 22.7 23.0
35.8 33.2
2 30.0 29.3 28.2
29.9 30.8
32.8 Starch content (mg glucose per g fr. wt.) 3 37.7 37.0
4 51.4 40.2 40.2 40.6 32.8
aMean values of two parallel culture flasks; exp. 1: growth period 10 days, prevention of sucrose exhaustion by adding sucrose
initial phase of the culture period [18 – 23]. P limita-tion of growth can, therefore, be expected in later phases only, when the cell concentration has reached a certain level and the vacuolar Pihas been
utilized. In a batch culture the Pi levels of the cells
are transiently increased after each subcultivation and P deficiency is restricted to the last cell divi-sions. Such periodic Pilimitation is tolerated by the
investigated D. deltoideacells, although these cells do not tolerate an uninterrupted growth limitation by insufficient P. P limitation of growth in the latest phase of the batch culture cycle can be even an advantage, since it improves the quality of the inoculum by prolonging the growth period and preventing rapid sucrose starvation [13]. A further reason to keep the Pi level of a basic mineral
medium low is the possible toxicity of Pi to the
inoculum which can result from excessive Piuptake
at low initial cell concentrations [18,19,24,25]. For the choice of the optimal P concentration in a plant cell culture medium not only the initial but also the final cell densities have to be considered. The results of this and further studies [24,26 – 29] suggest that a high density culture is usually P-lim-ited at the relatively low Pi concentration of the
MS-medium and further basic mineral media rec-ommended for plant suspension cultures [30,31]. The instability of uninterrupted growth at P limitation (Table 1, Fig. 1) found cannot be gener-alized, since P limitation has been used frequently to control cell density in chemostat and semicon-tinuous cultures [4,6 – 8,32 – 34]. In a chemostat, the culture studied here [11] and a furtherD. deltoidea culture strain (unpublished) were not able to grow uninterruptedly with a high specific growth rate on the standard MS-medium. We have shown here that the observed break-down is due to chronic P deficiency. Although we did not find reports on comparable findings in the literature, the observed break-down effect is not necessarily an exceptional case. Nondesired break-down of plant cell cultures after a period of rapid growth is not rare but usually remains unexplained.
Chemostat and semicontinuous cultures resem-ble organism populations maintained at constant rates of resource renewal, propagation, and re-moval of individuals. In the considered case, m is controlled by competition for Pi[20]. The
theoreti-cal treatment of this case shows that the cell density reaches a stable value (proportional to the P concentration) if the physiological affects of P
deficiency are reversible. Instabilities (oscillations, chaotic behaviour, break-down) are forecasted when a long lasting reduction of m develops in consequence of the physiological deficiency effects [35,36]. Since the cellular Pi content increased
strongly during the break-down period, the cells did not lose their capability to absorb Pibut could
not utilize the accumulated Pi. In the current state
we do not know the primary reason of this phenomenon.
At adequate P nutrition, the vacuolar Pi pool
may be the largest P fraction in plant tissues, whereas low Pi indicates P deficiency [18,19,37 –
39]. At SCC on the standard MS-medium the concentration of Piin the cell volume was less than
1 mM. Since the Piconcentration in the cytoplasm
has to be in the range of some mM [37], vacuolar Piwas very low or absent. The P content necessary
for the stabilization of uninterrupted growth of the investigated cell culture is more than 180mmol per g dry wt. Compared with the P content compatible with adequate growth of a plant shoot (about 60
mmol per g dry wt. [37,40]), this is a high require-ment.
It is known that excess Pi can effect
carbohy-drate partitioning and often results in a decreased starch content [18,37,41]. Growth, furostanol pro-duction and starch accumulation were not inhib-ited by up to 20 mM Pi. The observed insensitivity
of growth and carbon allocation to high external Pi
(Table 3) is probably due to the cells’ ability to regulate Piin the cytoplasm by its transport to the
vacuole and to control its uptake from the medium at high cellular Pi.
Although furostanol glycoside production byD. deltoidea cell cultures is coupled with growth [42], the furostanol content per g dry wt. remained stable and the volumetric product concentration increased markedly when the culture was trans-ferred to the stationary phase with additional P and sucrose. It may be concluded from our study that adequate P nutrition is important both for propagation stability and volumetric productivity, especially when a continuous production regime at high cell density is desired.
Acknowledgements
the Russian Ministry of Science and Technology. The authors thank Heidemarie Schneider for ex-cellent technical assistance.
References
[1] S.B. Wilson, P.J. King, H.E. Street, Studies on the growth in culture of plant cells. XXII. A versatile system for the large scale batch or continuous culture of plant cell suspension, J. Exp. Bot. 22 (1971) 177 – 207. [2] P.J. King, K.J. Mansfield, H.E. Street, Control of
growth and metabolism of cultured plant cells, Can. J. Bot. 51 (1973) 1807.
[3] P.J. King, Studies on the growth in culture of plant cells. XXII. Growth limitation by nitrate and glucose in chemostat cultures of Acer pseudoplatanus L., J. Exp. Bot. 28 (1977) 142 – 155.
[4] D.K. Dougall, K.W. Weyrauch, Growth and an-thocyanin production by carrot suspension cultures grown under chemostat conditions with phosphate as the limiting nutrient, Biotechnol. Bioeng. 22 (1980) 337 – 352.
[5] G. Wilson, Continuous culture of plant cells using the chemostat principle, in: A. Fiechter (Ed.), Plant Cell Cultures I, Akademie, Berlin, 1980, pp. 1 – 25.
[6] D.K. Dougall, S. LaBrake, G.H. Whitten, The effects of limiting nutrient, dilution rate, culture pH and tempera-ture on the yield constant and anthocyanin accumula-tion of carrot cells grown in semicontinuous chemostat cultures, Biotechnol. Bioeng. 25 (1983) 569 – 579. [7] D.K. Dougall, S. LaBrake, G.H. Whitten, Growth and
anthocyanin accumulation rates of carrot suspension cultures grown with excess nutrients after semicontinu-ous culture with different limiting nutrients at several dilution rates, pHs, and temperatures, Biotechnol. Bio-eng. 25 (1983) 581 – 594.
[8] S.J. Rokem, Continuous culture of plant cells, in: I.K. Vasil (Ed.), Cell Culture and Somatic Cell Genetics of Plants, Academic Press, New York, 1987, pp. 267 – 286. [9] A. Fleischer, C. Titel, R. Ehwald, The boron require-ment and cell wall properties of growing and stationary suspension-cultured Chenopodium album L. cells, Plant Physiol. 117 (1998) 1401 – 1410.
[10] A. Fleischer, M.A. O’Neill, R. Ehwald, The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II, Plant Physiol. 121 (1999) 829 – 838.
[11] O.F. Kandarakov, A.S. Vorob’ov, A.M. Nosov, Biosyn-thetic characteristics of D. deltoidea cell population grown in continuous culture, Russ. J. Plant Physiol. 41 (1994) 805 – 809.
[12] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 473 – 497.
[13] C. Titel, H. Woehlecke, I. Afifi, R. Ewald, Dynamics of limiting cell wall porosity in plant suspension cultures, Planta 203 (1997) 320 – 326.
[14] M.A. Abroshnikova (Sarcisova), R.G. Butenko, A.M. Singukhin, Tissue culture ofD.deltoidea is a producent of steroid saponins and genins, Rastitel’nye Resoursy 7 (1971) 517 – 524.
[15] J. Monod, La technique de culture continuee. Theorie et application, Ann. Inst. Pasteur 79 (1950) 390.
[16] I.S. Vasilieva, A.S. Vorob’ov, N.V. Gorskaya, A.Kh. Lipsky, K.G. Gurielidze, V.A. Paseshnichenko, Applica-tion of a spectrophotometric technique for determina-tion of oligofurostanosides in theD.deltoideaWall. cell culture, Prikl. Biokhim. Mikrobiol. 28 (1987) 692 – 696. [17] H. Woehlecke, R. Ehwald, Characterization of
size-per-meation limits of cell walls and porous separation mate-rials by high-performance size-exclusion chromatography, J. Chromatogr. A 708 (1995) 263 – 271. [18] K. Sakano, M. Matsumoto, Y. Yazaki, S. Kiyota, K. Okihara, Inorganic phosphate as a negative conditioning factor in plant cell culture, Plant Sci. 107 (1995) 117 – 124.
[19] K. Sakano, Y. Yazaki, K. Okihara, T. Mimura, S. Kiyota, Lack of control in inorganic phosphate uptake byCatharanthus roseus(L.) G. Don cells, Plant Physiol. 108 (1995) 295 – 302.
[20] K.-H. Knobloch, G. Beutnagel, J. Berlin, Influence of accumulated phosphate on the culture growth and for-mation of cinnamoyl putrescines in medium-induced cell suspension cultures of Nicotiana tabacum, Planta 153 (1981) 582 – 585.
[21] K.-H. Knobloch, Uptake of phosphate and its effect on phenylalanine ammonia-lyase activity and cinnamoyl putrescine accumulation in cell suspension cultures of
Nicotiana tabacum, Plant Cell Rep. 1 (1982) 128 – 130. [22] S. Amino, T. Fujimura, A. Komamine, Synchrony
in-duced by double phosphate starvation in a suspension culture ofCatharanthus roseus, Physiol. Plant. 59 (1983) 393 – 396.
[23] V. Srinivasan, L. Pestchanker, S. Moser, T.G. Hirasuna, R.A. Taticek, M.L. Shuler, Taxol production in bioreac-tors: kinetics of biomass accumulation, nutrient uptake, and taxol production by cell suspension of Taxus bac
-cata, Biotechnol. Bioeng. 47 (1995) 666 – 676.
[24] S. Liu, J.J. Zhong, Phosphate effect on production of ginseng saponin and polysaccharide by cell suspension cultures of Panax ginseng and Panax quinquefolium, Process Biochem. 33 (1998) 69 – 74.
[25] K. Shimogawara, H. Usuda, Uptake of inorganic phos-phate by suspension-cultured tobacco cells: kinetics and regulation by Pistarvation, Plant Cell Physiol. 36 (1995)
341 – 351.
[26] B. Tal, J.S. Rokem, I. Goldberg, Factors affecting growth and product formation in plant cells grown in continuous culture, Plant Cell Rep. 2 (1983) 219 – 222. [27] E. Battat, S.J. Rokem, I. Goldberg, Growth of D.
deltoideaat high sugar concentrations, Plant Cell Rep. 7 (1989) 652 – 654.
[28] J.J. Zhong, Q.X. Zhu, Effect of the initial phosphate concentration an cell growth and ginsenoside saponin production by suspended cultures ofPanax notoginseng, Appl. Biochem. Biotechnol. 55 (1995) 241 – 247. [29] Z.Y. Wen, J.J. Zhong, Effects of the initial phosphate
suspension-cultures of rice cells-a kinetic study, J. Ferment. Bioeng. 83 (1997) 381 – 385.
[30] O.L. Gamborg, Aromatic metabolism in plants. II En-zymes of the shikimate pathway in suspensions cultures of plant cells, Can. J. Biochem. 44 (1966) 791 – 799. [31] R.U. Schenk, A.C. Hildebrandt, Medium and
tech-niques for induction and growth of monocotyledons and dicotyledons plant cell culture, Can. J. Bot. 50 (1972) 199 – 204.
[32] G. Wilson, A simple and inexpensive design of chemostat enabling steady-state growth ofAcer pseudo
-platanus L. cells under phosphate limited condition, Ann. Bot. 40 (1976) 919.
[33] G. Wilson, C. Balague, Biosynthesis of anthraquinone by cells ofGalium mollugoL. grown in a chemostat with limiting sucrose or phosphate, J. Exp. Bot. 36 (1985) 485 – 493.
[34] A. Oostdam, L.H.W. van der Plas, A cell suspension of
Linum fla6um(L.) in phosphate limited continuous
cul-ture, Plant Cell Rep. 16 (1996) 188 – 191.
[35] R.M. May, Biological populations obeying difference equations: stable points, stable cycles and chaos, J. Theoret. Biol. 49 (1975) 511 – 524.
[36] T.S. Bellows, The descriptive properties of some models for density dependence, J. Anim. Ecol. 50 (1980) 139 – 156.
[37] H. Marschner, Mineral Nutrition of Higher Plants, Aca-demic Press, London, 1995, pp. 269 – 275.
[38] R.L. Bieleski, I.B. Ferguson, Physiology and metabolism of phosphate and its compounds, in: A. La¨uchli, R.L. Bieleski (Eds.), Enzyclopedia of Plant Physiology, Springer, Berlin, 1983, pp. 422 – 449 New Series, 15 A. [39] R.J. Robins, R.G. Ratcliffe, Intracellular distribution of
phosphate in cultured Humulus lupulus cells growing at elevated exogenous phosphate concentrations, Plant Cell Rep. 3 (1984) 234 – 236.
[40] E. Epstein, Mineral metabolism, in: J. Bonner, J.E. Varner (Eds.), Plant Biochemistry, Academic Press, Lon-don, 1965, pp. 438 – 466.
[41] D.A. Walker, S.P. Robinson, Chloroplast and cell. A contemporary view of photosynthetic carbon assimila-tion, Ber. Deutsch. Bot. Ges. 91 (1978) 513 – 526. [42] A.M. Nosov, V.N. Paukov, R.G. Butenko, Steroid
com-pounds of D. deltoidea Wall. during incubation of cul-ture in a microbiological fermentor, Prikl. Biokhim. Mikrobiol. 20 (1984) 119 – 124.