Short communication
Quorum sensing-disrupting compounds protect larvae of the
giant freshwater prawn
Macrobrachium rosenbergii
from
Vibrio harveyi
infection
Gde Sasmita Julyantoro Pande
a,b, Anne Aamdal Scheie
c, Tore Benneche
d, Mathieu Wille
a,
Patrick Sorgeloos
a, Peter Bossier
a, Tom Defoirdt
a,⁎
a
Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Rozier 44, 9000 Gent, Belgium b
Department of Aquatic Resources Management, Faculty of Marine Science and Fisheries, Udayana University, Bukit Jimbaran Campus, 80361 Bali, Indonesia c
Department of Oral Biology, Faculty of Dentistry, University of Oslo, Oslo, Norway dDepartment of Chemistry, Faculty of Chemistry, University of Oslo, Oslo, Norway
a b s t r a c t
a r t i c l e
i n f o
Article history:
Received 8 May 2013
Received in revised form 14 May 2013 Accepted 16 May 2013
Available online 23 May 2013
Keywords:
Quorum quenching Cinnamaldehyde Furanone Thiophenone Virulence Infection
Vibriosis outbreaks caused byVibrio harveyiand related species are amongst the major obstacles for the fur-ther expansion of giant freshwater prawn (Macrobrachium rosenbergii) larviculture.Vibrio harveyiregulates virulence gene expression through quorum sensing, bacterial cell-to-cell communication, and consequently, quorum sensing disruption has been suggested as an alternative strategy to control infections caused by these bacteria. Previous studies have shown that quorum sensing-disrupting compounds are able to disrupt quorum sensing inVibrio harveyi. In this study, we demonstrated that the quorum sensing-disrupting compounds cinnamaldehyde, (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone and (Z )-4-((5-(bromomethylene)-2-oxo-2,5-dihydrothiophen-3-yl)metoxy)-4-oxobutanoic acid increased the survival of giant freshwater prawn larvae when challenged to pathogenicVibrio harveyi. Ourin vivochallenge test showed that cinnamaldehyde and the thiophenone can protect the larvae fromVibrio harveyiinfection when dosed to the culture water at 1μM and 10μM, whereas the brominated furanone offered protection at 1μM but resulted in complete mortality
at 10μM. Although there were significant differences in survival between challenged larvae with and without addition of quorum sensing-disrupting compounds, there were no differences in growth (as determined by the larval stage index).
© 2013 Elsevier B.V. All rights reserved.
1. Introduction
The giant freshwater prawn,Macrobrachium rosenbergii, is one of the most important freshwater crustaceans from a commercial point of view. Freshwater prawn farming has expanded enormously since 1980, and in 2009 the global production reached a level of up to 80 times the production reported in 1980 (New and Nair, 2012). However, disease outbreaks are amongst the major obstacles to produce healthy and high quality seed for the further expansion of giant freshwater prawn culture (Nhan et al., 2010). Some studies have shown thatVibrio spp. are a major cause of disease (Kennedy et al., 2006; New et al., 2010; Tonguthai, 1997). In general, vibriosis is prevalent in the early life stages (eggs, larvae, and postlarvae) of giant freshwater prawn (Bhat and Singh, 1999), all of which need brackish water to survive (New et al., 2010). One of the species that has been isolated from affected giant freshwater prawn larvae is the luminescent bacteriumVibrio harveyi
(Bhat and Singh, 1999; Tonguthai, 1997).Harveyiclade vibrios
(includ-ingV. harveyiand closely related species such asV. campbelliiandV.
parahaemolyticus) are amongst the most important bacterial pathogens
of aquatic animals, and cause significant losses in the aquaculture in-dustry worldwide (Austin and Zhang, 2006; Ruwandeepika et al., 2012).
The frequent use of antibiotics to control vibriosis in hatcheries has led to the development and spread of antibiotic-resistant bacteria (Karunasagar et al., 1994; Moriarty, 1998), and alternative methods are needed to control these bacterial infections. Recently, it has become clear that the virulence ofV. harveyiis under control of quorum sensing, a regulatory mechanism based on secreting and sensing small signal molecules called autoinducers (Cao and Meighen, 1989; Chen et al., 2002; Defoirdt et al., 2008; Henke and Bassler, 2004; Natrah et al., 2011). We previously reported thatV. harveyiquorum sensing regulates its virulence towards giant freshwater prawn larvae (Pande et al., submitted for publication). Consequently, the application of quorum sensing-disrupting agents might be a valid strategy to control vibriosis in this species. Quorum sensing-disrupting compounds such as cinnamaldehyde, brominated furanones and brominated thiophenones
Aquaculture 406-407 (2013) 121–124
⁎ Corresponding author at: Laboratory of Aquaculture &ArtemiaReference Center, Rozier 44, B-9000 Gent, Belgium. Tel.: +32 9 264 37 54; fax: +32 9 264 41 93.
E-mail address:[email protected](T. Defoirdt).
0044-8486/$–see front matter © 2013 Elsevier B.V. All rights reserved.
http://dx.doi.org/10.1016/j.aquaculture.2013.05.015
Contents lists available atSciVerse ScienceDirect
Aquaculture
(Fig. 1) have been reported before to protect brine shrimp larvae fromV.
harveyi(Brackman et al., 2008; Defoirdt et al., 2007, 2012). In this study,
we aimed at investigating the impact of these compounds on the sur-vival and growth of giant freshwater prawn larvae when challenged to pathogenicV. harveyi.
2. Materials and methods
2.1. Bacterial strains and growth conditions
In this study we usedVibrio harveyiBB120 (=ATCC BAA-1116). The bacterium was stored at−80 °C in 40% glycerol and the stocks were streaked onto Luria-Bertani agar containing 12 g l−1synthetic sea salt
(LB12). After 24 h of incubation at 28 °C, a single colony was inoculated
into 5 ml LB12broth and incubated overnight at 28 °C under constant
agitation (100 min−1). Bacterial density was measured
spectrophoto-metrically at 600 nm.
2.2. Quorum sensing-disrupting compounds
Cinnamaldehyde (Sigma) was dissolved in distilled water at 1 mM and the brominated furanone (Z -)-4-bromo-5-(bromomethylene)-2(5H)-furanone (Sigma) was dissolved in methanol at 100μM. The brominated thiophenone (Z )-4-((5-(bromomethylene)-2-oxo-2,5-dihydrothiopen-3-yl)methoxy)-4-oxobutanoic acid was synthesized as described before (Defoirdt et al., 2012) and dissolved in ethanol at 10 mM. All compounds were stored at−20 °C.
2.3. Preparation of axenic Artemia nauplii as feed for the prawn larvae.
Decapsulation and hatching of axenicArtemianauplii for larval feed-ing were performed as described byMarques et al. (2004). 200 mg
Artemiacysts (Ocean Nutrition Europe, Essen, Belgium) were hydrated
in 18 ml distilled water for 1 h. Sterile cysts and nauplii were obtained via decapsulation using 660μl NaOH (32%) and 10 ml NaOCl (50%). During the reaction,filtered aeration was provided. The decapsulation process was carried out under a laminar flow hood. The reaction was stopped after 2 min by adding 14 ml Na2S2O3(10 g l−1). The
decapsulated cysts were washed with fresh autoclaved brackish water (12 g l−1synthetic sea salts) over a 100
μm sieve and transferred to two sterile falcone tubes, each containing 30 ml brackish water. The falcons with decapsulated cysts were incubated at 28 °C for 24 h on a rotor under constant light.
2.4. Giant freshwater prawn experiments
Giant freshwater prawn experiments were performed as described inPande et al. (submitted for publication). Briefly, prawn broodstock maintenance was performed according toCavalli et al. (2001) and water quality parameters were adjusted according to New (2003).
The larvae were obtained from a single oviparous female breeder. A ma-tured female which had just completed its pre-mating moult was mated with a hard-shelled male as described before (Baruah et al., 2009). The female with fertilized eggs was then maintained for 20 to 25 days to un-dergo embryonic development. When fully ripe (indicated by dark grey color of the eggs), the female was transferred to a hatching tank (30 l) containing slightly brackish water (containing 6 g l−1Instant Ocean
synthetic sea salt, Aquarium System Inc., Sarrebourg, France). The water temperature was maintained at 28 °C by a thermostat heater. After hatching, the newly hatched larvae with yolk were left for 24 h in the hatching tank. The next day, prawn larvae with absorbed yolk were distributed in groups of 25 larvae in 200 ml glass cones containing 100 ml fresh autoclaved brackish water (12 g l−1synthetic sea salts).
The glass cones were placed in a rectangular tank containing water maintained at 28 °C and were provided with aeration. The larvae were fed daily with 5Artemianauplii/larvae and acclimatized to the ex-perimental conditions for 24 h.
During the experiments, water quality parameters were kept at min-imum 5 mg l−1dissolved oxygen, maximum 0.5 mg l−1ammonium-N
and maximum 0.05 mg l−1nitrite-N. Prawn larvae were challenged
withVibrio harveyiby adding the strains at 106CFU ml−1to the culture
water on the day afterfirst feeding. The quorum sensing-disrupting compounds were also added to the culture water on the day afterfirst feeding. Survival was counted daily in the treatment challenged to wild type Vibrio harveyi without addition of the quorum sensing-disrupting compounds and the challenge test was stopped when more than 50% mortality was achieved. At this time point, larval survival was determined in all treatments by considering that only those larvae presenting movement of appendages were alive. The larval stage index (LSI) was estimated according toMaddox and Manzi (1976)by ran-domly sampling 5 larvae from each and calculated as:
LSI¼ΣSi=N
Si:stage of the larva ið ¼1 to 12Þ
N:the number of larvae examined:
2.5. Statistical data analysis
Statistical analyses were performed using the SPSS software, ver-sion 20. The larval survival data were arcsin transformed in order to satisfy normal distribution and homoscedasticity requirements. Data were analysed by one-way ANOVA, followed by Tukey multiple range tests with a significance level set at 0.05.
3. Results and discussion
In this study we investigated the impact of the addition of three differ-ent quorum sensing-disrupting compounds, including cinnamaldehyde, the brominated furanone (Z-)-4-bromo-5-(bromomethylene)-2(5H
)-Cinnamaldehyde (Z -)-4-bromo-5-(bromomethylene)-2(5H)-furanone
(Z)-4-((5-(bromomethylene)-2-oxo-2,5-dihydrothiophen-3-yl)methoxy) -4-oxobutanoic acid
Fig. 1.Structure of the quorum sensing-disrupting agents used in this study.
furanone, and the thiophenone (Z )-4-((5-(bromomethylene)-2-oxo-2,5-dihydrothiophen-3-yl)-4-oxobutanoic acid) on the virulence of
V. harveyitowards giant freshwater prawn larvae.In vivochallenge
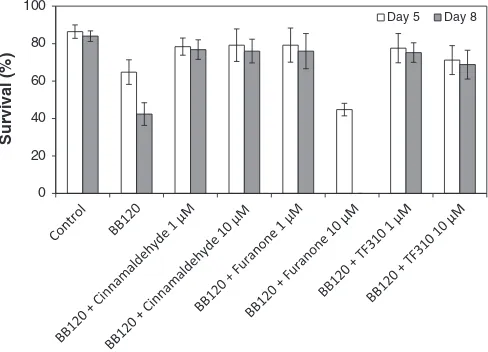
ex-periments revealed that the survival of challenged larvae significantly in-creased when these compounds were added to the culture water at 1μM, offering a complete protection (no significant difference in survival when compared to non-challenged larvae) (Fig. 2). Increasing the con-centration of the compounds to 10μM did not further increase the sur-vival of the larvae and 10μM of furanone even resulted in complete mortality of the larvae. Importantly, the quorum sensing-disrupting com-pounds showed no negative impact on the growth of surviving prawn larvae since no difference in LSI was observed between treated and untreated larvae (Fig. 3).
Our results are consistent with previous reports showing that cinnamaldehyde is able to protect brine shrimp larvae againstV. harveyi (Brackman et al., 2008) and burbot (Lota lota L.) larvae fromAeromonas
hydrophilaandAeromonas salmonicida(Natrah et al., 2012). Brominated
furanones have been reported before to protect brine shrimp larvae from pathogenicV. harveyi(Defoirdt et al., 2006) and rainbow trout
(Oncorhyncus mykiss) fromVibrio anguillarum(Rasch et al., 2004), and
the thiophenone compound has been reported before to protect brine shrimp larvae fromV. harveyi (Defoirdt et al., 2012). As far as we know, this study provides thefirst evidence of a protective effect of this kind of compounds in a commercial crustacean species. The con-centrations at which the compounds protected the giant freshwater prawn larvae were lower than those needed to protect brine shrimp for all three compounds tested (Brackman et al., 2008; Defoirdt et al., 2006, 2012). This might indicate that the vibrios are more vulnerable to quorum sensing disruption when during infection of giant freshwater prawn larvae when compared to brine shrimp. However, we cannot ex-clude the possibility that in addition to quorum sensing disruption, the compounds have other (yet unknown) activities towards either the lar-vae or the bacteria.
The observation that higher levels of furanone resulted in high mortality of the prawn larvae is also consistent with the literature, in that this kind of compounds is known to have considerable toxicity towards higher organisms (Defoirdt et al., 2006; Hentzer and Givskov, 2003). Apparently, giant freshwater prawn larvae are less tolerant to the furanone than brine shrimp larvae, which still survived at ~ 80μM (Defoirdt et al., 2006). As brominated furanones and bro-minated thiophenones are highly reactive molecules, we previously hypothesized that inactivation of essential proteins (caused by
binding of the compounds to nucleophilic amino acid residues) is re-sponsible for their toxicity, and that the presence of a side chain in the thiophenone compound reduces toxicity by limiting the access to some nucleophilic acid residues of essential proteins (due to steric hindrance) (Defoirdt et al., 2012). Indeed, the presence of a 3-alkyl side chain has been reported result in lower toxicity of brominated furanones to planktonic bacterial cells, without major impact on sensing-disrupting activity (Steenackers et al., 2010).
In conclusion, in this study, we found that the use of quorum sensing-disrupting compounds is a valid strategy to protect giant freshwater prawn larvae fromV. harveyiwithout negative effect on growth of the larvae. Further developing this kind of treatments will hopefully lead to a sustainable production of aquatic larvae in general and those of the giant freshwater prawn in specific.
Acknowledgments
We thank Bonnie Bassler for providing us withV. harveyiBB120. We also thank Gunnar Herstad for performing the synthesis of the brominated thiophenone. This work was funded by the Research Foundation of Flanders (FWO-Vlaanderen; project no 1.5.013.12N) and by the Directorate General of Higher Education of Indonesia through doctoral scholarship to Pande Gde Sasmita Julyantoro. Tom Defoirdt is a postdoctoral fellow of FWO-Vlaanderen.
References
Austin, B., Zhang, X.-H., 2006.Vibrio harveyi: a significant pathogen of marine verte-brates and inverteverte-brates. Letters in Applied Microbiology 43, 119–124.
Baruah, K., Cam, D.T.V., Dierckens, K., Wille, M., Defoirdt, T., Sorgeloos, P., Bossier, P., 2009.
In vivo effects of single or combined N-acyl homoserine lactone quorum sensing sig-nals on the performance ofMacrobrachium rosenbergiilarvae. Aquaculture 288, 233–238.
Bhat, S.G., Singh, I.S.B., 1999.Vibrionaceae associated with larvae and larval rearing sys-tems ofMacrobrachium rosenbergii: systematics and pathogenicity. Advances in shrimp biotechnology.National Centre for Genetic Engineering and Biotechnology, Bangkok 279.
Brackman, G., Defoirdt, T., Miyamoto, C., Bossier, P., Calenbergh, S.V., Nelis, H., Coenye, T., 2008.Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator luxR. BMC Microbiology 8 (149), 1–14.
Cao, J.G., Meighen, E.A., 1989. Purification and structural identification of an autoinducer for the luminescence system ofVibrio harveyi. Journal of Biological Chemistry 264, 21670–21676.
Cavalli, R.O., Lavens, P., Sorgeloos, P., 2001.Reproductive performance ofMacrobrachium rosenbergiifemale in captivity. Journal of the World Aquaculture Society 32 (1), 60–67.
Fig. 2.Percentage survival of giant freshwater prawn larvae after 5 and 8 days of challenge withVibrio harveyiBB120, with and without quorum sensing-disrupting compounds (average ± standard deviation of five replicates). Cinnamaldehyde, the furanone (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone, and the thiophenone (Z )-4-((5-(bromomethylene)-2-oxo-2,5-dihydrothiophen-3-yl)methoxy)-4-oxobutanoic acid were added to the culture water at the start of the experiment at 1 and 10μM.
0
Fig. 3.Larval stage index (LSI) of giant freshwater prawn larvae after 5 and 8 days of challenge withVibrio harveyiBB120, with and without quorum sensing-disrupting compounds (average ± standard deviation offive replicates). Cinnamaldehyde, the furanone (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone, and the thiophenone (Z)-4-((5-(bromomethylene)-2-oxo-2,5-dihydrothiophen-3-yl)methoxy)-4-oxobutanoic acid were added to the culture water at the start of the experiment at 1 and 10μM.
123
Chen, X., Schauder, S., Potier, N., Van Dorsselaer, A., Pelczer, I., Bassler, B.L., Hughson, F.M., 2002.Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545–549.
Defoirdt, T., Crab, R., Wood, T.K., Sorgeloos, P., Verstraete, W., Bossier, P., 2006.Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscanafrom pathogenicVibrio harveyi,Vibrio campbelli, andVibrio parahaemolyticus isolates. Applied and Environmental Microbiology 72 (9), 6419–6423.
Defoirdt, T., Miyamoto, C.M., Wood, T.K., Meighen, E.A., Sorgeloos, P., Verstraete, W., Bossier, P., 2007.The natural furanone (5Z)-4 bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyiby decreasing the DNA-binding activity of the transcriptional regula-tor protein luxR. Environmental Microbiology 9, 2486–2495.
Defoirdt, T., Boon, N., Sorgeloos, P., Verstraete, W., Bossier, P., 2008.Quorum sensing and quorum quenching inVibrio harveyi: lessons learned from in vivo work. ISME Journal 2, 19–26.
Defoirdt, T., Benneche, T., Brackman, G., Coenye, T., Sorgeloos, P., Scheie, A.A., 2012.A quorum sensing-disrupting brominated thiophenone with a promising therapeutic potential to treat luminescent vibriosis. PLoS One 7 (7), e41788.
Henke, J.M., Bassler, B.L., 2004.Three parallel quorum sensing systems regulate gene expression inVibrio harveyi. Journal of Bacteriology 186, 6902–6914.
Hentzer, M., Givskov, M., 2003.Pharmacological inhibition of quorum sensing for treat-ment of chronic bacterial infections. Journal of Clinical Investigation 112, 1300–1307.
Karunasagar, I., Pai, R., Malahti, G.R., Karunasagar, I., 1994.Mass mortality ofPenaeus monodonlarvae due to antibiotic-resistantVibrio harveyiinfection. Aquaculture 128, 203–209.
Kennedy, B., Venugopal, M.N., Karunasagar, I., Karunasagar, I., 2006.Bacterialflora associated with the giant freshwater prawnMacrobrachium rosenbergii, in the hatchery system. Aquaculture 261, 1156–1167.
Maddox, M.B., Manzi, J.J., 1976.The effects of algal supplements on static system culture onMacrobrachium rosenbergii(de Man) larvae. Proceedings of the World Mariculture Society 7, 677–698.
Marques, A., Dhont, J., Sorgeloos, P., Bossier, P., 2004.Evaluation of different yeast cell wall mutants and microalgae strains as feed for gnotobiotically-grown brine shrimpArtemia franciscana. Journal of Experimental Marine Biology and Ecology 312, 115–136.
Moriarty, D.J.W., 1998.Disease control in shrimp aquaculture with probiotic bacteria. In: Bell, C.R., Brylinsky, M., Johnson-Green, P. (Eds.), Microbial Biosystems: New
frontiers. Proceedings of the Eighth International Symposium on Microbial Ecology. Atlantic Canada Society for Microbial Ecology, Halifax, NS, Canada.
Natrah, F.M.I., Ruwandeepika, H.A.D., Pawar, S., Karunasagar, I., Sorgeloos, P., Bossier, P., Defoirdt, T., 2011.Regulation of virulence factors by quorum sensing inVibrio harveyi. Veterinary Microbiology 154, 124–129.
Natrah, F.M.I., Alam, M.I., Pawar, S., Harzevili, S., Nevejan, N., Boon, N., Sorgeloos, P., Bossier, P., Defoirdt, T., 2012.The impact of quorum sensing on the virulence of Aeromonas hydrophilaandAeromonas salmonicidatowards burbot (Lota lota L.) larvae. Veterinary Microbiology 159, 77–82.
New, M.B., 2003.Farming freshwater prawns: a manual for the culture of the giant river prawn,Macrobrachium rosenbergii. FAO Fisheries Technical Paper No. 428, pp. 145–146.
New, M.B., Nair, C.M., 2012.Review article: global scale of freshwater prawn farming. Aquaculture Research 43, 960–969.
New, M.B., Valenti, W.C., Tidwell, J.H., D’Abramo, L.R., Kutty, M.N., 2010.Freshwater prawn: biology and farming. Blackwell Publishing Ltd.
Nhan, D.T., Cam, D.T.V., Wille, M., Defoirdt, T., Bossier, P., Sorgeloos, P., 2010.Quorum quenching bacteria protectMacrobrachium rosenbergiilarvae fromVibrio harveyi infection. Journal of Applied Microbiology 109, 1007–1016.
Pande, G.S.J., Natrah, F.M.I., Sorgeloos, P., Bossier, P., Defoirdt, T., 2013.The threeVibrio harveyiquorum sensing signals have a different impact on virulence towards dif-ferent crustacean hosts (submitted for publication).
Rasch, M., Buch, C., Austin, B., Slierendrecht, J., Ekmann, K.S., Larsen, J.L., Johansen, C., Riedel, K., Eberl, L., Givskov, M., Gram, L., 2004.An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykissWalbaum). Systematic and Applied Microbiology 27, 350–359.
Ruwandeepika, H.A.D., Jayaweera, T.S.P., Bhowmick, P.P., Karunasagar, I., Bossier, P., Defoirdt, T., 2012.Pathogenesis, virulence factors and virulence regulation of vib-rios belonging to the Harveyi clade. Reviews in Aquaculture 4, 59–74.
Steenackers, H.P., Levin, J., Janssens, J.C., De Weerdt, A., Balzarini, J., Vanderleyden, J., De Vos, D.E., De Keersmaecker, S.C., 2010.Structure-activity relationship of brominated 3-alkyl-5-methylene-2(5H)-furanones and alkylmaleic anhydrides as inhibitors of Salmonellabiofilm formation and quorum sensing regulated bioluminescence in Vibrio harveyi. Bioorganic & Medicinal Chemistry 18, 5224–5233.
Tonguthai, K., 1997.Diseases of the freshwater prawn, Macrobrachium rosenbergii. The Aquatic Animal Health Research Institute Newsletter 4 (2), 1–9.