This
is
to certify
that
Teguh
Widodo
has
participated in
4:i"dMeeting
of
National
Working
Group on
Indonesia
Medicinal
INTERNATIONAL
CONFERENCE
ON MEDICINAL
PLANTS
Scientification of
Jamu
(Evidence-based Jamu Development):
A
Breakthrough
Program
from
Plant
to
Medicine
for
Health
Care
Purwokerto,
Indonesia
october
ll -
13,2012
as
Presenter
The
National
Working
Group
on
Indonesia
Medicinal Plant
Secretary General,
gf,RT[FlgAT'f,
,q
Plant
a
fenderal
Soedirman
University
Organizing
Comrnittee
Chairperson,
t
/11,..--rl>
l.,
lii i
i',:'
tl
\r/li
/l
Scientification
of
f
am u
(Evidence-based
f
amu
Development):
A
Breakthrough
Program
from
Plant
to
Medicine
for
Health
Care
lt f 'l
1.1
t )\
,tn
f
The 43'dMeeting
of
National
Working
Group
on
Indonesia
Medicinal
Plant
"Exploration, Conservation, Development,
rnd
Utilization of
lndonesian
Medicinal
Plant"
Penerbit
:UNIVERSITAS
IENDERAL
SOEDIRMAN
PURWOKERTO
/-"\
b?s)
\
e.:-7l
\
\"--.-'l
I
I
PROCEEDINGS OF
INTERNATIONAL
CONFERENCE ON
MEDICINAL
PLANTS
Scientification of
Jamu (EVidence.based Jamu
DeVeIopment):
A
Breakthrough
Program
lrom
Plant
to
Medicine
for
Health care
ln occasion
of
The
43th
National Meeting
of
National Working Group on Indonesia
'
Medicinal Plant
'il'"'-fi:::::3:::'"
Editors:
dr.
Agungsaprasetya Dwi
Laksana,M'5c',
PH' HenYEkowati,
M.Sc',APt'
Dr. Agus
Nuryanto,
M'Si'
Dr. PuliWidodo, M'Sc'
Rehana,
M.5i., APt'
Lay-out: Sarmoko Prima Andika Citra
Revo Almando Harris
Published
bY.
Ed
itor
Pera nca ng
Sampul
Penata Letak
Pracetak
dan Produksi
Perpustakaan Nasional Rl: Katalog Dalam
Terbitan
PROCEEDINGS OF
INTERNATIONAL
CONFERENCEON MEDICINAL PtANTS
Scientification
ofJamu
(Evidence-based Jamu
Development):A Breakthrough Program from
Plant
to
Medicine
for
Health
CareO
Universitas
Jenderal Soedirman
cetaka n
Pertama,
2012
llak
Cipta dilind
ungi Undang-undang
All Right
Reserved
Penerbit
@
UNIVERSITAS JENDERAL SOEDIRMAN
Jalan
Prof. Dr.
H.R.Boenyamin
708
Purwokerto
Kode
Pos53122 Kotak
Pos 115lelepon 635292 (Hunting) 638337, 638795
Faksimile
631802
www.u nsoed.ac.id
ISBN:
978-979'9204-80-6
vii
+27L
hal,29.7
cmx21
cm
Dilarang
keras
memfotokopi atau memperbanyak
sebagian
atau seluruh buku ini tanpa
seizin
tertulis dari penefbit
dr.
Agung Saprasetya
Dwi
Laksana, M.Sc.,
PH.Heny
Ekowati,
M.Sc.,
Apt.
Dr. Agus
Nuryanto, M.5i.
Dr. Puji
Widodo,
M.Sc.Rehana, M.Si.,
Apt.
Panitia
Sarmoko
Prima
Andika
Citra
Revo
Almando
Harris
Committee
sterring
committee:
Dr. Hj. Retno
Widiastuti,
M5
Indah
Yuning
Prapti,
SKM., M.Kes.
Prof. lr. Totok Agung
DH,
M.P.,
Ph.D.Prof.
Suwijiyo Pramono,
DEA.,Apt.
Prof.
Dr. lr. Suwarto,
MS.lr.
Agus
Margiwiyatno,
MS.,
Ph.DDr. Bambang
Heru Budianto,
MS.Dr.
lr. Akhmad Sodiq,
M.Sc., Agr.
Sugiyono,
Ph.DTuti
SriSuehesti,
M.5c.
Dr. Lesley Braun
Assoc.
Prof. Wandee
G ritsanapan
Chariperson:
Dra.
Warsinah,
M.Si.,
Apt.
iza), Turmeric (Curcuma domestica) and
n.J
Cing".
(Zingiberfficinale
cv. Rubrum) As Preventive of Nephrotoxicity Due to the Useof
DoxorubicinSoroyi
iiliwironi,
Ulya Rahn awati, Ayu Mayangtari,-Retna Pancaw.ati, Dina Arumi,Sarmoko
- .CombinationExtractCiandCurcumaxanthorrhizal01
As Anticardiotoxicity of Doxorubicin
Yohan Budi
Alint,
Wulan P. Rahayu, Agustin Damayanti, Ning Uswiyatun, Rika Triyanaputri, Heny97
Ekowati
a domestica, and Zingiber officinale Combination 106
Against Doxorubicin: A Hematological Study
Revo Almando Harris, Sornroko,
Itn! ilro.o!
(Psidiunlguajava)onAcuteToxicityRatLiverTissue
that Induced Glrrcose
Kristianto, A
S.f.
yEr.t".tt""
"f
Slimming Herbal X In Liver Function of Fernale Wistar RatGiva Oliviana Yudhista, Honrf Natiotul
Bororoh,Vitit
118
ffiicityStudyofExtractSecang(CaesalpiniasappanL)
127T. Sari
eSemenUsingGlucoseToleranceTestinAlbinoMaleRats Monica IVidyawati Irwan Set
i.
Yohanes Herntawaneleontidae Species (Myrmeleon sp') As an Oral Hypoglycemic Agent
Rahajeng P. Aryani, Sherliyana Novi.ta,
-N94i!t Ayunjtu'
llarwqEo
.
.
.
--N"pht"qrt""*
D*trativesas Potential Inhibitors of o-GlucosidaseActivity
142138
Mutia Hardhiyuna, Rilianawati,
Firdqtani,
Susi Kusununi Siska Andrina K.Eff""t
OfHyd-.y
G.oup Addition on 1,4 Naphtoquinon to Inhibit Alpha Glycoside Enzyme 147 Siska Andrina Kttsuntttstuti, Rilianawati, MutiaHu1!!yng'
Sus!@eenEthanolicLeafExtractofCaricaPapayaL.with
l)JHydroclorotiazid to Male White Rat
Ratih Febrina Puspi' Printananda
Zilni,
E".lr"ti""
"f
F"r-ulation
of Antidiabetic Herbal TableI of Andrographis panicr.r/a/a Ness15'7
Darntawan, Sri
F"r-"t"ti""
""d
Evaluation Metfomrin Hydrochloride Beads Using Matrix of Chitosan-Sodium162 Alginate Crosslinkage
Wahvu Kurniawan, Vitis Vini Fera, Prisci Perntanasari
F"r-rl.ti"r
"rd
Evaluation of Preparations Lotion Antioxidant Ethanolic Extract of Soursop Leaves(Annona nruricata L.)
Shinta Angresti, Iskandar Sobri, Tuti Sri $ulgst[
fo.-"tution
of Enteric Coated Tablets Containing Pineapple (Ananas Comosus Merr.) Fruit Juice 1'77 Teguh l(idodo, A , Ferry lrau,anI"ftu."..
ofVa.io*
Concentrations of PVP K-30 As a Binder on The Physical Quality of Cerme (Phyllantus acidus) Leaves Extract TabletKuncoro Foe, Teguh Widodo, Juleiry Selfiuni
184
Standirdization and Fonnulation of Antipyretic Tablet of Holy Basil Leaves Extract (Ocintum sanctLtm
L.)
Farida Lanawati Darsono, Yuliani, Sri
Harti
Effectiueness of Antifungi Galanga Essential Orl (Alpinia galunga) Preparations in Ointment 197
Tri Ayu Apriyani, Banu
Aii
lhijayanto, Ardhea Fredyantara, Testy Dwi Septiandini, SarmokoP"t."tt.t
""d
A*ty*
L.r"*ide
Fraction of Interest on Rumput Laut (Eryngium foetidunt)Against
202 Mosquito Larva Aedes aegtpti
Budi Untari, Herlina
epptl*ti*
of High Performance Liquid Chromatography in the Determination of Antifungal Drug in2t0
Samples
Dadan Hermawan, Nur Atiqah binti Abu Bakar, Wan
Aini
Wan lbrahim, Mohd Marsin Sanagi@iiofTriterpenoidandFlavonoidfromCentellaasiaticaLeafExtracts219
Harwoko, Esy Nansy, Suwijiyo Prantono
184
|
K. Foe et ol.Influence of Various Concentrations
of
Pvp K-30
AsA Binder on The Physical Quality
of
Cerme (Phyllanthus acidusl
Leaves
Extract Tablet
Kuncoro Foe*, Teguh Widodo, Juleiny Selfiani
Faculty of Pharmacy,
llidya
Mandala Catholic IJnirersity.Jl
Dinoyo 12-44 Surabaya* C o rre sp o ndin g a u t hor : ku n c orofo e@ya h o o. c o m
Abstract
A study on the inlluence o1'tablet lbrmulae containing cernre leaves extract using PVP K-30 as a binder in the concentrations
of 27o (lbrmula
A),3%
(tbrmula B),4% (lbrmula C) on the physical quality ol'tablet has been conducted. (lernte leavesextract was obtained by percolation method employing ethanol 960/o as a solvent. Tablets rvere prepared by wet granulation
nrethod arrd the physical quality ofresulting granules was evaluated including moisture content, repose angle, tlow rate, and
compressibility. Granules were then compressed to yield tablets of 650 nrg. in which each tablet contains 449 mg of dry extract of cerme leaves. The physical quality of resulting tablets was evaluated including rveiglrt unifonnities, hardness.
fiiability. disintegration time. dissolution. and a comparative qualitative determination of chernical contents of certne leaves
extract by TLC (Thin Layer Chronratography) in various samples conrnrencirtg frolrr viscous extract until tablet dosage fornr.
The hardness. friability. disintegration tinre. and dissolution data werc evaluated using contpletely randorlized design
ANOVA rvith a level of confidence of 95o/". and subsequentll' tbllorved b1 LSD (Least Signilicant Difl-erence) test. lt can be
concftrded Ihat cernte leaves extract could be formulated as a tablet arrd rrret the physical quality requirernents oftablet. There
\\€re no significant dill'erences orr the hardness and dissolution of three fbrntulae tablets (F""t.< l-'061"). On tlre otlter hand. the
liiabilit-r and disintegration tinre ofthree fornrulae tablets diflered significantll'(I"."r.) Frnr,r"). Fotrrtttla A rvas the selected one
osing ttt its shortest disintegration tirne and highest econonric value (least binder corrcerltlal.iorr).
Kcl
rvords: Cemte (Phvllanthus crcidus) leaves extract: Tablet tbrnrulation: Physical qualitl ol'tablet: Bindcr: PVP K-30.Background
Obesitl tna)
cause a serious problem sinceit
can lower someone's self'-esteem and result itr various diseasesincluding: cardiovascular.
gall
bladder. and diabetes. The causesol'obcsity.
anrong others. are excessive tbod collsunlption. exercise patterns. psychological and genetic I'actors. as rvcll as metabolic abnormalities. Various techniquesto
handle obesity include diet. exercise. psychothel'apy and nredication intervention using appetite suppressants (Gu1,ton. I 997).One
tlpe of
plant that can lorver the body weight and leduce the appetite is cerme (Phyllanthus acidtrs).ltis
knorvn that the viscous extract of ce,'nre leaves at a doseof
I
g/kg brr ma) reducebodl
rveight and epididimuslipid ot'albino Wistar rats. The administration ot'cerme leaves inlusion can also reduce the
bodl'weight
lvithoutclecreasing the appetite and this infusion is not toxic in rnice (Altlah. 2000). -fhe
leaves. bark and stem parts of cernte contain saponins. flavonoids. tannins and polyphenols (Hutapea. 1994:
Sastloamid.joyo.200l).
lt
rvas hypothesized that tannins ma1'clenronstrate slimnring efl'ectby
precipitatingr.nucosal proteins
in
the intestine and hence. reducing the absorptionof
ibod (Robinson. 1995). The content clf'tarrnins in cernte leaves extract can be detelrnined b1 pernranganonretr) titfation rnethod (Anonymous. l9tl9).
l:requent problerns arising
lionr
the adnrinistrationof
tladitional nrcdicine are unpleasant tastc and odor. lesspractical use and less precise dose (YapLrtra. 1989). Developn.rent ol'scicnce in plralmaceutical technologl, lield
lras rcsulted
in
a rapid developnrentof
the dosagelblnr.
Extract canbc
tblmulated into various dosage lblrnssuclr irs granules. tablets. capsules and
slrup.
Tablet is selected becauscit
can mask the unpleasantodor
andtastc..fablet
phlsico-chemicalll is
Inore stable than s1'rup.it
has a solid shapc and srnall volunre so thatit
iscasily packaged. stored and transported. and is not easily contar.ninated
bl
rricrobes (Allen el a|..2011).(
errrs
leaves extlact is hl,groscopic. sticky. pool corlpressibilitl'. has a chalactelistic taste and odor. Theretbre aerosil should be incorporated as a desiccant in the preparation ol'cermedrl
extract. Wet granulation method isselected
in
the
preparationof
tablet
!ince
the
chemical contentsin
cernrc leavesare relatively
stable to tempcrature and humidity. Binders play a signilicant rolein
'uvet granulation method. by bindingall
particles inProceedings International Conference on Medicinal plants I
20t2
|tablet tbrmulation, PVP K-30 can be employed in the solution lbrm or dry powder subsequently tbllowed by the addition
of
wetting liquid. The amountof
binder in the fbrmula can afi'ect the stredgth of granules and hence.influencing
the
physicalquality
of
the
resulting tablets. Cholit'ah (2003) reported thatPVP K-30
at
high concentrations increase the hardness and prolong the disintegration timeof
tablet. Thiswill
in turn reduce the amount of dissolved drug. Thus the determination of optimum concentrltion of PVP in the lbrmula is necessary.In this study. PVP K-30 at various concentrations
of
2, 3 and4Yo were employed as a binder in tablet lbrmulacontaining cerme leaves extract.
The
physicalquality
of
resultingtablets including
hardness- triability. disintegrationtime
and dissolution lvas observed.lt
is
expectedthat
cernre leaves extracttablet
tblmulacontbrming the physical qualitl, requirements of tablet could be obtained.
Material
and MethodsM ate ri al s and A ppara ! us
Cerme leaves were obtained liom'Nongkojajar region East.lava grown in a land
of
approximately 300 m abovesea levef with rainlhll of approximately 2271 mL/year. Priol to the study. cernte leaves r.vere characterized by the UPT Materia Medica Batu-galang East.lava Provincial Health Council.
The experimental materials
of
pharmaceutical grade included magnesium stearate. talcum. aerosil. PVP K-:]0.sodium starch
gllcolate.
dibasic calcium phosphate and kaolin.The
fbllorving materials. including KMnOr.H2SO4. oxalic acid. sodium hydloxide. iodine. FeClr. l-lCl and indigo carmine. \vefe
ot'plo
analytical grade.Equipments used in this
studl
r.vere tabletting machine single punch. tablet haldness tester (Schleuniger 6D-30 type. German)'). tablettiiabilitl
tester (Elr.vekaTA-3
type. Celmany). disintegration appalatus(Elneka
Z-l'3-l
t1 pe. (ierntan) ). Inoisture analyzer'(Sartorius
MA
30 t;-pe. Cermanl). analytical balance (Toledo MetlerAl. 20i
t1pe. Cerrnanl,). glass appafatus and otlrer supporting equipments.
P re pat'cr I i o n of C t'ucle,l lat e r i a
I
'l-he leal'es nete nashed ancl rinsed. and then dried in a shady aerated place.'fhe dlied leaves n'ere glounded and
sieved thlough a no. 4/8-mesh siever. Ex|t'aclion ,l'lethod
l'he
extractiorl method enrployed in this study was percolation using 967o etharrol. u,ith a Uou, fateof'I
mLintin.-l-he
lesultins pelcolate \{as evaporated on a water bath at a controlled temperature ol'less than 50
"C.I'uentr-live -eratns ol'aelosil rras added into the lesulting viscous extract. and dlied irr an oven a[ a ternperatLrre ol'50 "C. to obtain a clrv cxtt'itct subsecluentlr gloundcd and sier"ed thlough a-100 nresh siever'.
Dose Calcttltttion
('elzre lcaves
e\tlact
\\'as adnrinistered at a doseof I
g/kg br.r'ma) reduce the body rl'eight and epididinrus lipidas rvell as alter tligll.ceride level irr rats.
[)oseinhurnan= r'atdoscxconversionl'actor(=
l/1000x200x56)=
ll.2glT0kgdail;
'l'he body u,eight ol'Indonesian people is gener.alll, 60 kg
160/701
x
ll.2
g = 9.6 9/60 kg brvol'r,iscous extract daillDose :
[dly extract/viscous extfact] x dose ofviscous exrfacr (= 96.85/ll5
x 9.6) = 8.08 g/da1ln
this stud1. each tablet containeddrl
extractof
cerme leavesoi'
0.449,s andsix
tablets n,ere adnrinister.edthlee tinres
dailr.
Celnre leaves extract tablets u,as prepalcd into three dill'erent tbrmulaebl
rvet -eranulation method trsing PVPK-i0
at
concentrationsof
2% (FA).
3%(FB)
and 4Vo(FC)
asa
binder. Each tbrmula consistedof
| 0t) tablets and prepared in triplicates.Formula tablets are shown irr Table
l.
186
|
K. Foe et al.Table
l.Tablet
fblrnula of cerme leaves extt'acti*ul3
B('*)
rotmu-ll-C (m*)
l.
Cernte leaves extract2.
Dibasic calcium PhosPhate3.
Sodium stafch glycolate4.
PVP K-30 (2' 3 and 4o/o)5.
Talcum (4%)6.
Magnesiumstearate(lVo)449
I z)
32.5
l3
26 6.5 650 449 I 16.532.5 19.5 26 6.5 650 449
lt0
32.5 26 26 6.5 650I otal wel
Ta b I et tr4 a nufac tw' i ng P rocess
Dry
extfact was mixedwith
dibasic calciunr phosphate, sodium starch glycolate(5%)
until
honrogeneous' Asolution
of
pVp
K-30 was added to tbrm a damp gr.anule mass.then was sieved thlough a-16 ffresh siever and dr.iedin
an oven at a remperatu'eof
50 oC. Dried !ro,',ules were re-sieved th'ough a-20 mesh siever and thenmixed,"vith 4%o of talcum and l%o of maguesium stearate. The physical quality
of
nrixed granulesr'vas evaluated
including: moisture content,
flowabilitl'.
r'epose angle and conrpressibility index' Gt'anulesof
good quality rvet'e compressed using a tabletting nrachineu'ith
a-13 nrnr diatrleterof
molds arrd a tablet rveightof
650 mg'The
quality
of
r.esulting tablets was evaluated includirrg:u,eight a.d
size uniib'nrities-liiabilit}'
disintegration time and dissolution fate of tablets.TLC C hromatogram Prof les
Toe'aluatethestabilityofchenrical
conteuts itrcet'me leavesdurin-e.tabletnranulhcturingprocess'aTLCstudyu,as perfbrmed in the Stages of ext|action process. drl,ing pI.ocess. and tabletting.
Preparalion of Test SamPles
Extract pouders. granules and tablets
of
each tblrnula rlere lveighed (650 mg)' dissolved in96Yo ethanol up tol0
mL. fllter.ed. and the tlrst frltrate rvas discarded r,r,hereas the subsequent tlltrate r'vas collected' Ten microlitel'soffiltrate
rras applied onto a-GF:5a silica plate and eluated using a nrixtureofn-hexane:
ethyl acetate(4:l'
v/v)u,ith a development dlstance
of
8 cm. The spot rvas obserYed using visiblelight'
uv
254and
366 nm' Follou,ing the above obser.r,ation. the spot uas sprayed usingvanillin
sulphate as a spra;-ing reagent' driedand
obser.ved using visible light.
UV
254 and366 nrn. -fhe Rf value r'vas 0'2-0'5 cnr (Kirchner' 1978)'Results and Discussion
Thepartsofplantusedinthisstudywerelieshatrdgreencernte|eayes.Toensurethetypeofplantemployingin
this stud1. the cet.nre leaves were characterized by the UPT Materia Medica Batu-Malang East Java Provincial Health Council'
pr.ior
to
extr.action.cernte
leaves por'r'der rvas sub.lectto
severalcrude
standardizationtests
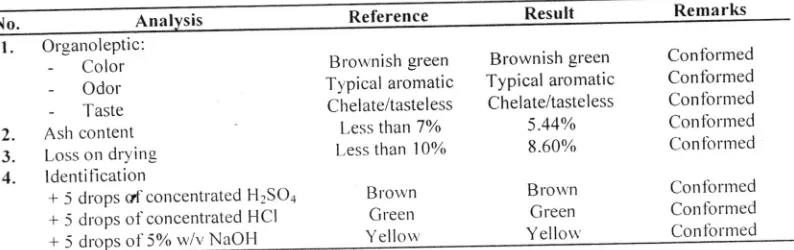
including: organoleptic. ash content. loss on dr-ying. and chernical identillcation^as shorvn in Table 2' The lesults indicated that the ct'ude rnatel'ial met all the requirements (Anonylnous' 1989)'Table 2. The results of crude material of cerne leaves porvder
Remarks Reference Result
No.
l.
OrganolePtic:-
Color-
Odor'-
Taste2.
Ash content3.
Loss on dlving4.
ldentiflcationBrortnish green
T1'pical at'omatic Chelate/tasteless l.ess tlran 77o
l,ess than l0olo
Brorvn Green
Yellow
Blownish green
Typical aromatic Chelate/tasteless 5.44% 8.60% Brou'n Green Yellorr' Confblmed Contbrmed Contbrmed Confbrrned Conlbrmed Contbnned Corrfbrmed Contblmed
+
i
drops t/-concentrated I-lrSO1Proceedings International Conference on Medicinal plants
20t2
The extraction process employed
in
this study was pelcolation using 96% ethanol.to
maintain the stability ol.active compounds in the crude material orving to the absence
of
heating process.Tht
testing result ol'viscousextfact is presented in Table 3.
lrcr
t"
Table 3, Examination results of viscous extract No. Observation Reference Remarks
l
2.
3.
Color' Odor
Brownish green
Typical aromatic Viscous
Contbrmed Confbrmecl Confbrnred
Aerosil was added into the resulting viscous extracts and subsequently dried in an
o'en
r.r,ith a temper.atureof
50 "C until dry. This resulting dry extract was used as an active ingledient in the tablet manuf'acturing process.
Each tablet contained 449 mg of dry extract.
wet
granulation method using PVP K-30 as a binder at concentratior-rsof2yo(FA).3%
(FB). and 4% (FC) rras selected' To identily the compessibilit.v- and flou,ability charactelistics of gmnules. seyeral tests \\,ere pertbrnrecl.as shosn in Table 4.
Table 4. The quality of granules No. Parameters utrements FA
t.
2.
3.
4.
Moisture content Flolv tinre
Repose angle
Conrplessibilit1,(%)
2-5%
<
| 0 seconds20-40"
5-l8o/o
4.48 + 0.20
9. t5 + 0.03
3t.45+0.03
13.73
*
0.59FB 4.44 + 0.22 7.77 + 0.10 31.78 +
0.0i
14.t0 + 0.72
FC 4.75
r
0.42 9.44r
0.ti
3 l. t5r
0.06I
i.80
+ 0.56fhe results shoued that FA. FB and FC rnet the quality standar.d of good grat.lules" narrrelr the ntoisture content
ol'2-5o/o (Bandelin
&
Shangrau- 1989:Voigt,
1995), theflou,time
of
less tlranl0
seconds (Voi-er. 1995). therepose angle
ot'20-40'(Marshall.
1996). compressibility of5-lg%
(Fierse&
FIagen. 1996).Good quality o1'tablets should meet the quality requirements as desired. including uei_shr and dose unifbrmities. lack ot'incompatibilities. att|active appeafance. as well as possible and etllcient large scalc
producti.,
(Bandelin
&
Shang|ar'r'. 1989). The examination results of the qualitv ot'c,er.rze lear,es exl.ract rabler ar.e shorinin
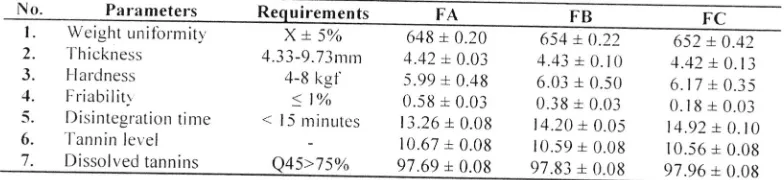
l.able 5.Based on data shown
in'fable
5- FA. FB and FC demonstrated good r.r,eight uniibrnrit-r since none of'the tabletsshorted r'r'eight deviations
of
mot'e than 5o4 andall of
thern passed the sizeunilbrrnitl
test.'l-he diamelers.1. labfet \\'ereof'
no mot'e than three times and of no less than 413 tinres of'tablet thickness (AnonVnrous. 1979).Table 5. 'l'he clualitl testing ol'rablc-ts
No. P:rr:r meters en ts FA
xt50h
648 + 0.204.i3-9.73mrn
4.42 + 0.03FB 654 + 0.22 4.43 L 0. t0
652 + 0.42 1.42 + 0.13
FC
l.
WeightLrnifbrmit-r,2.
'l hickness3.
l lardness1.
Friabilitr5.
Disinteglationtinte6.
'l'annin ler, el4-8
kgf
5.99 + 0.48 6.03 +0.50
6.17r
0.35<
l%ol5
minr0.58
r
0.03 0.38 .L 0.03 0.18r
0.03: |
/e
rr.Jdi (r.Uj
U.jE :L0.0j
0.lg
t
0.03nrinutes
13.26rr.zo:t
+0.08
U.UU
14.20,r l4.2U,t0.05
0.05
14.92 14.92 + + 0. 0. l(l0
10.67 +
0.08
t0.59 +0.08
10.i6 r v.Jv{
! 0.08w.vo087.
Dissolvedtannins
e45>75%
97.69 +0.0g
97.gj +0.08
97.96
*
0.0g.l
ablet ha|clness is the lb|ce reqtrired to break or-crush tablets and expressed in a kilo_ur.anr lbrce r,rnit. Accor.ding
to
Pat'rot (197 1)a
good hardnessof
tablet shouldvary
betneen4-8
kgl.
'l'lre
r.esLrlts sho*,edan
increased concentration ol'PVP K--i0 may enhance the hardness oltablets although they <tid notdifler.signiticantll
188 I K. Foe et o,.
Tablet ti.iability test is used to deterrnine the exrerrt
ot
tablet lesistance to'uvardiiiction
and shocks experiencedduring manuf'acturing, packagirrg ancl transportation.l-he t'esults shorved that FA, FB and FC had good
{iiability
because its value wasof
less thanl%
(Banker&
Anderson, 1996). Statistical analysis by orre wayANoVA
showed no signiflcant dillerence in the tablettiiabilitl
anrong lbrmulae (p<0'0'j)'
Disintegration tirne is an important parameter
tbl
arr oral tablet sinceit
may atfectthe oral bioavailability' Tabletis claimed
to
be completely crushedif'the
renrairring preparatiorrs lel1 on the apprratus scleen is a soft masswhich does not have a clear.cor.e. Tablet disintegratiotl time should be of no mot'e than
l5
minutes (Anonynrous'1979).
The
expefimental results demonstratedthat
FA- FB
and
FC
nretthe
requirenrents'An
increasedconcentrationofpVpK-30prolongedthedisinteg|atiorrtime
ot'cerme leavesextractsandtherewasasignificantdift-erence in the disinteglation time among tablet forrnulae by one r'vay
ANOVA
(p <0'05)'Dissolution test results showed that FA. FB and FC met the requi|empnts acoording to Shargel
&
Yrr (1999)' thatis the
per.centageof
released drugis
75%o rvithin45
minutes. Thet'e wasno
signiticant dill'erencein
tabletdissolution among lbrmulae
by
one $,ayANOVA
(p<0.05) andthis
may be dueto
a small ditl'e|encein
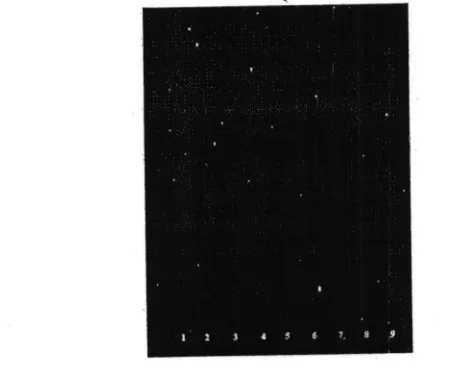
the concentrations of PVP K-30 as a binder among FA' FB and FC'Figure
l.
chromatogramof
constitutents ttt cernrc leaves extract, from crude materialsuntil
tablets lbllorving observation underUV
366.Notes:l.
crude materials;2. viscous
extract;3'
Dry extract:4'
FAgranu|es: 5. FB granules: 6. FC granules:7. FA tab|etl 8. FB tab.let; 9. FC tab|et.
The
chromatogranr protilesof
tabletsduring
nranut'acturing plocess: crude materials, extracts, g|anules and tablets using a-GF25a silica plate as a stationarl phase and a mixtureof
n-hexane : ethyl acetate(4:l'
r'/r') as amobile phase u'ith vanillin as a spraying feagent catr be seen in Figure
l'
The
resultsof
the
chromatogram dernonstrated thattlre
chemical constituentsin
cernre leaves'tiom
crude materials until tablets. u,ere relatively unchangcd.'l'annins spot of purple color w'hich was used as a rnarker (RI' 0.20 and 0.50) rvas identitled in-the.extracts. e\tract porvder. granules and tablets'The tannins contents
in
crude matelials. dry e)itracls. granules, and tablets were2'97Yo,l5'33%'
l0'63%'
and 10.63% respectively. The cgntent ol'tanninsin
the extlact rvas lbund to be higher than thatin
crude mate|ials due to a relatively long extr.action process ol'actir,e constitutentsin
the preparation o1'extfacts' The addition ot' tablet excipients resulted in a decrease in tannins content. Tannins contents in granules and tablets did not dil'f'ersignilicantly
since tannins were resistant to heat and rrroistut'e. T rus wet granulation process did not leduce thc content of tannins in the samPles.All
tbrnrulaeof A. B.
andc
showed good ph1'sical qualityof
tablets. Horvever, tbrmulaA
demonstrated :rslrorter. disintegration time than those
of
lbr.nrulacIl
and C. Thtrsit
is expected that tbrrrrulaA rvill
disintegt'irtcI
l'aster and be readily absorbed
in
the body so thatit
will
exhibit albrmulae B and C.
Proceedings International Conlerence on Medicjnal Aants
J
1g9
more rapid pharmacological effect than
Conclusion
cefme leaves extract courd be lbrmurated into
a tabre{ dosage
tbrrr
\ihich
eets the requir.cmentsofphlsical
quality
oftablet
The additionofPVP
K-30as a binder at co-ncentrations
ol
2.i
and 4yo may resurt in a tabrelwh ich meets the fequ irements. The
serected rbrmu ra \vas FA with
pvp
K-i'
at a concentration of270.
References
Altiyah
H,
r992' pemberianInts
Daun_cerm€ terhadap Bemt Badan dan
Uii
roksisitas pada Hepal Mencir. Skripsi Sarjana Farmasi, Lembaga penelitian L|NAIR.Arren' Jr'
LV,
popovichNc
andAnseflrC,20r
r- pharmaceuticar Dos,ge Form and Drug Deliver;, systems.9,r,
.
ed, )?.5-255,33,
LippincottWiliams
&
Wif f
t"r. pr,i[a"f
pf,i'l. "Usn.Anonyrnous' r979, Farmakope rndonesia. ed.
tt.6-s.
oepanemen["r"r,""
Repubrik rndonesia. Jakarla. Anon)'mo us' I ggg' Materia Medika rndonesia V.:
r-sa.5"p"i",n",t
-i-esetratan nepuurir Indonesia. Jakana.Anonrmous' L995'Farmargrpe Indonesia.
Ed.
IV.
.)rr-ioai..
;;;;;men
KcseharanRepubrik
Ind.rnesi' FJcndelin.F.,.
Shangrcu. R.F.. I9S9.-L pn1p1q5scd ri,blet.br,.",'gr"nul",,on.
In: I,hJfmaceutical Dusase
fofrl
Tabler. Volume
i.
Liebernrrn. I.l.A.. Lachmun. t..a
Sci,nanz..t.8.
I l,.J\r
.
ti0_19i.
Mrrcell Dcklcl
Inc.. Ne\.\, york.
tlankcr..
C.S.
Anderson. N.R.. I996.,.fablclsln:
l.hc.[.hcof
itnd practice ol.lnduslr.jal pjrarnracr. Lachnriln. j..
Lieberman. H.A.
&
Kanis. J.L-1Eds.). 3,,i edjrion.ZOS;+;.'i"*
i"oig.r.
phitadetphia. crholithh' 2003' pengaruh Konseni:asi,p)1p -[ -J, {) :gbrsaj
p",rei["i"ih"d"p
Mul.
Fisik ].abret Ekstr.ak Rinrpan' Kunyit (Cufcuma domistica Val.). Skr.ipsi FakultasI,i
"l"-.i
il"if."
w jd-\r Mandala Surabaya.Kirchner. .1.C.. 1978. Phenolcar.boxl
lic Acid. In:
pcn1. It.S..(;;.1-:ii;il;
La\er
chronr arograph)
.
2,,d Edition..
362-363. A -Wiley- lnterscience publicarion.Nern,york-l
ierue'[
'
'
Hagen- TA..
r996. prefbrnruration. rn: Trr.rhco'1
and pmctrce or Industricr pharmacy. Lachman-L.. Lieberman. H.A.&
Kani8. J.L. (Eds.). i,J edition.rii_rlo L.,
Fcbiger.. phitadetphia.cuvron'
A .c;
1997- Fisiorogi KeJokre'an (setia\\af'r.i. ;;';;;;.
Edisi
9.
l16-i19.
pener.bitBukLr
Kedokteran ECC. Jakar1a.
Hutapea' I
'
l9g4'
rventaris Tanaman obat Indonesia r. Depanerren Kesehatan Repubrik Indonesia. JakartaMa'shal.
K.,
r996.
compression and consoridarion"1E,,a.,=ii""iior.
1",-'ri.
ii.*
""a'il.i"',l*.,
Industrial pharmacy. Lachman.
1..
Lieberman.g.n.-a-l_ig
.t.1.
(Ed;J.
j;
"if,f"i. ;;:;;..,_:;
Febiger, philadelphia.
t"tt"t
i*;;';hPhamaceutical
rechnologl
Fundancntat pharmaceutics. 3.d ed. 73-86. Burgess publishingRobinson, T.- 1995, Kanduncan Kimia Organik Tumbuhan Tinggi. Edisi 6- 7t_72.
ITB. Bandung. Rowe
R'..'
sheskey. pJ'
&-euinn
M E.. 2009. Handbook"i'p'l".nrri"ri*r
Excipicnrs. 6,h edition. 58 r-585. Pharmaceutical press, l_ondon UK.Sastroamidjoio, A.S.. 2001, Obat
Asli
Indonesia. Dian Rak).ar..takaflaSharget L and Yu
Bc
l999. Appried Biopharmacer,ricsTil;"r;;;"1'r
"d
.
r69-20r.Mccra\\-Hir.
Londor).Voight'
R.
r995.
Buku petaiaran T'eknorogi Fur,na.i.{
s;;,;;-n'i;i
sii
peneqe.ar,r. Edisi5. cadiah
Madr Universitv Pt.ess. Yogyakana.Yapurra.
T..
1989. Tanaman Berkhasiat Obat di Indonesia..tilid