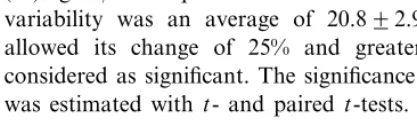Sensitivity of striate neurons to Y-like figures: experiment
and simulation
Igor A. Shevelev *
Department of Sensory Physiology,Institute of Higher Ner6ous Acti6ity and Neurophysiology,Russian Academy of Sciences, 5-a Butlero6a Street,Moscow117865,Russia
Abstract
Under stimulation of the receptive fields (RF) of neurons in the cat area 17 by flashing Y-like figures of different shape and orientation, the sensitivity to these figures was revealed in 72% of the studied cells, while 62% of units were sensitive to cross-like figures as well. Tuning to Y-like figures was typically selective to their shape and orientation, but in some cases it was invariant to these features. Response magnitudes to single bar, Y-like figure and cross were positively correlated. Simulation showed that the disinhibition might be a sufficient mechanism for effective detection of Y-like figures in a classical receptive field. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cat; Cross; Feature; Invariance; Neuron; Orientation; Shape; Visual cortex; Y-like figure
www.elsevier.com/locate/biosystems
1. Introduction
After studies of Hubel and Wiesel (1962, 1965) it was commonly accepted that in the area 17 of the cat visual cortex nearly all units generate a maximal response to a single light bar with pre-ferred orientation (Hammond and Andrews, 1978; Chapman and Stryker, 1992; Eysel et al., 1998). Meanwhile, theoretically, a system for image pro-cessing must also include units selective to line crossings at different angles (Marr, 1970; Marr and Hildreth, 1980). Hubel and Wiesel were the first to describe a neuron of V3 sensitive to corner (Hubel and Wiesel, 1965).
The sensitivity of units in the cat area 17 (V1) to flashing cross or corner of different shape and orientation centered in the receptive field (RF) was studied. It was found that about 1/3 of neurons showed, on average, 3-fold increase of response to an optimal figure as compared with preferred single bar (Shevelev et al., 1994, 1995, 1998a,b, 1999; Lazareva et al., 1995, 1998; Shev-elev, 1998). Similar results were reported under stimulation of the central and peripheral parts of the RF with two grids of orthogonal orientations (Sillito et al., 1995).
In the present study there were two aims: to find and describe neurons of the cat striate cortex (area 17) which would be more sensitive to Y-like figures than to a single bar or cross, and to analyze by simulation a sufficiency of a disin-hibitory (inhibition of inhibition) mechanism of RF for this sensitivity.
* Tel.: +7-95-3344281; fax:+7-95-3388500.
E-mail address:[email protected] (I.A. Shevelev).
centered in the RF of the unit. Bar or figure was viewed monocularly by the eye contralateral to the hemisphere from which a unit activity was recorded. Stimuli of different orientations and shape (an angle between its lines) with 22.5° step were presented repetitively in pseudo-randomized order (multi-histogram mode) using specially de-veloped software (Fig. 1). The length of the bar or lines of the figures was 3 – 8°, their width 0.06 – 0.2°. The centers of single bar and line-branching in a Y-like figure were always located in the excitatory center of the RF. For each combina-tion of the shape and orientacombina-tion the responses in 10 – 20 trials were collected. Orientation tuning and tuning to the shape of a figure were estimated by the mean number of discharges in the unit response. Tuning to a figure was compared with orientation tuning to a single bar (control) which was plotted several times during the recording period for each neuron. The sensitivity index (SI):figure/bar response ratio was calculated. Its variability was an average of 20.892.9% that allowed its change of 25% and greater to be considered as significant. The significance of data was estimated witht- and paired t-tests.
3. Results
3.1. General effects of stimulation with Y-like figures
In 28/39 of the studied neurons (72%) the sensi-tivity to Y-like figures was found — their SI was equal to or higher than 1.25. Among them, nine cells were sensitive only to this figure (their re-sponses to cross and single bar were significantly lower). Seven units were predominantly sensitive to Y-like figure (responses to cross were lower), while ten cells showed preference to cross, but responded to Y-like figure rather well. The re-maining two neurons revealed an equal sensitivity to these stimuli.
3.2. Neurons — detectors of the Y-like figures
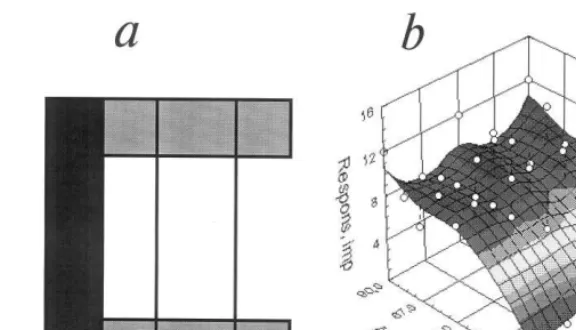
Responses of the striate cells to Y-like figure were typically selective: they depended on its shape and orientation. Fig. 2 demonstrates a three-dimensional plot of the on-response tuning of the cell with simple RF recorded at a cortical depth of about 840 mm and tested by a flashing
light bar, cross and Y-like figure with lines 6×
0.2°. The RF shows the preference for the orienta-tion of 135° of a single bar (Bar) and the absence of response to a cross (A), while for Y-like figure with the long line orientation at 135° and the angle between the ‘branches’ of 90° the response is markedly increased as compared with the bar (B). In another example (Fig. 3), the effect of invari-ance of neuron tuning to the shape of the Y-like
Fig. 2. Tuning of striate neuron to a stationary flash-presented bar (‘Bar’), cross (A) and Y-like figure (B) shown on the three-dimensional plots of the mean response magnitude (imp./400 ms) in on-response to all possible combinations of figure shape (angle between lines in deg.) and orientation (deg.) with a step of 22.5°. The response plane was calculated with a smoothing algorithm (least squares) therefore some raw data points (open circles) are situated above or below this surface. At zero angle between the branches of a figure (Bar) the conventional orientation tuning to a single light bar of 8×0.1° can be seen (horizontal corresponds to 0°, vertical to 90°). The mean level of background activity is not shown here because it is invisible in the used scale. Absence of the sensitivity to cross (A) and selective sensitivity to the Y-like figure shape and orientation (B) is shown. For more details see text.
figure is demonstrated. This cell with complex RF was not sensitive to the cross (A), while tuning to Y-like figure with orientation 90° did not depend practically on the angle between its branches (B) in the range 45 – 135°. In some cells, the invariance of tuning could be expressed only for the orienta-tion of branches. It was found that striate neurons preferred the Y-like figure with a between-branch angle of 67.5° most frequently (see also: Shevelev, 1998; Shevelev et al., 1998b, 1999 for corners), while the preferred crosses are of 45 and 90° angles. The difference in the shape preference in the units of cross- and Y-group was significant (PB0.05).
3.3. Sensiti6ity of tuning to Y-like figures and crosses
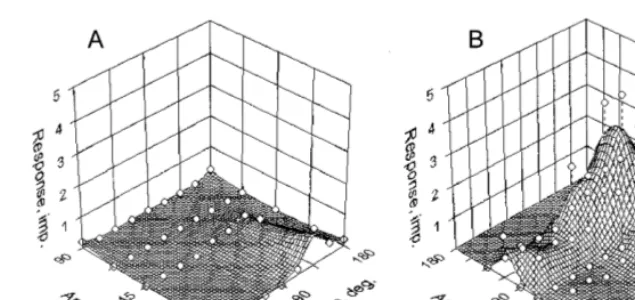
Correlation of the sensitivity index to Y-like figure and cross is shown in Fig. 4. Vertical and horizontal dashed lines delimit non-sensitivity zones (sectors a, e and f), where the index is less than 1.25, while the dashed diagonal (c) corre-sponds to SIs equal for both figures. It can be seen that SIs for both figures are different in 87% of neurons. There are 28/39 cells (72%) sensitive
to Y-like figures (a – d) and 24/39 units (62%) sensitive to cross (b – e). Nine neurons sensitive exclusively to Y-like figure (a), 19 cells tuned to both figures (b – d), and five units sensitive only to cross (e) have been found. From 19 neurons tuned to both figures, seven preferred the Y-like one (b), ten-the cross (d), while two units had equal sensi-tivity to both of them (c). Among studied cells, only six (15.4%) did not reveal any sensitivity to tested figures (f).
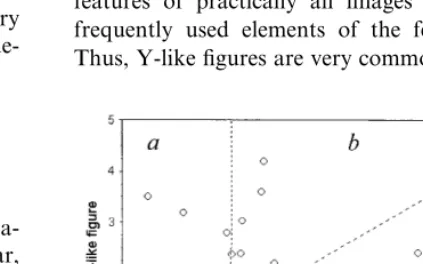
3.4. Simulation of the sensiti6ity to figures
Fig. 3. Absence of sensitivity of the striate neuron to cross (A) and its invariant sensitivity to a shape of the Y-like figure in the range 45 – 135° (B). Other details as in Fig. 2.
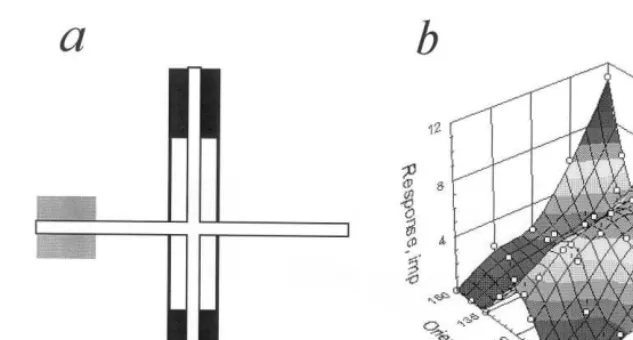
zone (a), while sharp orientation tuning — with narrow end-inhibitory zone(s). Contrary to that neuron with long disinhibitory zone (Fig. 6a) revealed invariance to a figure shape, while wide excitatory and end-stopping inhibitory zones provide for invariance to its orientation. Finally, combination of these properties ensures tuning invariance to both shape and orientation (Fig. 6b). The simulation showed that position of disin-hibitory zone determines preferred shape of the figure. Thus, it is shown that the disinhibitory mechanism may be sufficient for an effective de-tection of Y-like figure in a classical RF.
4. Discussion
A gap between knowledge of the 1st-order fea-ture extraction in V1 (orientation of a single bar, a line or an edge) and highest-order feature pro-cessing in IT cortex (faces and other complex objects) became evident. Where the features of an intermediate complexity are processed — that is the question to be solved experimentally for ad-vancement in understanding of visual recognition. It could be expected that the data on high sensi-tivity of the neurons in the cat area 17 to a cross, a corner (Shevelev et al., 1993, 1994, 1995, 1998a,b, 1999; Lazareva et al., 1995, 1998; Shev-elev, 1998) and a Y-like figure, as well as to a local orientation discontinuity (Sillito et al., 1995) would allow this gap to be filled. Moreover, these
data revise the wide-spread views on the role of the area 17 in the visual processing: in addition to detection of only the 1st-order shape features (orientation of single lines and contrast borders), the 2nd-order feature extraction (line crossing and branching) is also assumed to be a function of this area.
This property of striate neurons seems natural because lines crossing and branching are the key features of practically all images and the most frequently used elements of the feature library. Thus, Y-like figures are very common in an image
Fig. 4. Correlation between the sensitivity indices (SI): figure/
Fig. 5. (a) The scheme of the simulated receptive fields (RF) with narrow excitatory zone (white), small disinhibitory zone (gray) and two end-stopping inhibitory zones (black); (b) 3-D response plot of the simulated neuron vs. orientation and shape (angle between bars) of the Y-like figure. Details as in Fig. 2.
of a tree (branching of a crown), and in any rectangular three-dimensional object (converging facets).
What intracortical mechanisms could underlie the sensitivity to an Y-like figure? It was supposed that they might be as follows: excitatory conver-gence to the studied cell of two neurons with different preferred orientations, inhibitory and fa-cilitatory (or disinhibitory) interaction between central and peripheral RF zones, as well as com-bination of these factors. Excitatory convergence as a reason for cross tuning was discussed previ-ously (Shevelev et al., 1994, 1995, 1998a,b, 1999; Shevelev, 1998) and was suggested to be involved in one-fifth – one-fourth part of cases with double orientation tuning to a single bar.
In other cells contribution of recurrent excita-tion (Nelson and Frost, 1985; Volgushev et al., 1993; Sillito et al., 1995) might be one of the mechanisms of a figure-selectivity. Sillito et al. (1995) suggested that orientation discontinuity en-hances a neuronal output by the active peripheral facilitatory mechanism due to recurrent cortical excitation.
An inhibitory interaction between the central RF zone and its surroundings (Sillito, 1975; Ham-mond and Andrews, 1978; Nelson and Frost, 1978; Maske et al., 1986; Hata et al., 1988; Gilbert and Wiesel, 1990; DeAngelis et al., 1992;
Eysel, 1992; Volgushev et al., 1993; Shevelev et al., 1998a; Worgotter et al., 1998) may be one more typical mechanism of sensitivity to figures. The study of Shevelev et al. (1998a) demonstrated a substantial and different contribution of inhibi-tion to cross-sensitivity, which is generated or enhanced in 33% of cortical neurons and sup-pressed or diminished in 39% of cases. It is well known also that although striate neurons respond to stimuli mainly within their classical RF, these responses can be modified by ‘contextual’ stimuli overlying the surrounding area (Nelson and Frost, 1978, 1985; Hata et al., 1988; Gilbert and Wiesel, 1990; Eysel, 1992).
Fig. 6. (a) The same, as in Fig. 5 for another simulated neuron with wide excitatory zone (white), high disinhibitory zone (black) and two wide end-stopping inhibitory zones (gray). (b) Response plot of this simulated neuron. Other details as in Fig. 2.
al., 1998). In particular, it was shown that sensi-tivity to crosses might be determined in many cases only by the peripheral mechanism of RF that may be of a disinhibitory nature.
Acknowledgements
The study was partly supported by the Russian Foundation for Basic Research (grant no. 99-04-48207).
References
Chapman, B., Stryker, M.P., 1992. Origin of orientation tun-ing in the visual cortex. Curr. Opin. Neurobiol. 2, 498 – 501.
DeAngelis, G.C., Robson, J.G., Ohzawa, I., Freeman, R.D., 1992. Organization of suppression in receptive fields of neurons in cat visual cortex. J. Neurophysiol. 68, 144 – 163. Eysel, U.T., 1992. Lateral inhibitory interactions in area 17 and 18 of the cat visual cortex. Progr. Brain Res. 90, 407 – 422.
Eysel, U.T., Pape, H.C., Van Schayck, R., 1986. Excitatory and differential disinhibitory actions of acetylcholine in the lateral geniculate nucleus of the cat. J. Physiol. (Lond.) 370, 233 – 254.
Eysel, U.T., Shevelev, I.A., Lazareva, N.A., Sharaev, G.A., 1998. Orientation tuning and receptive field structure in cat striate neurons during local blockade of intracortical inhi-bition. Neuroscience 84, 25 – 36.
Gilbert, C.D., Wiesel, T.N., 1990. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vision Res. 30, 1689 – 1701. Hammond, P., Andrews, D.P., 1978. Orientation tuning of
cells in areas 17 and 18 of the cat’s visual cortex. Exp. Brain Res. 31, 341 – 351.
Hata, Y., Tsumoto, T., Sato, H., 1988. Inhibition contributes to orientation selectivity in visual cortex of cat. Nature 335, 815 – 817.
Hubel, D.H., Wiesel, T.N., 1962. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. (Lond.) 160, 106 – 154.
Hubel, D.H., Wiesel, T.N., 1965. Receptive fields and func-tional architecture in two non-striate visual areas (18 and 19) of the cat. J. Neurophysiol. 28, 229 – 289.
Lazareva, N.A., Shevelev, I.A., Novikova, R.V., Tikhomirov, A.S., Sharaev, G.A., 1995. Selective sensitivity of cat stri-ate neurons to cross-like figures and angles of different orientations. Neurophysiology 27, 403 – 412.
Lazareva, N.A., Shevelev, I.A., Sharaev, G.A., Tikhomirov, A.S., Sharaev, G.A., 1998. Sensitivity of neurons of cat visual cortex to cross-like figures under stimulation of the center and periphery of receptive field. Zh. Vyssh. Nerv. Deiat. im. I.P.Pavlova 48, 485 – 495.
Li, C.Y., Zhou, Y.X., Pei, X., Qiu, F.T., Tang, C.Q., Xu, X.Z., 1992. Extensive disinhibitory region beyond the clas-sical receptive field of cat retinal ganglion cells. Vision Res. 32, 219 – 228.
Marr, D., 1970. A theory for the cerebral cortex. Proc. R. Soc. B (Lond.) 176, 161 – 234.
Marr, D., Hildreth, C.E., 1980. A theory of edge detection. Proc. R. Soc. B (Lond.) 204, 301 – 328.
Nelson, J.I., Frost, B.J., 1978. Orientation-selective inhibition from beyond the classical visual receptive field. Brain Res. 139, 359 – 365.
Nelson, J.I., Frost, B.J., 1985. Intracortical facilitation among co-oriented, coaxially aligned simple cells in cat striate cortex. Exp. Brain Res. 61, 54 – 61.
Saltykov, K.A., Shevelev, I.A., 1999. A model of a neuronal network for detection of single bands and cross-like figures. Neurosci. Behav. Physiol. 29, 385 – 392.
Shevelev, I.A., 1998. Second-order features extraction in the visual cortex: selective and invariant sensitivity of neurons to the shape and orientation of crosses and corners. BioSystems 48, 195 – 204.
Shevelev, I.A., Lazareva, N.A., Novikova, R.V., Tikhomirov, A.S., Sharaev, G.A., 1993. Tuning of neurons in the cat visual cortex for detection of cross-like figures. Neurophys-iology 1, 362 – 365.
Shevelev, I.A., Lazareva, N.A., Novikova, R.V., Tikhomirov, A.S., Sharaev, G.A., 1994. Double orientation tuning of units in cat visual cortex. Neuroscience 61, 965 – 973. Shevelev, I.A., Novikova, R.V., Lazareva, N.A., Sharaev,
G.A., Tikhomirov, A.S., 1995. Sensitivity to cross-like figures in the cat striate neurons. Neuroscience 69, 51 – 57. Shevelev, I.A., Jirmann, K.U., Sharaev, G.A., Eysel, U.T., 1998a. Contribution of GABAeric inhibition to sensitivity
to cross-like figures in striate cortex. NeuroReport 9, 3153 – 3157.
Shevelev, I.A., Lazareva, N.A., Sharaev, G.A., Novikova, R.V., Tikhomirov, A.S., 1998b. Selective and invariant sensitivity to crosses and corners in cat striate neurons. Neuroscience 84, 713 – 721.
Shevelev, I.A., Lazareva, N.A., Sharaev, G.A., Novikova, R.V., Tikhomirov, A.S., 1999. Interrelation of tuning char-acteristics to bar, cross and corner in striate neurons. Neuroscience 88, 17 – 25.
Sillito, A.M., 1975. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J. Physiol. (Lond.) 250, 305 – 329. Sillito, A.M., Grieve, K.L., Jones, H.E., Cudeiro, J., Davis, J.,
1995. Visual cortical mechanisms detecting focal orienta-tion discontinuities. Nature 378, 492 – 496.
Volgushev, M., Pei, X., Vidyasagar, T.R., Creutzfeldt, O.D., 1993. Excitation and inhibition in orientation selectivity of cat visual cortex neurons revealed by whole-cell recordings in vivo. Vis. Neurosci. 10, 1151 – 1155.
Worgotter, F., Nelle, E., Li, B., Wang, L., Diao, Y., 1998. A possible basic cortical microcircuit called ‘cascaded inhibi-tion’. Results from cortical network models and recording experiments from striate simple cells. Exp. Brain Res. 122, 318 – 332.