ENVIRONMENTAL SCIENCE,ENGINEERING AND TECHNOLOGY SERIES
F
LUID
W
ASTE
D
ISPOSAL
E
NVIRONMENTAL
S
CIENCE
,
E
NGINEERING
AND
T
ECHNOLOGY
S
ERIES
Nitrous Oxide Emissions Research Progress
Adam I. Sheldon and Edward P. Barnhart (Editors)
2009. ISBN: 978-1-60692-267-5
Fundamentals and Applications of Biosorption Isotherms, Kinetics and Thermodynamics Yu Liu and Jianlong Wang (Editors)
2009. ISBN: 978-1-60741-169-7
Environmental Effects of Off-Highway Vehicles Douglas S. Ouren, Christopher Haas, Cynthia P. Melcher, Susan C. Stewart,
Phadrea D. Ponds, Natalie R. Sexton Lucy Burris, Tammy Fancher and Timothy E. Reeve (Editors) 2009. ISBN: 978-1-60741-097-3
Alfred O. Owino and Joseph O. Oyugi 2009. ISBN: 978-1-60741-367-7
Forest Canopies: Forest Production, Ecosystem Health
and Climate Conditions Jason D. Creighton and Paul J. Roney
(Editors)
2009. ISBN: 978-1-60741-457-5
Soil Fertility
Derek P. Lucero and Joseph E. Boggs (Editors)
2009. ISBN: 978-1-60741-466-7
Handbook of Environmental Policy Johannes Meijer and Arjan der Berg
Process Engineering in Plant-Based Products
Hongzhang Chen
2009. ISBN: 978-1-60741-962-4
Buildings and the Environment Jonas Nemecek and Patrik Schulz
(Editors)
2009. ISBN:978-1-60876-128-9
Tree Growth: Influences, Layers and Types
Wesley P. Karam (Editor) 2009. ISBN:978-1-60741-784-2
Syngas: Production Methods, Post Treatment and Economics
Adorjan Kurucz and Izsak Bencik (Editors)
2009. ISBN:978-1-60741-841-2
Potential of Activated
Sludge Utilization
Xiaoyi Yang
2010. ISBN: 978-1-60876-019-0
Psychological Approaches
to Sustainability:
Current Trends in Theory,
Research and Applications
Victor Corral-Verdugo, Cirilo H. Garcia-Cadena
and Martha Frias-Armenta (Editors) 2010. ISBN: 978-1-60876-356-6
Carbon Capture and Storage including Coal-Fired Power Plants
Todd P. Carington (Editor) 2009. ISBN: 978-1-60741-196-3
Process Engineering in Plant-Based Products
Hongzhang Chen 2009. ISBN:978-1-60741-962-4
ENVIRONMENTAL SCIENCE,ENGINEERING AND TECHNOLOGY SERIES
F
LUID
W
ASTE
D
ISPOSAL
K
AY
W.
C
ANTON
E
DITOR
Nova Science Publishers, Inc.
Copyright © 2010 by Nova Science Publishers, Inc.
All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means: electronic, electrostatic, magnetic, tape, mechanical photocopying, recording or otherwise without the written permission of the Publisher.
For permission to use material from this book please contact us: Telephone 631-231-7269; Fax 631-231-8175
Web Site: http://www.novapublishers.com
NOTICE TO THE READER
The Publisher has taken reasonable care in the preparation of this book, but makes no expressed or implied warranty of any kind and assumes no responsibility for any errors or omissions. No liability is assumed for incidental or consequential damages in connection with or arising out of information contained in this book. The Publisher shall not be liable for any special,
consequential, or exemplary damages resulting, in whole or in part, from the readers‘ use of, or
reliance upon, this material.
Independent verification should be sought for any data, advice or recommendations contained in this book. In addition, no responsibility is assumed by the publisher for any injury and/or damage to persons or property arising from any methods, products, instructions, ideas or otherwise contained in this publication.
This publication is designed to provide accurate and authoritative information with regard to the subject matter covered herein. It is sold with the clear understanding that the Publisher is not engaged in rendering legal or any other professional services. If legal or any other expert assistance is required, the services of a competent person should be sought. FROM A DECLARATION OF PARTICIPANTS JOINTLY ADOPTED BY A COMMITTEE OF THE AMERICAN BAR ASSOCIATION AND A COMMITTEE OF PUBLISHERS.
LIBRARY OF CONGRESS CATALOGING-IN-PUBLICATION DATA
Fluid waste disposal / editor, Kay W. Canton. p. cm.
Includes index.
ISBN 978-1-61122-590-7 (eBook) 1. Sewage disposal. I. Canton, Kay W. TD741.F55 2009
628.3--dc22
2009037445
C
ONTENTS
Preface ix
Chapter 1 Treatment of Wastewater by Electrocoagulation Method
and the Effect of Low Cost Supporting Electrolytes 1
Lazare Etiégni, K. Senelwa, B. K. Balozi, K. Ofosu-Asiedu, A. Yitambé, D. O. Oricho and B. O. Orori
Chapter 2 Application of Sulphate-Reducing Bacteria
in Biological Treatment Wastewaters 49
Dorota Wolicka
Chapter 3 Utilization of Water and Wastewater Sludge
for Production of Lightweight-Stabilized Ceramsite 83
Zou Jinlong, Yu Xiujuan, Dai Ying and Xu Guoren
Chapter 4 Modelling and Observation of Produced
Formation Water (PFW) at Sea 113
D. Cianelli, L. Manfra, E. Zambianchi, C. Maggi and A. M. Cicero
Chapter 5 Disposal of Sulfur Dioxide Generated in Industries
Using Eco-Friendly Biotechnological Process – A Review 137
A. Gangagni Rao and P.N. Sarma
Chapter 6 Novel Biological Nitrogen-Removal Processes:
Applications and Perspectives 155
J.L. Campos, J.R. Vázquez-Padín, M. Figueroa, C. Fajardo, A. Mosquera-Corral and R. Méndez
Chapter 7 Application of Microbial Melanoidin-Decomposing
Activity (MDA) for Treatment of Molasses Wastewater 181
Suntud Sirianuntapiboon and Sadahiro Ohmomo
Chapter 8 Wastewaters from Olive Oil Industry:
Characterization and Treatment 199
Contents viii
Chapter 9 Usability of Boron Doped Diamond Electrodes in the Field
of Waste Water Treatment and Tap Water Disinfection 217
Hannes Menapace, Stefan Weiß, Markus Fellerer, Martin Treschnitzer and Josef Adam
Chapter 10 Utilization of Biosolids as Fertilization Agents on Agricultural Land: Do the Obvious Benefits of Recycling Organic Matter and Nutrients
Outweigh the Potential Risks? 237
Veronica Arthurson
Chapter 11 Integrated Approach for Domestic Wastewater
Treatment in Decentralized Sectors 249
Rani Devi and R. P. Dahiya
Chapter 12 Biodegradation Characteristics of Wastewaters 265
Fatos Germirli Babuna and Derin Orhon
Chapter 13 Batch Treatment of a Coffee Factory Effluent for Colour Removal Using a Combination of
Electro-Coagulation and Different Supporting Electrolytes 279
L. Etiégni, D. O. Oricho, K. Senelwa B. O. Orori, B. K. Balozi, K. Ofosu-Asieduand A. Yitambé
Chapter 14 Water as a Scarce Resource: Potential for Future Conflicts 297
M. A. Babu
Chapter 15 Recycling Wastewater After Hemodialysis: An Environmental and Cost Benefits Analysis
for Alternative Water Sources in Arid Regions 307
Faissal Tarrass, Meryem Benjelloun and Omar Benjelloun
Chapter 16 Pb (II) Ions Removal by Dried Rhizopus Oligosporus
Biomass Produced from Food Processing Wastewater 317
H. Duygu Ozsoy and J. Hans van Leeuwen
Chapter 17 Control of Plasticizers in Drinking Water,
Effluents and Surface Waters 331
Rosa Mosteo, Judith Sarasa, M. Peña Ormad and Jose Luis Ovelleiro
P
REFACE
Wastewater is any water that has been adversely affected in quality by anthropogenic influence. It comprises liquid waste discharged by domestic residences, commercial properties, industry, and/or agriculture and can encompass a wide range of potential contaminants and concentrations. In the most common usage, it refers to the municipal wastewater that contains a broad spectrum of contaminants resulting from the mixing of wastewaters from different sources. With the dwindling available water resources in the world coupled with high population growth, pressure is being exerted on water and wastewater plant managers the world over to find cost-effective methods to treat a wide range of wastewater pollutants in a diverse range of situations. This new and important book gathers the latest research from around the globe on fluid waste disposal with a focus on such topics as: wastewaters from the olive industry, application of sulphate-reducing bacteria in biological treatment wastewaters, electrocoagulation treatment method, usability of boron doped diamond electrodes in wastewater treatment and others.
Kay W. Canton x
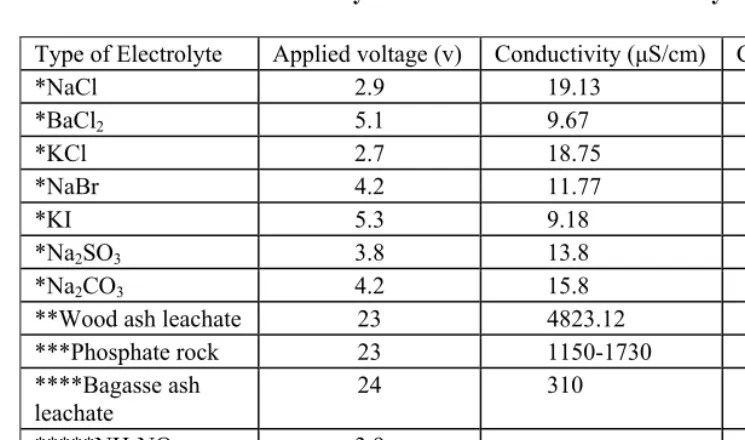
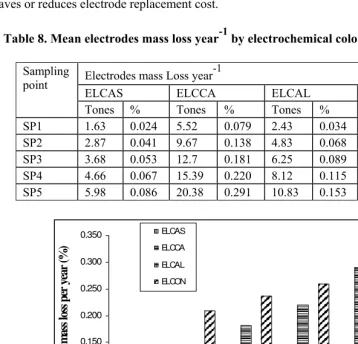
the electrocoagulation process uneconomical. The inclusion of supporting electrolyte such as NaCl achieves this. One of the methods pioneered by researchers at Moi University in Kenya is the use of wood ash leachate as supporting electrolyte which in some cases could reduce energy consumption by as much as 80%. Other supporting electrolytes tested are ash from bagasse and from coffee husks. These supporting electrolytes are relatively inexpensive, but they all generally result in large amount of coagulated sludge. Other supporting electrolytes such phosphate rock are less effective than wood ash, but they yield almost 50% less sludge after electrocoagulation. Most of the supporting electrolytes have an added advantage of reducing other wastewater pollution parameters such as BOD, COD, TSS, TS, turbidity, pH and color. Because of the inherent benefits of these low cost supporting electrolytes, electro-chemical methods could be a credible alternative to more traditional wastewater treatment approaches.
Chapter 3 - Disposal of wastewater treatment sludge (WWTS) and drinking-water treatment sludge (DWTS) is one of the most important environmental issues nowadays. Traditional options for WWTS and DWTS management, such as landfilling, incineration, etc., are no longer acceptable because they can cause many environmental problems. Conversion of WWTS and DWTS into useful resources or materials is of great interest and must be intensely investigated. To attain this goal, WWTS and DWTS were used as components for making ceramsite. Part I: SiO2 and Al2O3 were the major acidic oxides in WWTS and DWTS, so their effect on characteristics of ceramsite was investigated. Results show that WWTS and DWTS can be utilized as resources for producing ceramsite with optimal contents of SiO2 and Al2O3 ranging from 14–26% and 22.5–45%, respectively. Bloating and crystallization in ceramsite above 900
℃
are caused by the oxidation and volatilization of inorganic substances. Higher strength ceramsite with less Na-Ca feldspars and amorphous silica and more densified surfaces can be obtained at 18%≤Al2O3≤26% andγ0%≤SiO2≤45%. Part II: Fe2O3 and CaO were the major basic oxides, so their effect on characteristics of ceramsite was also investigated. The optimal contents of Fe2O3 and CaO are in the range of 5%–8% and 2.75%–7%, respectively. Higher strength ceramsite with more complex crystalline phases and fewer pores can be obtained at 6%≤Fe2O3≤8%. Lower strength ceramsite with more pores and amorphous phases can be obtained at 5%≤CaO≤7%, which implies that excessive Ca2+ exceeds the needed ions for producing electrical neutrality of silicate networks. Part III: To investigate stabilization of heavy metals in ceramsite, leaching tests were conducted to find out the effect of sintering temperature, pH, and oxidative condition. Results show that sintering exhibits good binding capacity for Cd, Cr, Cu, and Pb and leaching contents of heavy metals will not change above 1000
℃
. Main compounds of heavy metals are crocoite, chrome oxide, cadmium silicate, and copper oxide, which prove that stronger chemical bonds are formed between these heavy metals and the components. Leaching contents of heavy metals decrease as pH increases and increase as H2O2 concentration increases. Leaching results indicate that even subjected to rigorous leaching conditions, the crystalline structures still exhibit good chemically binding capacity for heavy metals and it is environmentally safe to use ceramsite in civil and construction fields. It is concluded from the 3 parts that utilization of WWTS and DWTS can produce high performance ceramsite, in accordance with the concept of sustainable development.Preface xi
water are brought to the surface along with the hydrocarbons. These waters include the
‗formation water‘, that lies underneath the hydrocarbon layer, and ‗additional water‘ usually injected into the reservoirs to help force the oil to the surface. Both formation and injected waters, named ―produced formation waters‖ (PF→s), are separated from the hydrocarbons onboard offshore platforms and then disposed into the marine environment through ocean diffusers. PFWs contain several contaminants and represent one of the main sources of marine environment pollution associated with oil and gas production.
This makes the study of PFW fate of paramount importance for a proper management of environmental resources as well as for planning and optimizing the discharge and monitoring procedures.
In the first part of this chapter we provide a detailed description of the chemical characteristics of PFWs and their potential toxic effects and review the mixing processes governing their dispersion in the marine environment. In the second part of the work we briefly review past efforts in observing and modelling PFW spreading in the ocean. Finally, we propose a multidisciplinary approach, integrating in situ observations and numerical modelling, to assess dispersion of PFWs in space and time. As a case study we will refer to the results of a previous study conducted in the Northern Adriatic Sea, a sub-basin of the Mediterranean Sea, where a number of offshore natural gas (CH4) extraction platforms are currently active.
Chapter 5 - Sulfur dioxide (SO2) is a known pollutant and responsible for various ill effects on living and living organisms. SO2 emissions can be reduced by using non-conventional energy sources or using non-conventional fuels containing less sulfur. However, under the present circumstances SO2 emissions cannot be completely avoided due to the reasons of rapid industrialization. Various technologies are available for the removal of SO2 from flue and waste gases. Most of these technologies fall under the category of physical, chemical or thermal. All these technologies generate secondary pollutants ending up in disposal problems and also cost prohibitive. Biotechnology offers relatively cheaper solutions for the conventional problems. Due to this reason, biotechnology is making in roads into the conventional treatment processes in all the fields. Over the last decade, efforts have been made to develop biotechnological alternatives to conventional physico- chemical processes for the removal of SO2 from flue gases known as Biological flue gas desulphurization (BIO-FGD).SO2 from flue gas can be absorbed in a suitable organic media. In the aqueous phase SO2 would be converted to sulfite and some part may again be converted to sulfate due to the presence of dissolved oxygen. Therefore, the aqueous phase will be having both sulfate and sulfite, which can be reduced to sulfide using Sulfate Reducing Bacteria (SRB) under anaerobic conditions. The sulfide formed in the anaerobic reactor could be converted to elemental sulfur using Sulfur Oxidizing Bacteria (SOB) under partial microbial aerobic conditions. The elemental sulfur can be used either as a soil conditioner or raw material for industrial applications. Therefore, BIO-FGD process could be an environmentally benign and economically viable alternative for the disposal of SO2 emitted from the industries especially from power plants and refineries. The present article reviews the state of art of BIO-FGD process.
Kay W. Canton xii
not suitable to treat wastewater with a low COD/N ratio because it involves the addition of an external organic carbon source and, therefore, an increase of the operational costs.
Several alternative processes for nitrogen removal can be applied in order to reduce
partially (―nitrite route‖) or totally (anammox, autotrophic denitrification) the organic matter required. Such processes suppose not only an economical way to treat these wastewaters but they are also more environmentally friendly technologies (lower production of CO2, N2O and sludge; lower energy consumption). Up to now, they were basically applied to the return sludge line of municipal wastewater treatment plants (WWTPs). However, these processes could even be implemented in the actual WWTPs in order to achieve more compact and energy efficient systems.
Their potential advantages can make them also feasible technologies to treat polluted ground water or to remove nitrogen compounds from recirculating aquaculture systems.
Chapter 7 - This review will discuss the melanoidin-decomposing activity (MDA) among microorganisms. The focus will be on the potential use of the microbial-MDA to treat the wastewater discharged from factories using molasses as the raw material (molasses wastewater: MWW) because molasses is one of the most useful raw materials in various types of industries, such as the fermentation and animal feed industries. However, the wastewater discharged from factories using molasses contains a large amount of dark brown pigment, melanoidin pigment: MP, which is poorly decomposed and/or decolorized by normal biological treatment processes, such as the activated sludge or anaerobic treatment systems (anaerobic pond or anaerobic contact digester), because, the microorganisms in those wastewater treatment systems showed very poor MDA. The distribution of MDA among microorganisms and the mechanism of decomposing activities, in particular, were reviewed. Also, the application of the isolated strains having the MDA to treat molasses wastewater in the wastewater treatment plant was tested.
Preface xiii
(w/w), at OMW pH and environmental temperature. The final average value of COD obtained next to 370 mg L-1 (%CODremoval = 86.2%), and the water obtained can be destined to irrigation or disposed directly to the municipal wastewater system for their tertiary treatment. OMW from three-phase process does not allow direct chemical and biological purification for its content in phenolic compounds and generally used natural and forced evaporation process. Another way of using is the application of OMW nutrients to the growth of microorganisms such as microalgae.
Chapter 9 - Over the past few years one main focus on the research efforts at the Institute for Sustainable Waste Management and Technology (IAE) has been on possible applications for reactors with boron doped diamond electrodes (BDD) in the field of (waste) water treatment. This article deals with the technical construction of the electrodes used (continuous reactor with a different number of plate electrodes), which were produced by a spin-off of the institute. The electrodes consist of conductible industrial diamond particles (< 250 µm), which are mechanically implanted on a fluoride plastic substrate. These electrodes showed a high mechanical and chemical stability in different test runs. At the institute, treatment methods for micro pollutants (e.g. pharmaceuticals and complexing agents) were developed with electrochemical oxidation by BDD. In this case test runs were made on laboratory scale and technical scale treatment units and elimination rates up to 99 % were achieved. In this
project the analytic is partly provided by the ―Umweltbundesamt GmbH‖ (UBA), one of the
project partners. This agency has been a project partner in different studies about pharmaceuticals in the ecosystem. These techniques could also be used for the waste water treatment of alpine cabins. Pilot projects have been set up. On the basis of these results a follow-up project was launched last October, in which an alternative treatment process for oil-in-water emulsions and mixtures was developed by the usage of electrochemical oxidation with BDD. A third possible application is the disinfection of drinking water from contaminated ground and spring water. In this process oxidation agents like ozone or OH radicals produced in situ by the BDD reactor from the treated water are used to eliminate bacterial contaminants (for example e. coli) in the water.
Chapter 10 - Treatment of wastewater, commonly performed at municipal sewage plants, generates sanitized water and sewage sludge. Anaerobic degradation of sewage sludge results in the production of different gases, including the economically valuable methane, and digested residue (biosolids) with potential value as a crop fertilizer. Traditionally, digested sewage sludge is disposed either into water, onto or into the earth or into the air. However, alternative exploitation of digested sewage sludge in agriculture has several advantages over commercial fertilizers, including environmental aspects benefiting agricultural sustainability and increased crop yield. Additionally, residue utilization is nearly always a cheaper option than disposal costs.
Kay W. Canton xiv
present a modified microbial community composition after some time and, hence, a modified ecosystem function.
At the end of the present chapter, we discuss whether the potential risks of recycling biosolids to agricultural cropland are acceptable for consumers, producers and scientific expertise, in view of the resulting alterations in soil microbial diversity, activity and accompanying functions. Furthermore, optimal ways of managing the recycling process to achieve the most favourable balance of benefits and risks for the community are highlighted.
Chapter 11 - The purpose of the present study was to design an integrated wastewater treatment system for a nalla (riverlet) flowing through Indian Institute of Technology Delhi (IITD), India, besides its cost estimation and comparison with the conventional wastewater treatment system. The design parameters for integrated aeration-cum-adsorption tank were worked out for 240 m3 / d flow rate of the wastewater. The important parameters used for the design included initial COD and BOD concentration in the influent, treatment time, adsorbent dose, pH, adsorbent particle size and the desired COD and BOD in the effluent after treatment as prescribed by Central Pollution Control Board, (CPCB) Delhi, India. All the design parameters of this system were similar to those of conventional system except for the replacement of aeration tank in conventional system by the aeration-cum-adsorption tank. The concentration of COD and BOD of the treated effluent by the integrated system were well within the permissible limits of CPCB standards (for COD it is 100 ppm and for BOD of 30 ppm) to discharge in the canal for irrigation purpose. It was worth mentioning here that the adsorbents used in the present study were based on discarded materials which were available free of cost. Of course, the cost of their transportation and processing should have been taken into account.
The total cost estimated for the conventional system and the adsorption based system would be Rs. 198,312 and Rs. 141,275 respectively (including civil work, machinery, labour, adsorbent and miscellaneous). The cost difference for the two systems would be approximately Rs 57,037.
This design of integrated system has resulted into saving of cost by 28 % over the conventional system. Thus, it is a good approach for saving of conventional energy in addition to saving the cost of treatment and can be applicable for any country for decen-tralized sector. Moreover, it is an open ended research and we can recommend more research by changing the adsorbents types and operating parameters to improve the model.
Preface xv
with domestic sewage and end-of-pipe industrial effluents are evaluated in terms of their biodegradation characteristics.
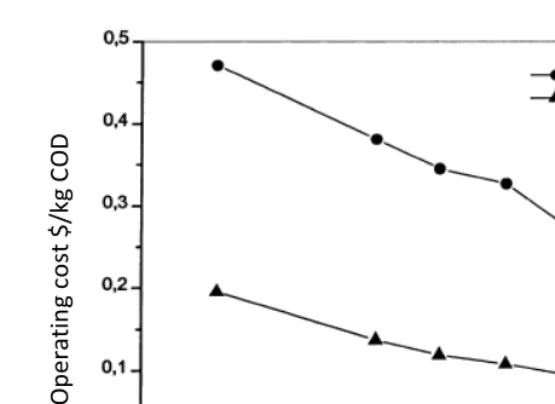
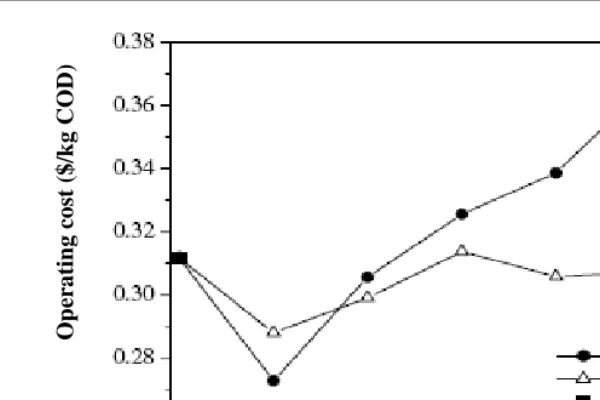
Chapter 13 - In the present study, two types of colour removal systems were tested on effluent samples collected from a coffee pulping factory which discharged on average 15 m3 of wastewater daily with a colour index of about 2500 OH that was too high for direct discharge into a river in Kenya. The two colour removal systems used were: (i) electrolysis combined with wood ash or coffee husks leachate and (ii) electrolysis combined with phosphate rock solutions at a rate of 0.5 g/l to 4g/l. Phosphate rock is often used as agricultural liming agent. The surface area of the electrodes was set at close to 75 m2/m3 of effluent with a current density of 1,200 mA/m2. The experiments were laid out in a stratified random sampling design and the data were analysed using the Statistical Package for Social Scientists (SPSS) computer programme version 10.0. Electrolysis combined with phosphate rock (ELPHOS) proved to be the best process in terms of power consumption (68% reduction) compared with the 57% reduction by electrolysis combined with wood ash (ELCAS) and the 58% reduction by electrolysis combined with coffee husks ash (ELCHAS). Besides the 100% colour removal, ELPHOS also reduced other effluent physico-chemical parameters such as BOD, COD, TSS and TS by 79%, 80%, 69%, and 88% respectively. The analysis of ELPHOS treated wastewater showed that the mill could discharge an effluent that meets local discharge standards for colour requirements. It is recommended that recycling of the treated water by ELPHOS back to the factory for cleaning and washing purposes be considered since the quality meets the requirement for uses of fresh water for cleaning purposes. Furthermore, calculation of power consumption based on a scale-up batch reactor of 15 m3 proved less expensive to treat the factory effluent than a set of 12 one 100-L reactors similar to the one used in the field.
Chapter 14 - The major aim of this paper is to review the major problems of water resources in the developing countries. It is based on problems related to population growth and pollution and how these are more likely to lead to future conflicts. We know that fresh water is only 3 % of the total global water and 78% of this is in glaciers. This makes it a scarce and precious resource which must be sustainably managed. The paper also analyses some of the already existing and potential conflicts based on water resources. It reviews the potential threats to Ugandan water resources and problems which are most likely to occur as a result of these threats. Factors hindering treatment of wastewater as a remedy to pollution in developing countries have also been discussed. The methodology used in this paper is based on literature review of the most current issues that affect water resources world-wide. The review is limited to scientific facts and no political factors affecting water resources have been included.
It has been found that although Uganda is endowed with 66km2/year of renewable water resources, population increase, deforestation, degradation of wetlands and pollution are major threats to its water resources. Problems associated with water quality and quantities are more likely to result into internal conflicts which are bound to spread beyond Ugandan borders.
Kay W. Canton xvi
comparison of intended cost with that of seawater desalination, which is widely used in irrigation.
Chapter 16 - Heavy metal pollution is a serious problem in many developed and developing countries. Lead had been recognized as a particularly toxic metal and comes into water bodies mainly from metallurgical, battery, metal plating, mining and alloy industries. In order to minimize the impacts of this metal on human health, animals and the environment, lead-contaminated water and wastewater need to be treated before discharge to water bodies.
This chapter concerns an investigation of potential usage of corn-processing wastewater as a new alternative low-cost substrate to produce biosorbent and evaluate this biosorbent to remove Pb(II) ions from aqueous solutions. For this aim, Rhizopus oligosporus cultivated on corn-processing wastewater and dried biomass of these fungi was used as an adsorbent. The adsorption experiments were conducted in a batch process and the effects of contact time (1-48 hours), initial pH (2-7), initial metal ion concentration (20-100 mg L-1) and adsorbent dosage (0.5-5 g L-1) on the adsorption were investigated. Pb (II) ion concentrations before and after adsorption were measured using Inductively Coupled Plasma-Mass Spectrometry. Maximum adsorption capacity was achieved at pH 5.0. The isothermal data of dried fungal biomass could be described well by the Langmuir equation and monolayer capacity had a mean value of 59.88 mg g-1. The pseudo-second order reaction model provided the best description of the data with a correlation coefficient 0.99 for different initial metal concentrations. This result indicates that chemical sorption might be the basic mechanism for this adsorption process and Fourier Transform Infrared Spectroscopy analyses showed that amide I and hydroxyl groups play an important role in binding Pb (II).
Because of the high activation capacity of adsorbent and low cost of process dried R. oligosporus biomass presents a good potential as an alternative material for removal of Pb (II) ions from the aqueous solutions.
Chapter 17 - The main objective of this research work is to determine the presence of di(2-ethylhexyl) phthalate, di(2-ethylhexyl) adipate and diisodecyl phthalate, in different water samples (drinking waters, effluents and surface waters). Different analytical methods were studied in order to know the best methodology for the quantification of these compounds. Solid-liquid and liquid extraction were investigated and finally the liquid-liquid extraction and analysis by gas chromatography followed by mass spectroscopy was chosen because of offering the highest recovery rate. In the whole of this research study, the control of background pollution by reagents and material was extremely important. The problem of background pollution is more serious in the trace analysis of phthalates and adipates as a consequence of their presence in almost all equipment and reagents used in the laboratory.
In: Fluid Waste Disposal ISBN: 978-1-60741-915-0 Editor: Kay W. Canton, pp. 1-48 © 2010 Nova Science Publishers, Inc.
Chapter 1
T
REATMENT OF
W
ASTEWATER BY
E
LECTROCOAGULATION
M
ETHOD AND THE
E
FFECT
OF
L
OW
C
OST
S
UPPORTING
E
LECTROLYTES
Lazare Etiégni
1*, K. Senelwa
1, B. K. Balozi
1, K. Ofosu-Asiedu
2,
A. Yitambé
3, D. O. Oricho
1, and B. O. Orori
11Moi University, Department of Forestry & Wood Science, P. O. Box 1125 Eldoret, Kenya.
2 J.I.C., Dept. of Chem. Eng. Box 10099, Jubail Industrial City-31961, Kingdom of Saudi Arabia.
3
Kenyatta University, Department of Public Health P.O Box 43844-00100 Nairobi, Kenya
A
BSTRACTCoagulation and flocculation are traditional methods of treating of polluted water. Electrocoagulation (EC) presents a robust novel and innovative alternative in which a sacrificial metal anode doses water electrochemically. This has the major advantage of providing active cations required for coagulation, without necessarily increasing the salinity of the water. Electrocoagulation is a complex process with a multitude of mechanisms operating synergistically to remove pollutants from water. A wide variety of opinions exist in the literature for key mechanisms and reactor configurations. A lack of a systematic approach has resulted in a myriad of designs for electrocoagulation reactors without due consideration of the complexity of the system. A systematic, holistic approach is required to understand electrocoagulation and its controlling parameters (pH, temperature, conductivity, current density). This will enable a priori prediction of the treatment of various pollutant types. Electrocoagulation involves applying a current across electrodes in water. This results in the dissolution of the anode (either aluminum or iron). These ions then form hydroxides which complex with and/or absorb contaminants and precipitate out. The precipitate with the contaminants can then be
*
Lazare Etiégni, K. Senelwa, B. K. Balozi et al. 2
removed from the water by settling and decantation or filtration. EC has the potential to be applied in many other areas besides the textile and semiconductor industry. It has been successfully tested in the pulp and paper industry, as well as tea and coffee processing. However over electrical potential within electrodes during electrocoagulation normally causes extra voltage, which wastes energy. There have been attempts to reduce this extra voltage which, in these days of World energy crisis, will render the electrocoagulation process uneconomical. The inclusion of supporting electrolyte such as NaCl achieves this. One of the methods pioneered by researchers at Moi University in Kenya is the use of wood ash leachate as supporting electrolyte which in some cases could reduce energy consumption by as much as 80%. Other supporting electrolytes tested are ash from bagasse and from coffee husks. These supporting electrolytes are relatively inexpensive, but they all generally result in large amount of coagulated sludge. Other supporting electrolytes such phosphate rock are less effective than wood ash, but they yield almost 50% less sludge after electrocoagulation. Most of the supporting electrolytes have an added advantage of reducing other wastewater pollution parameters such as BOD, COD, TSS, TS, turbidity, pH and color. Because of the inherent benefits of these low cost supporting electrolytes, electro-chemical methods could be a credible alternative to more traditional wastewater treatment approaches.
I
NTRODUCTIONWith the dwindling availability of water resources in the World coupled with high population growth, pressure is being exerted on water and wastewater plant managers the world over to find cost-effective methods to treat a wide range of wastewater pollutants in a diverse range of situations. Traditionally coagulation, flocculation and lagooning have been used as chemical and biological processes with varying degrees of success to treat polluted waters. However a more cost-effective and proven method to clean an ever widening range of water pollutants, on-site, and with minimum additives, is required for sustainable water and wastewater management. Electrocoagulation treatment of water seems to fit this description.
Colloidal dispersions in water or wastewater often referred to as sols consist of discrete particles held in suspension by their extreme small size (1-200 nm), state of hydration (chemical combination with water), and surface electric charge. The chemistry of coagulation and flocculation is primarily based on the electrical properties of the particles. Like charges repel each other while opposite charges attract. Particles finer than 0.1 µm (1x10-7 m) in water or wastewater remain continuously in motion due to electrostatic charges (often negative) which cause them to repel each other.
There are two types of colloids - hydrophilic and hydrophobic. Hydrophilic are readily dispersed in water and their stability depends on the affinity for water rather than the slight negative charge they possess. Hydrophobic colloids on the other hand have no affinity for water and their stability depends on the charge they possess, usually positive. The electrostatic repulsion between the colloidal particles leads to a stable sol. The surface or primary charge of colloidal particles comes from charged groups within the particles or the adsorption of charged particles. The sign and magnitude of the surface charge depends on the character of colloids, the pH (the lower the pH the more positive the charge becomes), the ionic strength and the characteristics of the water or wastewater.
Treatment of Wastewater by Electrocoagulation Method… 3
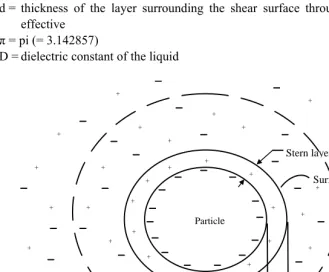
theory applied to hydrophobic colloids (Figure 1), and can be estimated using Smoluchowski‘s (1872-1917) electrokinetic mobility equation:
ε
μ =
(1)
where μ = the electrophoretic mobility = zeta potential
ε = the electric permittivity
= the viscosity of the water or wastewater
Zeta potential can also be calculated using the following relationship for electrostatic force
D = dielectric constant of the liquid
Lazare Etiégni, K. Senelwa, B. K. Balozi et al. 4
The diffused double layer (Figure 1) consists of two parts: an inner region, also referred to as Stern layer, which includes ions bound relatively strongly to the surface (including specifically adsorbed ions) and an outer, diffuse or movable region in which the ion distribution is determined by a balance of electrostatic forces and random thermal motion. The potential in this region, therefore, decays as the distance from the surface increases until, at sufficient distance, it reaches the bulk solution value, conventionally taken to be zero. The repulsive force of the charged double layer scatters particles thus preventing agglomeration. Particles with high zeta potential have a very stable sol.
The zeta potential is the overall charge a particle acquires in a specific medium. In other words, it is a measure of the magnitude of electrical charge surrounding the colloidal particles. The magnitude of the zeta potential gives an indication of the potential stability of the colloidal system. Zeta potential can be equated to the amount of repulsive force which keeps the particles in suspension. If the zeta potential is large, then more coagulants will be needed to destabilize colloidal particles. If all the particles have a large negative or positive zeta potential they will repel each other and there is dispersion stability. When particles have low zeta potential values, there is no force to prevent the particles coming together and there is dispersion instability. A dividing line between stable and unsable aqueous dispersions is generally taken at either +30 or -30mV.
F
ACTORSA
FFECTINGZ
ETAP
OTENTIALThere are several factors that can affect zeta potential
1. pH
In aqueous media, the pH of a sample is one of the most important factors that affect its zeta potential. A zeta potential value on its own without defining the solution conditions is virtually meaningless. A zeta potential versus pH curve will be higher or positive at low pH and lower or negative at high pH. There may be a point where the plot passes through zero zeta potential. This point is called the isoelectric point and is very important from a practical consideration. It is normally the point where the colloidal system is least stable.
2. Conductivity
Treatment of Wastewater by Electrocoagulation Method… 5
change in the value of the isoelectric point. The specific adsorption of ions onto a particle surface, even at low concentrations, can have a dramatic effect on the zeta potential of the particle dispersion. In some cases, specific ion adsorption can lead to charge reversal of the surface.
3. Concentration of a Formulation Component
The effect of the concentration of a formulation component on the zeta potential can give information to assist in formulating a product to give maximum stability. The influence of known contaminants on the zeta potential of a sample can be a powerful tool in formulating the product to resist flocculation for example.
C
OAGULATIONSchulze, in 1882, first showed that colloidal systems could be destabilized by the addition of ions having a charge opposite to that of the colloid (Benefield et al., 1982). Coagulation in water or wastewater chemistry is a process in which a chemical referred to as a coagulant is added to destabilize dispersed colloidal particles so that they agglomerate. Coagulation experiments using natural products such as Moringa oleifera have also been tried with varying degrees of success (Kasser et al., 1990; Ogutveren et al., 1994; Ndabigengesere et al., 1995; Mohammed, 2001; Bhuptawat and Chaudhari, 2003). The objectives of coagulation are to (i) destabilize suspended and colloidal particles to enhance their removal through sedimentation and filtration and (ii) to precipitate dissolved maters i.e. PO43-, color, natural organic matter (NOM). Coagulation process may require several reaction steps: (i) hydrolysis of multivalent metal ions; (ii) adsorption of hydrolysis species at the solid-solution interface for the destabilization of colloidal particle (reduction of zeta potential); (iii) aggregation of destabilized particles by interparticle bridging; (iv) aggregation of destabilized particles by particle transport and van der Waals‘ forcesś (v) ―aging‖ of flocs formed in the processś and (vi) precipitation of metal hydroxides (Stumm and O‘Melia, 19ζ8).
E
LECTROCOAGULATIONLazare Etiégni, K. Senelwa, B. K. Balozi et al. 6
W
ITHI
RONE
LECTRODESIn acidic medium,
Anode: Fe(s) Fe2+(aq) + 2e- (Equation 3)
4Fe2+(aq) + 10 H2O(l) + O2 (g) δ Fe (OH)3(s) + 8H+ (aq) (Equation 4)
Cathode: 2H+ (aq) + 2e- H2 (g) (Equation 5)
Overall: 4 Fe(s) + 10 H2O(l) + O2 (g) δFe(OH)2(s) + 4H2 (g) (Equation 6)
In alkaline medium,
Anode: Fe(s) Fe2+ (aq) + 2e- (Equation 7)
Fe2+(aq) + 2OH-(aq) Fe(OH)2(s) (Equation 8)
Cathode: 2H2O(l) + 2e- H2(g) + 2OH-(aq) (Equation 9)
Overall: Fe (s) + 2H2O(l) Fe(OH)2(s) + H2(g) (Equation 10)
Floc + H2 (g) Floats (Equation 11)
W
ITHA
LUMINUME
LECTRODESIn acidic medium
Anode: Al (s) Al3+ + 3e- (Equation 12)
2Al3+ (aq) + 4H2O(l) + O2 (g) 2Al(OH)3(s) + 2H+ (aq) (Equation 13)
Cathode: 2H+ (aq) + 2e- H2 (g) (Equation 14)
Overall: 2Al(s) + 4H2O(l) + O2 (g) 2Al(OH)3(s) + 2H2(g)(Equation 15)
In alkaline medium
Anode: Al (s) Al3+ + 3e- (Equation 16)
Al3+ (aq) + 3OH- (aq) Al(OH)3(s) (Equation 17)
Cathode: 2H2O(l) + 2e- H2(g) + 2OH-(aq) (Equation 18)
Treatment of Wastewater by Electrocoagulation Method… 7
The cation hydrolyses in water to form a hydroxide. The following equations (20 to 23) are an illustration of this phenomenon in the case of aluminum:
Al3+ + H2O AlOH2+ + H+ (Equation 20)
pH AlOH2+ + H2O Al(OH)2+ + H+ (Equation 21)
Al(OH)2+ + H2O Al(OH)30 + H+ (Equation 22) Al(OH)30 + H2O Al(OH)4- + H+ (Equation 23)
E
LECTROCOAGULATIONM
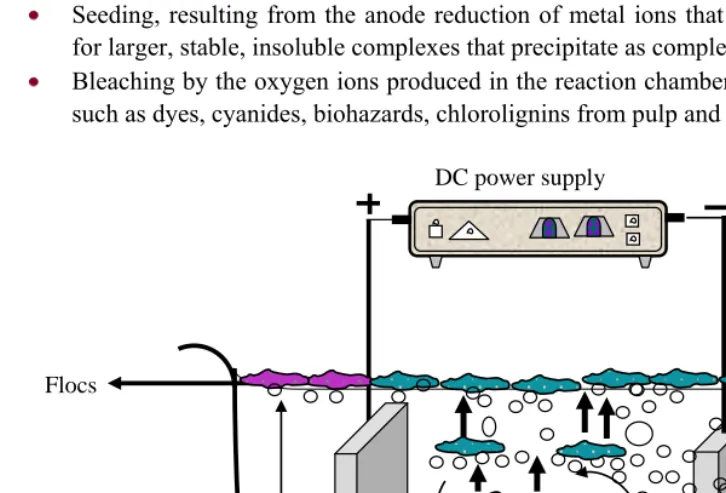
ECHANISMSThe electrocoagulation overall mechanism is a combination of mechanisms that operate concurrently or in series but synergistically. The main mechanism may vary throughout the dynamic process as the reaction progresses, and will almost certainly shift with changes in operating and environmental parameters and pollutant types. Highly charged cations destabilize any colloidal particles by the formation of polyvalent polyhydroxide complexes. These complexes have high adsorption properties, forming aggregates with pollutants. The pollutants presumably act as a ligand to bind with iron or aluminum ions resulting in the formation of amorphous polymeric complexes (hydroxo-complexes). These compounds with a large specific surface area are very active and able to coagulate and adsorb pollutants soon after their in situ generation (Rajeshwar and Ibanez 1997; Scott, 2001). Besides the generation of polyvalent cations described above, electrocoagulation includes also the production of electrolysis gases that are hydrogen and oxygen (Equation 5, 6, 9, 10, 14, 15 & 19).
Evolution of hydrogen gas aids in mixing and flocculation. Once the floc is generated, the electrolytic gas binds to and creates a buoyant force on the floc leading to its flotation and ultimately to the removal of the pollutant as a floc - foam layer at the liquid surface (Equation 11). Other flocs that are heavier settle at the bottom of the reactor.
There are many ways in which species can interact in solution:
1. Migration to an oppositely charged electrode (electrophoresis) and aggregation due to charge neutralization.
2. The cation or hydroxyl ion (OH-) forms a precipitate with the pollutant.
3. The metallic cation interacts with OH- to form a hydroxide, which has high adsorption properties thus bonding to the pollutant (bridge coagulation).
4. The hydroxides form larger lattice-like structures that sweep through the water (sweep coagulation).
5. Oxidation of pollutants to less toxic species.
6. Removal by electroflotation and adhesion to bubbles (Figure 2).
Lazare Etiégni, K. Senelwa, B. K. Balozi et al. 8
which electrochemically dissolved aluminum (from the anode) into solution, reacting this with the hydroxyl ion (from the cathode) to form aluminum hydroxide. The hydroxide flocculates and coagulates the suspended solids purifying the water. A similar process was used in Britain in 1956 for which iron electrodes were used to treat river water (Matteson and Dobson, 1995).
Because of its capability to remove several types of water pollutants, the recent thirty years have seen an explosion of journal article reports on electrocoagulation methods probably due to new and more stringent environmental regulations on a wide range of water and wastewater pollutants. This has further translated into a number of electrocoagulation devices, designed to purify water or wastewater, being put on the market.
R
EACTIONS WITHIN THEE
LECTROCOAGULATIONR
EACTORSeveral distinct electrochemical reactions are produced independently within the electrocoagulation reactor. They are as follows:
Emulsion breaking, resulting from the oxygen and hydrogen ions that bond into the water receptor sites creating a water insoluble complex that separate water from pollutants.
Seeding, resulting from the anode reduction of metal ions that become new centers for larger, stable, insoluble complexes that precipitate as complex metal ions.
Bleaching by the oxygen ions produced in the reaction chamber oxidizing pollutants such as dyes, cyanides, biohazards, chlorolignins from pulp and paper mill effluent.
C
athode
H2
Flocs
Sediments
DC power supply
Anode
Coagulation & Flocculation
Treatment of Wastewater by Electrocoagulation Method… 9
Electron flooding of the water that eliminates the polar effect of the water complex, allowing colloidal materials to precipitate. The increase of electrons creates an osmotic pressure that ruptures bacteria, cysts, and viruses.
Oxidation reduction reactions that are forced to their natural end point within the reactor which speeds up the natural process.
Electrocoagulation induced pH swings toward neutral although this will not always be the case and will depend on the type of electrolyte used.
T
YPE OFE
LECTRODESElectrode material can subtancially affect the performance of an electrocoagulation reactor. The heart of EC is the dimensionally stable oxygen evolution anode which is usually expensive. The anode material determines the cation introduced into solution. Several researchers have studied the choice of electrode material with a variety of theories as to the preference of a particular material. The most common electrodes were aluminum or iron plates as described by Vik et al. (1984) and Novikova and Shkorbatova (1982). Do and Chen (1994) have compared the performance of iron and aluminum electrodes for removing color from dye-containing solutions. Their conclusion was that the optimal electrocoagulation conditions varied with the choice of iron or aluminum electrodes, which in turn was determined by initial pollutant concentration and pollutant type.
S
TIRRINGR
ATEBazrafshan et al. (2008), while comparing chromium removal efficiency with iron and aluminum electrodes, showed that removal efficiency of chromium with aluminum electrodes was lower than chromium removal efficiency with iron electrodes. Metal consumption equally was much lower with aluminum than with iron electrodes. Conversely, power consumption was lower with aluminum than with iron electrodes for the same concentration of pollutant. However, as the chromium concentration in the solution increased to 500.0 mg/L , the consumption of the electrode reduced, but efficient chromium removal occurred due to the large amount of flocs formation that helped sweep away chromium. For example, iron electrode consumption for the initial concentration of 5.0 mg/l and voltage of 40 V was 9.01 g while for an initial concentration of 500.0 mg/L it was 7.70 g (Bazrafshan et al., 2008). The highest efficiency of chromium removal (for both iron and aluminum electrodes) was measured in acidic medium (pH = 3) for an initial chromium concentration of 500.0 mg/L and at lower concentrations, the removal efficiency was almost complete at all pH values. At high chromium concentration, however, the complete removal would have required longer time i.e. higher power consumption.
Some researchers have investigated the relationship between ―size‖ of the cation introduced and removal efficiency of organic waste (Baklan and Kolesnikova, 1996; Vlyssides et al., 1997). The size of the cation produced (10-γ0μm for Fe3+ compared to 0.05-1
Lazare Etiégni, K. Senelwa, B. K. Balozi et al. 10
as the only measure. Hulser et al. (1996) observed that electrocoagulation is strongly enhanced at aluminum surfaces in comparison to steel. This is attributed to a higher efficiency due to the in situ formation of dispersed aluminum-hydroxide complexes through hydrolysis of the aluminate ion, which does not occur with steel electrodes.
Tsai et al. (1997) employed Fe and Al anodes to simultaneously utilize electrocoagulation, responsible for removal of high molecules, and oxidation during treatment of a raw leachate. Iron anodes provided better COD removal at low applied voltages than did aluminum (Englehardt et al., 2006). As a general statement the efficiency of aluminum or iron electrodes will depend on the specific type of pollutant and also on the different set of operating parameters (Kobya et al., 2003).
E
LECTRODEP
ASSIVATIONOne of the greatest operational issues with electrocoagulation is electrode passivation. The passivation of electrodes is of concern for the longevity of the process. Passivation of aluminum electrodes has been widely reported in the literature (Nikolaev et al., 1982; Osipenko and Pogorelyi, 1977). The latter also observed that during electrocoagulation with iron electrodes, deposits of calcium carbonate and magnesium hydroxide were formed at the cathode and an oxide layer was formed at the anode. Nikolaev et al. (1982) investigated various methods of preventing electrode passivation and suggested the following options for its control:
Changing polarity of the electrode; Hydromechanical cleaning; Introducing inhibiting agents;
Mechanical cleaning of the electrodes.
According to these researchers, the most efficient and reliable method of electrode maintenance was to periodically mechanically clean the electrodes or wash the electrodes with 8% sulfuric acid between runs in batch which for large-scale, continuous processes is a challenging issue. Corrosion promoters such as Cl- ions have been found to induce thinning of passive layer, enhance dissolution and promote depassivation (spontaneous depassivation).
Treatment of Wastewater by Electrocoagulation Method… 11
thermal resistance and a wide electrochemical potential window in aqueous solutions. Above all they can provide very high current efficiencies. Diamond coated electrodes have been investigated worldwide over the past number of years with notable results (Fryda et a l., 2003). It is possible to vary electrical properties of diamond from semiconductor (very wide band gap) to close to metallically conductive by varying the boron doping level (1019-1021 cm-3).
The most important electrochemical properties of BDD electrodes are their very high corrosion stability in electrochemical applications and their extremely high overvoltage for water electrolysis (Fryda et a l., 1999). This large working potential window in aqueous electrolytes provides the possibility of producing strong oxidizing solutions with extremely high efficiency. As reported by Michaud and Comninellis (2000), compared to other electrode materials, BDD electrodes produce hydroxyl radicals on their surface with higher current efficiency. These hydroxyl radicals completely mineralize organic impurities in water or wastewater, such as oil, cooling fluid, toxic compounds (Tennakone et a l., 1995). As diamond electrodes are both stable as anodes and cathodes, it is possible to reverse polarity in order to prevent calcium build-up on the electrode surface. Through the use of diamond electrodes, it is possible to obtain an electrochemical process which, without the addition of further chemicals, results in an environmentally friendly and relatively maintenance-free method for the treatment of waste water. Nonetheless, despite the promising results with respect to effectiveness and energy efficiency which have been demonstrated for wastewater treatment, electrosynthesis and electroplating, BDD electrodes remain extremely expensive.
A new anode coated IrOx−Sb2O5−SnO2 onto titanium has also been proposed
(Xueming et a l., 2002). Accelerated life test showed that the electrochemical stability of the Ti/IrOx−Sb2O5−SnO2 anode containing only 2.5 mol % of IrOx nominally in the
activated coating was even higher than that of the conventional Ti/IrOx anode. Its service
life for electroflotation application is predicted to be about 20 years. Voltametric investigation demonstrated that the Ti/IrOx−Sb2O5−SnO2 anode could provide fast
electron transfer. The present anode had a fork-like design and arranged in an interlocking manner with the cathode with a similar shape. Such an innovation in electrode configuration and arrangement is claimed to allow bubbles produced at both electrodes to be dispersed into wastewater flow quickly and, therefore, enhances the effective contact between bubbles and particles, favorable for high flotation efficiency. In addition, the novel electrode system reduces the interelectrode gap to 2 mm, a spacing that is technically difficult for a conventional electrode system (Xueming et a l., 2002). This small gap results in a significant energy saving. Easy maintenance is another advantage of this novel electrode system.
A
REAS OFA
PPLICATION OFE
LECTROCOAGULATIONLazare Etiégni, K. Senelwa, B. K. Balozi et al. 12
arsenic bearing wastewater, and chemical mechanical polishing wastewater. The electrocoagulation process can successfully remove a wide range of pollutants in a much shorter time than conventional treatment methods (Ogutveren et al., 1994; Kongsricharoern and Polprasert, 1995, ). They include: removal of metals, oil, BOD, TSS, TDS, FOG, color etc., from wastewater before final disposal, thus reducing or eliminating discharge surcharges; reconditioning antifreeze by removing oil, dirt, and metals; reconditioning brine chiller water by removing bacteria and fat; pretreatment before membrane technologies such as reverse osmosis, ultrafiltration and nanofiltration; preconditioning boiler makeup water by removing silica, hardness, TSS; reconditioning boiler blow down by removing dissolved solids eliminating the need for boiler chemical treatment; recycling water, allowing closed loop systems; harvesting protein, fat and fiber from food processor waste streams; de-watering sewage sludge and stabilizing heavy metals in sewage, lowering freight and allowing sludge to be land applied; conditioning and polishing drinking water; removing chlorine and bacteria before water discharge or reuse (Cenkin and Belevstev ,1985; Biswas and Lazarescu,1991; Browning,1996; Adhoum et al., 2004).
C
OLORColor is found mostly in surface waters, although some groundwater inside deep wells may also contain color that is noticeable (APHA-AWWA, 1992; AWWA, 1999). Many domestic and industrial wastewaters are rarely colorless and the color levels depend on the industrial process and the age of the wastewater i.e. the travel time in the collection and treatment system (Kim et a l., 2005). The pulping and bleaching of wood for example generally produce large amounts of wastewaters that contain lignin derivatives and other dissolved wood by-products. Lignin derivatives which are usually brownish in color remain resistant to biological degradation during wastewater treatment. The brownish color of a pulp and paper mill effluent is mainly attributed to products of lignin polymerization formed during pulping and bleaching operations. These chromophoric groups are mainly quinonic types with conjugated double bonds originating from pulping processes (Luner et a l., 1970). When disposed of into natural watercourses, they add color which persists for great distance. Additionally, colored effluents from pulp and paper mills for example result in reduced photosynthetic activity, increased long term BOD, increased water treatment cost for users downstream, and increased toxicity (Springer et a l., 1995).
Treatment of Wastewater by Electrocoagulation Method… 13
A
DVANTAGES OFE
LECTROCOAGULATION(EC)
Electrocoagulation has several advantages that are as follows:
EC produces effluent with less total dissolved solids (TDS) content compared to chemical treatments. If this water is reused, the low TDS level contributes to a lower water recovery cost.
EC requires simple equipment and is easy to operate with sufficient operational latitude to handle most problems encountered during its running.
Wastewater treated by EC can give palatable, clear, colorless and odorless water.
Sludge formed by EC tends to be readily settable and easy to de-water, because it is composed of mainly metallic oxides/hydroxides.
Flocs formed by EC are similar to chemical floc, except that EC floc tends to be much larger, contains less bound water, is acid-resistant and more stable, and therefore, can be separated faster by filtration.
The EC process can remove the smallest colloidal particles, because the applied electric field sets them in faster motion, thereby facilitating their agglomeration and subsequent coagulation.
The EC process often avoids uses of chemicals and so there may be no problem of neutralizing excess chemicals and no possibility of secondary pollution caused by chemical substances added at high concentration as when chemical coagulation of wastewater is used alone.
The gas bubbles produced during electrolysis can carry the pollutant to the top of the solution where it can be more easily concentrated, collected and removed.
The electrolytic processes in the EC cell are controlled electrically and with no moving parts, thus requiring less maintenance.
D
ISADVANTAGES OFE
LECTROCOAGULATION(EC)
Lazare Etiégni, K. Senelwa, B. K. Balozi et al. 14
process as a whole. The use of new materials, different electrode types and arrangements (Pretorius et al., 1991; Mameri et al., 1998) and more sophisticated reactor operational strategies (such as periodic polarity reversal of the electrodes mentioned above) have led to significant reductions in the impact of passivation. The issue, however, is still seen as a serious potential limitation for applications where a low-cost, low maintenance water treatment facility is required.
D
ESIGN OFE
LECTROCOAGULATIONU
NITSThe inherent complexity of the electrocoagulation reactions makes it difficult to model and control this process. Adequate scale-up parameters, a systematic approach to the optimization and a priori prediction for the performance of the electrocoagulation reactor are yet to be established. A literature survey reveals that previously each ―new‖ system has been considered separately on an individual basis. The key driver for the development of any particular application of this process has generally been the removal of a specific pollutant i.e. color, heavy metal, COD, tannin etc. There has been little or no attempt to provide a holistic approach to electrocoagulation. Consequently, despite more than a century‘s worth of applications, many of them deemed successful, the science and engineering behind EC reactor design is still largely empirical and heuristic. It has failed to take full advantage of the potential success and incorporation in the understanding behind the science of electrocoagulation.
A literature review indicates that EC reactors can be configured as batch or continuous and that the majority of reactors reported so far fall in the latter category with continuous feed and outflow operating under pseudo-steady state.
Electrocoagulation systems require amperage to treat the water. The amount of amperage drawn is dependent upon the conductivity of the water or wastewater. If the water is not conductive then no amperage will be used. The system should be designed with adequate wiring and electrical capacity to deliver adequate amperage if needed by a particular water stream.
P
HYSICALD
ESIGNI
SSUESThere has been a range of laboratory, pilot and industrial scale electrocoagulation units produced. The designs range from fully integrated units to ‗stand alone‘ reactors. The electrocoagulation process has been combined with many units including microfiltration, dissolved air flotation (DAF), sand filtration and electroflotation. Obviously, pre- and post- water treatment impacts significantly on the performance of the electrocoagulation reactor. The design of the electrocoagulation process influences its operation and efficiency (Holt et al., 2005). The design phase should consider the following physical factors:
Continuous versus batch operation Reactor geometry
Treatment of Wastewater by Electrocoagulation Method… 15
G
EOMETRYGeometry of the reactor affects operational parameters including bubble path, flotation effectiveness, floc formation, fluid flow regime and mixing/settling characteristics. From the literature, the most common approach involves plate electrodes (aluminum or iron) and continuous operation. Water is dosed with dissolved metal ions as it passes through the electrocoagulation cell. A downstream unit is often required to separate pollutant and water.
S
CALE-U
PI
SSUESOne of the cornerstones of chemical engineering is to establish key scale-up parameters to define the relationships between laboratory and full-scale equipment.
The surface area to volume ratio (S/V) is a significant scale-up parameter.
Electrode area influences current density, position and rate of cation dosing, as well as bubble production and bubble path length. Mameri et al. (1998) reported that as the S/V ratio increases the optimal current density decreases.
However, the S/V ratio was not widely reported. Some of the values reported are listed in Table 1 below:
The values reported here seem empirical with no specific criteria for their choice. A more rigorous and consistent approach is clearly required to establish a set of design characteristics for Electrocoagulation reactors. The prime differentiator between pollutant removal by settling or flotation would seem to be the current density employed in the reactor. A low current produces a low bubble density, leading to a low upward momentum flux—conditions that encourage sedimentation over flotation (Holt et al., 2002). As the current is increased, so does the bubble density resulting in a greater upwards momentum flux and thus more likely removal by flotation.
Other researchers such as Zolotukhin (1989) scaled up an electrocoagulation-flotation system from laboratory to industrial scale. The following dimensionless scale-up parameters have been chosen to ensure correct sizing and proportioning of the reactors:
Reynolds number – indication of the fluid flow regime; Froude number – indication of buoyancy;
Weber criteria – indication of the surface tension; Gas saturation similarity;
Geometric similarity.
Table 1. S/V values reported in the literature.
Reference (Author) Year S/V (m2/m3)
Amosov et al. 1976 30.8
Osipenko and Pogorelyi 1977 18.8 Novikova and Shkorbatova 1982 42.5
Orori et al. 2005 80.0
Oricho et al. 2008 75.5
Lazare Etiégni, K. Senelwa, B. K. Balozi et al. 16
Horizontal Flow
Vertical flow
Figure 3. Types of electrodes set-up during EC.
Electrodes during EC can be set up as parallel vertical or horizontal sheets as can be seen in Figure 3.
The turbulence generated by the gases at the anode and cathode can be used in both types of flow. However, vertical flow allows for more improved separation by electroflotation as compared with horizontal flow.
F
ACTORSA
FFECTINGE
LECTROCOAGULATIONP
ROCESSESSeveral studies have shown that electrocoagulation is quite complex and may be affected by several operating parameters such as pollutant concentrations, initial pH, electrical potential voltage, COD, turbidity, pollutant type and concentration, bubble size and position, floc stability and agglomerate, size and type of supporting electrolyte. The complexity and number of possible interactions are highlighted in Figure 2.