Risk factors for first-ever acute ischemic non-embolic
stroke in elderly individuals
Haralampos J. Milionis
a, Evangelos Liberopoulos
a, John Goudevenos
a,
Eleni T. Bairaktari
b, Konstantinos Seferiadis
b, Moses S. Elisaf
a,*
aDepartment of Internal Medicine, Medical School, University of Ioannina, 451 10 Ioannina, Greece bLaboratory of Biochemistry, University Hospital of Ioannina, Ioannina, Greece
Received 25 September 2003; received in revised form 31 December 2003; accepted 8 January 2004 Available online 2 April 2004
Abstract
Background:Stroke is a leading cause of mortality and subsequent serious long-term physical and mental disability among survivors. In the elderly, ischemic stroke accounts for more than 80% of all strokes.Objectives: To identify major risk factors for a first-ever acute ischemic/non-embolic stroke in individuals older than 70 years.Methods:A population-based case-control study of patients admitted to the University Hospital of Ioannina, Epirus, Greece, due to first-ever ischemic/non-embolic stroke from March 1997 to January 2002. All patients were subjected to brain CT and had their serum lipids and biochemical metabolic parameters determined within 24 h from the onset of symptoms.Results:A total of 163 (aged > 70 years) consecutive stroke patients and 166 apparently healthy volunteers were studied. An atherogenic lipid profile and metabolic disturbances were more prevalent in the patient group than in stroke-free controls. Multivariate logistic regression analysis identified diabetes mellitus (odds ratio (OR), 1.92; 95% CI, 1.02 – 3.63), triglycerides (TG) (OR, 1.16; 95% CI, 1.09 – 1.22), HDL-cholesterol (OR, 0.57; 95% CI, 0.43 – 0.76), apo A – I (OR, 0.80; 95% CI, 0.70 – 0.92), lipoprotein(a) [LP(a)] (OR, 1.51; 95% CI, 1.25 – 1.79), uric acid (OR, 1.30; 95% CI, 1.06 – 1.59) albumin (OR, 0.38; 95% CI, 0.20 – 0.70) fibrinogen (OR, 1.10; 95% CI, 1.05 – 1.13) and the metabolic syndrome (OR 2.48, 95% CI, 1.16 – 5.29) as significantly associated with ischemic/non-embolic stroke.Conclusion:
Ischemic non-embolic stroke in the elderly is associated with dyslipidemia and several predictor metabolic factors, which could be substantially modified by lifestyle changes and therapeutic intervention.
D2004 Elsevier Ireland Ltd. All rights reserved.
Keywords:First-ever acute ischemic non-embolic stroke; Elderly individuals; Dyslipidemia
1. Introduction
Throughout the industrialized world, a remarkable in-crease in the proportion of the ‘‘elderly’’ population, however this term is defined, has taken place [1]. Therefore, it is becoming increasingly important for societies to quantify the burden of illness in their ageing populations[2]. Stroke is one of the leading causes of mortality in the developed countries leading to serious long-term physical and mental disabilities among survivors [3]. In the elderly, more than 80% of all strokes have an ischemic etiology. There is some debate as to whether hyperlipidemia is causally associated with stroke[4]. Although, there is limited evidence of proven benefit from
correcting the ‘‘deranged variables’’, it would be clinically useful to identify, monitor and appropriately manage any modifiable risk factors for stroke in elderly populations[5].
The aim of our study was to identify major risk factors for a first-ever acute ischemic/non-embolic stroke in indi-viduals older than 70 years of age.
2. Subjects and methods
We conducted a population-based case-control study in the prefecture of Ioannina, Epirus, a region of Northwestern Greece with about 170,000 inhabitants. A total of 163 elderly patients (88 men and 75 women) who were consec-utively hospitalized over a 5-year period for first-ever acute ischemic/non-embolic stroke were studied (March 1997 to January 2002). Enrollment criteria were as follows: (i)
0167-5273/$ - see front matterD2004 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.ijcard.2004.01.013
* Corresponding author. Tel.: 97509; fax: +30-26510-97016.
E-mail address:hmilioni@cc.uoi.gr (M.S. Elisaf).
patients diagnosed to suffer a first-ever episode of acute cerebral ischemia (fatal/non-fatal cerebral infarctions, and transient ischemic attacks), (ii) subjects older than 70 years of age, (iii), subjects residing in the prefecture of Ioannina, and (iv) patients reaching the Emergency Department of the University Hospital of Ioannina within 12 h from the onset of symptoms. Diagnosis of acute ischemic stroke was established by clinical evaluation and confirmed by com-puted tomography[6].
One hundred and sixty-six apparently healthy volun-teers (87 men and 79 women) annually evaluated in the primary care setting (Public Primary Care Health Center facilities) in the prefecture of Ioannina comprised the control group. About 2000 individuals routinely receive health care (in terms of check up, lab tests and drug prescribing) in these facilities every year. The control group was made up of individuals consecutively evaluated during the study period; subjects eligible for the control group were older than 70 years of age, resided in the prefecture of Ioannina, and had no previous history of cardiovascular disease. All subjects gave informed consent to use information concerning their hospitalization and the study protocol was approved by the Institutional Ethics Committee.
Subjects with a history of a previous stroke, coronary events (angina or myocardial infarction), active infections, neoplasia, renal or liver disease, thyroid dysfunction, chron-ic obstructive pulmonary disease, chronchron-ic inflammatory bowel disease, and a history of excessive alcohol consump-tion were excluded from the study. Stroke patients and controls with an identifiable embolic source (i.e. atrial fibrillation, heart valve disease, patients receiving anticoag-ulant treatment) were also excluded [7]. Subjects were included in the study on the basis of complete laboratory analyses.
According to their medical records, 77 stroke patients and 60 controls were on antihypertensive treatment, includ-ing diuretics, beta-blockers, ACE inhibitors and calcium antagonists; 41 patients and 29 controls were treated for diabetes mellitus with oral hypoglycemic agents (biguanide or sulfornylurea)Finsulin, while 12 subjects in the patient group and 15 subjects in the control group were taking aspirin. None of the participants was receiving lipid-lower-ing treatment, such as statins and fibrates durlipid-lower-ing the study period.
Hypertensive patients were recorded according to med-ical history and relevant drug treatment. Diabetes mellitus was coded as present if a subject was treated for diabetes as well as by fasting blood glucose measurements (>126 mg/ dl). The diagnosis of metabolic syndrome was made when three or more of the following risk determinants were present: abdominal obesity (waist circumference >102 cm for men and >88 cm for women), triglycerides z150 mg/dl,
low HDL-cholesterol (i.e. < 40 mg/dl for men and < 50 mg/ dl for women), blood pressure z130/z85 mm Hg, and
fasting glucosez110 mg/dl (8).
3. Laboratory investigations
Upon admission blood samples were drawn for complete blood count, plasma fibrinogen, serum creatinine, urea and electrolytes, iron, ferritin, uric acid, albumin, total bilirubin, thyroid stimulating hormone (TSH), total T3, and free thyroxine (free T4) determinations[9].
Blood samples for glucose and lipid determination were drawn after overnight fasting.
All biochemical analyses were performed by commer-cially available standardized methods within 24 h after stroke onset.
Plasma fibrinogen levels were measured by the Clauss method as previously described [10].
The laboratory determinations were carried out by auto-mated chemical analysis in our laboratory using Olympus AU 560 analyzer. Specifically, serum samples were ana-lyzed by using ion-sensitive electrodes for sodium, potassi-um and chloride, for calcipotassi-um, and photometric assays for phosphorus and magnesium. The glutamate dehydrogenase (GLDH) method was used for the determination of urea levels, and a modification of the Jaffe´-method for creatinine. Serum total protein concentrations were measured by the Biuret method, and serum albumin by the bromocresol green (BCG) method. Glucose was measured by the hexo-kinase method, and serum iron levels were measured by the TPTZ (2,4,6-Tri [2 pyridyl]-5-tiazine) method. Total biliru-bin, was measured by the diazonium tetrafluoroborate (DPD) method. A chemiluminescent microparticle immu-noassay (CMIA) was used for the determination of serum ferritin (Architect Ferritin assay, Abbot Laboratories, Abbott Park, IL, USA).
Total T3 (reference range 0.6 – 1.7 ng/ml), free T4 (reference range 0.7 – 2.7 ng/dl) and TSH (reference range 0.2 – 4.8 mU/l) were measured by immunoassay on an AxSYM analyzer (Abbot Laboratory). The sensitivities of the assays were calculated to be 0.3 ng/ml, 0.4 ng/dl and 0.03 mU/l, respectively.
4. Statistical analysis
Values were expressed as meanFS.D., except for age, Lp(a), and fibrinogen which were expressed in terms of median and range. A comparison of continuous variables was performed by an unpaired two-tailed Student’s test, whilev2-tests were used for categorical variables. Because of the highly skewed distribution of Lp(a), the non-para-metric Mann – Whitney U test was applied to discriminate differences in Lp(a) levels between patients and controls.
The strength of associations between serum parameters (lipid and non-lipid) and acute ischemic/non-embolic stroke were assessed by means of logistic regression analysis (backward stepwise likelihood ratio) comparing stroke and control subjects. Two models were used for analysis: a first model which included age, gender, BMI, the presence of hypertension, diabetes mellitus, metabolic syndrome, smok-ing, and lipidemic parameters [TC, TG, HDL, apo A – I, apo B, Lp(a)] and a second model which, in addition, included fibrinogen and other metabolic parameters, such as uric acid, albumin, total bilirubin and ferritin.
Significance levels were set atp< 0.05 in all cases. SPSS 10.0 for Windows (SPSS, 1989 – 1999), and Statistica (1998, Statsoft, Tulsa, OK, USA) were used to perform statistical analysis.
5. Results
The clinical characteristics of the study population are shown inTable 1. Body weight and smoking habits were similar among patients and controls. The prevalence of hypertension and diabetes mellitus was greater in stroke patients compared to the control group. However, there were no statistically significant differences among groups(Table 1). Metabolic syndrome was more frequent among patients suffering first-ever acute ischemic stroke than in the control group (46.0% vs. 15.7%, p< 0.001; Table 1). Among patients with the metabolic syndrome, 52 (69.4%) had three
of its features, 16 (21.3%) had four, and 7 (9.3%) had all five features; in the control group, 19 (73.1%) subjects had three, 6 (23.1%) had four and 1 (3.8%) had all five features of the metabolic syndrome.
Stroke patients exhibited a more atherogenic lipid profile compared to controls (Table 2). Specifically, both groups had similar serum TC levels LDL-C and apo B levels. Nevertheless, stroke patients had higher values of the atherogenic risk ratio (TC/HDL-C, 5.5F1.8 vs. 4.2F1.2, p< 0.001), TG [175F74 mg/dl (2.0F0.8 mmol/l) vs. 125F80 mg/dl (1.4F0.9 mmol/l), p< 0.001], and Lp(a) (median value, 15.7 vs. 7.1 mg/dl,p< 0.001), whereas they displayed lower concentrations of HDL-C [40F11 mg/dl (1.0F0.3 mmol/l) vs. 51F11 mg/dl (1.3F0.3 mmol/l), p< 0.001], and apo A – I (130F24 vs. 150F22 mg/dl, p< 0.001) (Table 2).
Stroke patients had increased serum uric acid [5.6F1.7 mg/dl (333.1F101.1 Amol/l) vs. 4.8F1.4 mg/dl (285.5F83.3 Amol/l), p< 0.001] and plasma fibrinogen
Table 1
Clinical characteristics of the study population Stroke patients
Hypertension 77 (47.2%) 61 (36.7%) 0.07 Diabetes mellitus 46 (28.2%) 34 (20.5%) 0.1 Metabolic syndromea 75 (46.0%) 26 (15.7) < 0.001
Age is expressed as median (range), and body mass index as meansFS.D.
aMetabolic syndrome is defined in the text.
Table 2
Laboratory parameters in the study population Parameters* Stroke patients Fibrinogen (mg/dl) 432(132 – 633) 302(114 – 608) < 0.001 Uric acid (mg/dl) 5.6F1.7 4.8F1.4 < 0.001
Albumin (g/dl) 3.8F0.5 4.1F0.6 < 0.001 Total Bilirubin (mg/dl) 0.7F0.3 0.8F0.3 < 0.02 Iron (Ag/dl) 64.4F29.4 63.9F27.1 NS
Ferritin (ng/ml) 42.9F18.9 41.6F17.3 NS TSH (AU/ml) 1.8F1.1 1.7F1.2 NS Sodium (mmol/l) 138.5F3.5 138.9F3.0 NS Calcium (mg/dl) 8.4F0.9 8.5F1.0 NS Magnesium (mEq/l) 1.5F0.2 1.5F0.2 NS Phosphorus (mg/dl) 3.2F0.8 3.4F0.7 NS TC: total cholesterol, TG: triglycerides, Apo: apolipoprotein, Lp(a): lipoprotein(a). Values represent meansFS.D., except for Lp(a) and fibrinogen where median and ranges are shown.
*To convert data from mg/dl to mmol/l divide TC, LDL-C, and HDL-C by 38.7 and TG by 88.6. To convert data from mg/dl to g/l divide fibrinogen by 100. To convert data from g/dl to g/l, multiply albumin by 10. To convert mg/dl toAmol/l, multiply uric acid by 59.5, creatinine by 88.4, and total
bilirubin by 17.10. To convertAg/dl toAmol/l, multiply iron by 0.18. To
convert data fromAU/ml to mU/l, multiply TSH by 1.0. To convert data
levels [median value 432 mg/dl (4.3 g/l) vs. 302 mg/dl (3.0 g/l),p< 0.001] compared to controls (Table 2). There were no statistically significant differences in fibrinogen levels between men and women either in the patients’ group [median value 444 mg/dl (4.4 g/l) in men vs. 412 mg/dl (4.1 g/l) in women] or in the control group [median value 311 mg/dl (3.1 g/l) in men vs. 293 mg/dl (2.9 g/l) in women]. In contrast, serum albumin and total bilirubin levels were lower in the patients’ group [3.8F0.5 g/dl (38F5 g/l) vs. 4.1F0.6 g/dl (41F6 g/l), p< 0.001 for albumin; 0.7F0.3 mg/dl (12.0F4.8 Amol/l) vs. 0.8F0.3 mg/dl (13.2F4.4 Amol/l), p< 0.02 for bilirubin]. No sig-nificant differences were shown for serum creatinine, urea and electrolytes, iron and ferritin levels(Table 2).
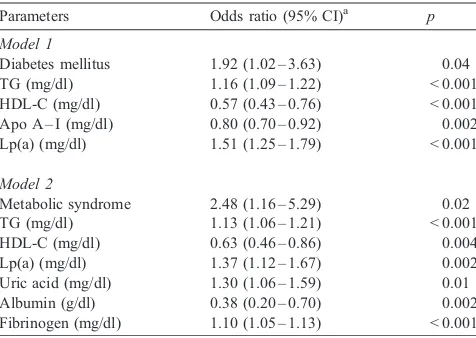
In logistic regression analysis model 1, TG, HDL-C, apo A – I, and Lp(a) levels were the major lipidemic parameters to be strongly associated with stroke among elderly subjects (Table 3). The presence of diabetes mellitus was associated with a two-fold stroke risk (odds ratio (OR), 1.92; 95% confidence interval, 1.02 – 3.63). In the second model (which also included fibrinogen and serum metabolic parameters, such as uric acid, albumin, total bilirubin, and ferritin) strong associations were evident between ischemic stroke and TG, HDL-C, Lp(a), fibrinogen, uric acid and albumin, but not for total bilirubin (Table 3). It is of importance that in this model, metabolic syndrome (which represents a cluster of metabolic disturbances, including impaired glucose tolerance, dyslipidemia, insulin resistance, hypertension, and upper body obesity) was identified as significantly associated with ischemic/non-embolic stroke (odds ratio, 2.48; 95% confidence interval, 1.16 – 5.29).
6. Discussion
It has been reported that in Japan and China, the age standardized annual death rate from stroke is greater than that of coronary heart disease (CHD), whereas in Northern Europe and the USA the CHD associated mortality is three to four times greater than the stroke related deaths[12]. In contrast, in Mediterranean countries, deaths as a result of both CHD and stroke display an approximate 1:1 ratio [12,13]. Serum lipid values, smoking, alcohol intake, and traditional diets may account for these differences[14]. Our study was held in the prefecture of Ioannina, Epirus (North-western Greece), a non-industrialized region of the country where at least the elderly (which comprise about 17% of the population) lead their lives in a more traditional way. In this area, eating habits in the elderly population remained unchanged for years (Mediterranean diet, including olive oil and minimal saturated fat consumption, close to NCEP step I diet), while smoking is strictly considered as a ‘‘male privilege’’ [12,13,15]. This is in contrast to a more west-ernized way of living followed by urban younger population residing in the city of Ioannina. Surprisingly, smoking was not identified as a significant risk factor for ischemic stroke. This could be explained by the small number of smokers among study participants, especially women.
Hypertension and diabetes rates were relatively high in the study population. It is well documented that the prev-alence of hypertension rises with advancing age. For exam-ple in the Framingham cohort, in those aged 70 to 79, almost 50% had borderline hypertension[16]. Moreover, the institution of the novel criteria introduced by the American Diabetes Association for the diagnosis of diabetes mellitus leads to an increased number of subjects at risk [17]. The presence of diabetes mellitus was associated with a two-fold stroke risk in our study. All things considered, a gradual decline in the physiologic reserve of all organ systems, vulnerability to degenerative diseases and polypharmacy are common problems associated with old age[5].
In the present study, stroke patients exhibited a more atherogenic lipid profile compared to CHD-free controls. Whether lipid abnormalities are causally associated with a higher incidence of stroke remains controversial [18,19]. Early studies did not suggest that raised serum TC levels consistently predict stroke-related mortality [20,21]. Fur-thermore, in some studies, TC levels are strongly but inversely related to stroke death [22]. Nevertheless, the presence of a link between hyperlipidemia and stroke is supported by the growing evidence that lipid-lowering treatment (especially statins) can significantly reduce the risk of stroke [23 – 27]. In the recently published Heart Protection Study (HPS), in which large numbers of older individuals (men and women) were included, simvastatin treatment produced a favorable effect on blood lipids and on vascular disease outcomes [28]. There was a definite and substantial reduction in ischemic stroke, including transient ischemic attacks, whereas lipid-lowering therapy was not
Table 3
Multivariate comparison of cases and controls by logistic regression analysis (backward stepwise likelihood ratio)
Parameters Odds ratio (95% CI)a
p
Model 1
Diabetes mellitus 1.92 (1.02 – 3.63) 0.04 TG (mg/dl) 1.16 (1.09 – 1.22) < 0.001 HDL-C (mg/dl) 0.57 (0.43 – 0.76) < 0.001 Apo A – I (mg/dl) 0.80 (0.70 – 0.92) 0.002 Lp(a) (mg/dl) 1.51 (1.25 – 1.79) < 0.001
Model 2
Metabolic syndrome 2.48 (1.16 – 5.29) 0.02 TG (mg/dl) 1.13 (1.06 – 1.21) < 0.001 HDL-C (mg/dl) 0.63 (0.46 – 0.86) 0.004 Lp(a) (mg/dl) 1.37 (1.12 – 1.67) 0.002 Uric acid (mg/dl) 1.30 (1.06 – 1.59) 0.01 Albumin (g/dl) 0.38 (0.20 – 0.70) 0.002 Fibrinogen (mg/dl) 1.10 (1.05 – 1.13) < 0.001 First model included age, gender, BMI, the presence hypertension, diabetes, metabolic syndrome and smoking as well as lipidemic parameters [TC, TG, HDL, apo A – I, apo B, Lp(a)]. Second model, in addition, included fibrinogen, uric acid, albumin, total bilirubin and ferritin.
aValues represent odds ratios per 10 mg/dl changes in TG, HDL-C,
associated with a decrease in the risk of hemorrhagic stroke [28]. Very low TC levels have been associated with an increased risk for hemorrhagic strokes [23]. However, evidence from recent statin trials, in which patients reached very low LDL-C levels, showed a decrease in strokes [29,30].
Total-C, LDL-C and apoB levels were similar among stroke patients and controls. It was the TG, HDL and apoA levels, which significantly differed between the two groups. It has been shown that postprandial hypertriglyceridemia is associated with carotid artery atherosclerosis[31]. However, there is a controversy regarding the association between serum TG levels and stroke [32]. Nonetheless, in the Copenhagen City Heart Study, a log linear association between serum TG levels and non-hemorrhagic stroke was found, which was independent of age and gender [33]. In general, in the majority of studies, an inverse association between HDL-C and stroke risk has been documented[32]. In the Northern Manhattan Stroke Study, increased levels of HDL-C are associated with reduced risk of ischemic stroke in the elderly and among different racial or ethnic groups [34]. These findings are in concordance with evidence relating these lipid parameters to stroke and strongly support HDL-C and TG as important modifiable stroke risk factors [35].
Stroke patients had remarkably higher Lp(a) levels as compared to controls. Elevated LDL and Lp(a) levels have been associated with an increased risk of non-hemorrhagic stroke[36,37]. Lp(a), a predictor of atherosclerotic disease, has been proposed as a link between lipids and hemostasis [38]. The accumulation of Lp(a) molecules has been dem-onstrated in the arterial walls of both human coronary and cerebral vessels. However, the evidence that Lp(a) is a strong predictor for ischemic stroke is contradictory [37,39]. Methodological problems in the determination of Lp(a) levels might contribute to the existing confusion in attributing risk to Lp(a) [11,38,39]. It has also been sug-gested that the coexistence of other risk factors (such as dyslipidemia or raised homocysteine values) reinforces the atherosclerotic potential of Lp(a)[37,38].
Stroke patients had significantly higher fibrinogen levels compared to controls. This finding is in agreement with evidence suggesting that fibrinogen is a powerful predictor of vascular events in healthy populations as well as and patients with cardiovascular disease[40]. Plasma fibrinogen levels are associated with an increased stroke risk and are considered of significant prognostic influence in stroke patients[41,42]. However, measurement of fibrinogen lev-els is subjected to certain methodological limitations [10,40]. In the present study, we followed the recommen-dations most authorities advocate that fibrinogen levels should be measured within 24 h from the onset of symptoms [10,40,42,43]. Although high fibrinogen levels have been reported to be accounted for by environmental and genetic influence, its expression is regulated by interleukin-6 and impaired by transforming growth factor-h similar to other
acute-phase proteins[40]. This raises the question whether raised plasma fibrinogen is the epiphenomenon of the severity of the vascular injury taking place in the brain. Therefore, although relevant from a therapeutic point of view (i.e. measure fibrinogen levels to identify high risk subjects), the prognostic value of fibrinogen is still of little clinical relevance[40 – 43].
Increased uric acid levels have been reported to be a predictor of stroke [44,45]. This is in agreement with our findings. Although an independent association between elevated uric acid serum levels and increased atherosclerotic disease or mortality is evident in some studies, others suggest that this association is due to concurrent risk factors related to the polymetabolic syndrome (i.e. hypertension, diuretic use, insulin resistance, hyperlipidemia, obesity) [46,47]. It has been proposed that uric acid may increase platelet adhesiveness and urate crystals may induce platelet lysis, thus accelerating thrombogenesis[48]. Uric acid may also play a role in the formation of free radicals and oxidative stress[49,50].
Interestingly, serum albumin and total bilirubin levels were lower in stroke patients compared to controls. This inverse relationship between serum albumin and stroke risk may reflect the nutritional status and well-being, which are important factors for the development and prognosis of a cerebrovascular event [51,52]. There is also evidence that higher levels of total serum bilirubin are associated with a lower risk for cardiovascular disease[53]. This was limited to men in the Framingham study [54]. Bilirubin is a well-known antioxidant and may protect LDL from oxidation [55]. Furthermore, bilirubin appears to be cytoprotective to rat hepatocytes, human erythrocytes and human monocytes when these cells are exposed to oxyradicals[55]. It has also been reported that higher levels of bilirubin are inversely associated with the presence of atheromatous plaques in the carotid arteries [56]. Thus, higher bilirubin levels seem to protect against stroke in the elderly.
Iron stores, as measured by serum ferritin levels, were considered as a risk factor for CHD. However, recent evidence does not support this hypothesis [57]. According to our findings, there were no significant differences in serum ferritin concentrations between stroke patients and controls.
Metabolic syndrome represents a ‘‘constellation’’ of lipid and no-lipid risk factors and is closely linked to a general-ized metabolic disorder refer to as insulin resistance[8,58]. It is of import that stroke patients in our study were more frequently diagnosed as suffering from metabolic syndrome than CHD-free controls and its presence was associated with a three-fold stroke risk. Since the metabolic syndrome is considered a potential secondary target of lipid-lowering treatment, its recognition in the elderly merits management of its underlying causes.
clin-ical problems and/or metabolic disturbances in the elderly patient should be considered as the result of a disease process rather than the result of ‘‘just getting old’’. There-fore, identification of high-risk subjects warrants manage-ment with lifestyle changes and appropriate treatmanage-ment.
References
[1] Buckley BM. Healthy ageing: ageing safely. Eur Heart J Suppl 2001; 3(Suppl. N):N6 – 10.
[2] Hankey GJ, Warlow CP. Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations. Lancet 1999;354:1457 – 63.
[3] Bonita R. Epidemiology of stroke. Lancet 1992;339:342 – 4. [4] Futterman LG, Lemberg L. Stroke risk, cholesterol and statins. Am J
Crit Care 1999;8:416 – 9.
[5] Avorn J. Unhealthy ageing: functional and socio-economic impact. Eur Heart J Suppl 2001;3(Suppl. N):N3 – 5.
[6] Moonis M, Fisher M. Imaging of acute stroke. Cerebrovasc Dis 2001; 11:143 – 50.
[7] Brickner ME. Cardioembolic stroke. Am J Med 1996;100:465 – 74. [8] Executive summary of the third report of the National Cholesterol
Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). J Am Med Assoc 2001;285:2486 – 97.
[9] Bruckert E, Giral P, Chadarevian R, Turpin G. Low free-thyroxine levels are a risk factor for subclinical atherosclerosis in euthyroid hyperlipidemic patients. J Cardiovasc Risk 1999;6:327 – 31. [10] Goudevenos JA, Bairaktari ETh, Chatzidimou KG, Milionis HJ,
Mikhailidis DP, Elisaf MS. The effect of atorvastatin on serum lipids, lipoprotein (a) and plasma fibrinogen levels in primary dyslipidaemia. A pilot study involving serial sampling. Curr Med Res Opin 2001;6: 269 – 75.
[11] Lippi G, Guidi G. Standardization and clinical management of lipo-protein (a) measurements. Clin Chem Lab Med 1998;36:5 – 16. [12] World Health Organization. Tobacco or health: a global status report.
Geneva: World Health Organization; 1997.
[13] Chimonas ET. Mortality trends and main causes of death in the Greek population. Curr Med Res Opin 2001;17:27 – 33.
[14] Reed DM. The paradox of high risk of stroke in populations with low risk of coronary heart disease. Am J Epidemiol 1990;131:579 – 88. [15] Summary of the second report of the National Cholesterol
Educatio-nal Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. J Am Med Assoc 1993;269:3015 – 23.
[16] Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham Study. Arch Intern Med 2001;161:2343 – 50.
[17] Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and clas-sification of diabetes mellitus. Diabetes Care 1997;20:1183 – 97. [18] Demchuk AM, Hess DC, Brass LM, Yatsu FM. Is cholesterol a risk
factor for stroke? Yes. Arch Neurol 1999;56:1518 – 20.
[19] Landau WM. Is cholesterol a risk factor for stroke? No. Arch Neurol 1999;56:1521 – 4.
[20] Rastenyte D, Tuomilehto J, Domarkiene S, Cepaitis Z, Reklaitiene R. Risk factors for death from stroke in middle-aged Lithuanian men: results from a 20-year prospective study. Stroke 1996;27:672 – 6. [21] Palmer A, Bulpitt C, Beevers G, et al. Risk factors for ischaemic
heart disease and stroke mortality in young and old hypertensive patients. J Hum Hypertens 1995;9:695 – 7.
[22] Menotti A, Blackburn H, Kromhout D, et al. The inverse relation of average population blood pressure and stroke mortality rates in the seven countries study: a paradox. Eur J Epidemiol 1997;13:379 – 86.
[23] Papadakis JA, Mikhailidis DP, Winder AF. Lipids and stroke: ne-glect of a useful preventive measure? Cardiovasc Res 1998;40: 265 – 71.
[24] Blauw GJ, Lagaay AM, Smelt AH, Westendorp RG. Stroke, statins, and cholesterol. A meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke 1997; 28:946 – 50.
[25] Lewis SJ, Moye LA, Sacks FM, et al., for the CARE Investigators. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) Trial. Ann Intern Med 1998;129:681 – 9.
[26] Pedersen TR, Kjekshus J, Pyorala K, et al. Effect of simvastatin on ischaemic signs and symptoms in the Scandinavian Simvastatin Sur-vival Study (4S). Am J Cardiol 1998;81:333 – 5.
[27] White HD, Simes RJ, Anderson NE, et al. Pravastatin therapy and the risk of stroke. N Engl J Med 2000;343:317 – 26.
[28] Heart Protection Study Collaborative Group. MRC/BHF Heart Protec-tion Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002;360: 7 – 22.
[29] Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with Atorvastatin to the National Cholesterol Educational Program Goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) Study. Curr Med Res Opin 2002;18:220 – 8.
[30] Sever PS, Dahlo¨f B, Poulter NR, et al., for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hyper-tensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised con-trolled trial. Lancet 2003;361:1149 – 58.
[31] Ryu JE, Howard G, Craven TE, Bond MG, Hagaman AP, Crouse 3rd JR. Postprandial triglyceridemia and carotid atherosclerosis in middle-aged subjects. Stroke 1992;23:823 – 8.
[32] Rizos E, Mikhailidis DP. Are high density lipoprotein (HDL) and triglyceride levels relevant in stroke prevention? Cardiovasc Res 2001;52:199 – 207.
[33] Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high-density lipoprotein cholesterol, and triglycerides on risk of ce-rebrovascular disease: the Copenhagen City Heart Study. Br Med J 1994;309:11 – 5.
[34] Sacco RL, Benson RT, Kargman DE, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly. The Northern Manhat-tan Stroke Study. J Am Med Assoc 2001;285:2729 – 35.
[35] Tanne D, Koren-Morag N, Graff E, Goldbourt U. Blood lipids and first-ever ischemic stroke/transient ischemic attack in the Bezafibrate Infarction Prevention (BIP) Registry: high triglycerides constitute an independent risk factor. Circulation 2001;104:2892 – 7.
[36] Bostom AG, Cupples LA, Jenner JL, et al. Elevated plasma lipopro-tein (a) and premature coronary heart disease in Framingham men: a prospective study. J Am Med Assoc 1996;276:544 – 8.
[37] Milionis HJ, Winder AF, Mikhailidis DP. Lipoprotein (a) and stroke. J Clin Pathol 2000;53:487 – 96.
[38] Hobbs HH, White AL. Lipoprotein (a): intrigues and insights. Curr Opin Lipidol 1999;10:225 – 36.
[39] Luc G, Bard JM, Arveiler D, et al., on behalf of the PRIME Study Group. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis 2002;163:377 – 84.
[40] Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plas-ma fibrinogen to predict stroke and myocardial infarction. Arterioscler Thromb Vasc Biol 1999;19:1368 – 77.
[41] Folsom AR, Rosamond WD, Shahar E, et al., for the Atherosclerosis Risk in Communities (ARIC) Study Investigators. Prospective study of markers of hemostatic function with risk of ischemic stroke. Cir-culation 1999;100:736 – 42.
C-reactive protein and fibrinogen levels in ischemic stroke. Stroke 2001;32:133 – 8.
[43] Di Napoli M, Papa F, for the Villi Pini Stroke Data Bank Investiga-tors. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke 2002;33:1763 – 71.
[44] Matias-Guiu J, Alvarez J, Insa R, et al. Ischemic stroke in young adults: II. Analysis of risk factors in the etiological subgroups. Acta Neurol Scand 1990;81:314 – 7.
[45] Longo-Mbenza B, Luila EL, Mbete P, Vita EK. Is hyperuricemia a risk factor of stroke and coronary heart disease among Africans? Int J Cardiol 1999;71:17 – 22.
[46] Fang J, Alderman MH. Serum uric acid and cardiovascular mortality. The NHANES I epidemiologic follow-up study, 1971 – 1992. J Am Med Assoc 2000;283:2404 – 10.
[47] Lee J, Sparrow D, Vokonas PS, Landsberg L, Weiss ST. Uric acid and coronary heart disease risk: evidence for a role of uric acid in the obesity-insulin resistance syndrome. The Normative Aging Study. Am J Epidemiol 1995;142:288 – 94.
[48] Ginsberg MH, Kozin F, O’Malley M, McCarty DJ. Release of platelet constituents by monosodium urate crystals. J Clin Invest 1977;60: 999 – 1007.
[49] Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78: 6858 – 62.
[50] Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric
acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis 2000;148:131 – 9.
[51] Gillum RF. Assessment of serum albumin concentration as a risk factor for stroke and coronary disease in African Americans and whites. J Natl Med Assoc 2000;92:3 – 9.
[52] Gariballa SE, Parker SG, Taub N, Castleden CM. Influence of nutri-tional status on clinical outcome after acute stroke. Am J Clin Nutr 1998;68:275 – 81.
[53] Hunt SC, Kronenberg F, Eckfeldt JH, Hopkins PN, Myers RH, Heiss G. Association of plasma bilirubin with coronary heart disease and segregation of bilirubin as a major gene trait: the NHLBI family heart study. Atherosclerosis 2001;154:747 – 54.
[54] Djousse L, Levy D, Cupples LA, Evans JC, D’Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham Offspring Study. Am J Cardiol 2001;87:1196 – 200. [55] Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidation of
human low density lipoprotein by unconjugated and conjugated bilir-ubins. Biochem Pharmacol 1996;51:859 – 62.
[56] Ishizaka N, Ishizaka Y, Takahashi E, Yamakado M, Hashimoto H. High serum bilirubin level is inversely associated with the presence of carotid plaque. Stroke 2001;32:581 – 3.
[57] Pilote L, Joseph L, Be´lisle P, Robinson K, van Lente F, Tager IB. Iron stores and coronary artery disease: a clinical application of a method to incorporate measurement error of the exposure in a logistic regres-sion model. J Clin Epidemiol 2000;53:809 – 16.