Dietary fish oil reduces intercellular adhesion molecule 1 and
scavenger receptor expression on murine macrophages
Elizabeth A. Miles *, Fiona A. Wallace, Philip C. Calder
Institute of Human Nutrition,Uni6ersity of Southampton,Bassett Crescent East,Southampton SO16 7PX, UK
Received 11 June 1999; received in revised form 11 October 1999; accepted 28 October 1999
Abstract
During atherogenesis, a pathological accumulation of lipids occurs within aortic intimal macrophages through uptake of oxidised low-density lipoprotein (LDL) via scavenger receptors. Here we investigate whether some of the anti-atherosclerotic effects ascribed to dietary fish oil are mediated through effects on macrophage intercellular adhesion molecule 1 (ICAM-1) and scavenger receptor expression. Mice were fed on a low fat diet (containing 25 g/kg corn oil) or on high fat diets containing 200 g/kg coconut oil, safflower oil or fish oil. Thioglycollate-elicited peritoneal macrophages were analysed for fatty acid composition by gas chromatography. Macrophage scavenger receptor A (MSR-A) type I+type II and ICAM-1 expression were measured by flow cytometry and the levels of mRNA coding for MSR-A type I, MSR-A type II and ICAM-1 were measured by reverse-transcription polymerase chain reaction. Feeding mice diets enriched with different fats resulted in significant changes in the fatty acid profile of macrophages, which reflected the fatty acid compositions of the diets. Macrophages from the fish oil fed mice had the lowest expression of ICAM-1 and MSR-A at the level of both mRNA and cell surface expression. The reduced expression of ICAM-1 and MSR-A on macrophages from mice fed on a fish oil-rich diet supports our hypothesis that part of the protective effect of fish oil against atherosclerosis might be due to an altered macrophage phenotype and function ameliorating macrophage-induced plaque formation. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Scavenger receptor; Intercellular adhesion molecule 1; Fish oil; Atherosclerosis; Murine peritoneal macrophage
www.elsevier.com/locate/atherosclerosis
1. Introduction
Two key initial events within the arterial wall during early atherogenesis are the recruitment and differentia-tion of circulating monocytes, and the uptake of choles-terol by these tissue macrophages to become lipid-laden foam cells. Intercellular adhesion molecule 1 (ICAM-1) is involved in leukocyte-endothelial interactions and extravasation of monocytes. ICAM-1 is strongly ex-pressed on macrophages within atherosclerotic plaques where it has a role in leukocyte-leukocyte interactions [1,2]. Disruption of the ICAM-1 gene in C57Bl6 mice
fed on a high-fat diet results in reduced development of fatty streaks compared with mice with the wild type ICAM-1 gene and thus protects against the atheroscle-rotic process [3]. Tissue macrophages within the aortic intima take up cholesterol through binding and uptake of oxidised low-density lipoprotein (LDL) to become the lipid-laden foam cells characteristic of early atherosclerotic lesions [4,5]. This oxidised LDL uptake is mediated via macrophage scavenger receptors [6]. Macrophage scavenger receptor A (MSR-A) was the first of the scavenger receptors to be characterised [7]. It has three splice variants, two of which are active as oxidised LDL receptors (type I and type II) [7,8]. MSR-A expression is upregulated in animals fed high cholesterol diets [9] and it has been demonstrated in human atherosclerotic lesions where it co-localizes with oxidised LDL [10]. The expression of MSR-A type I and type II in human atherosclerotic lesions is restricted to macrophage-derived foam cells with very little ex-pression by smooth muscle cells or aortic endothelium Abbre6iations: Ac-LDL, acetylated low-density lipoprotein; CO,
coconut oil; FO, fish oil; ICAM-1, intercellular adhesion molecule 1; LDL, low-density lipoprotein; LF, low fat; MSR-A type I, macrophage scavenger receptor A type I; MSR-A type II, macrophage scavenger receptor A type II; SO, safflower oil.
* Corresponding author. Tel.:+44-1703-594-689; fax: + 44-1703-595-489.
E-mail address:[email protected] (E.A. Miles).
[11]. The role for MSR-A in plaque formation is indi-cated by studies showing that MSR-A knockout mice fed on a high cholesterol diet have a significantly reduced development of atherosclerotic plaques [12,13]. Increased intake of oily fish or fish oils containing long chain n-3 polyunsaturated fatty acids confers pro-tection against cardiovascular and ischaemic heart dis-ease in man [14,15]. Addition of fish oil to a diet high in saturated fats reduced the development of atheroscle-rotic plaques in mice [16], while fish oil given orally to Watanabe heritable hyperlipidaemic rabbits resulted in a significant reduction of aortic cholesterol content and a lowered development of aortic atherosclerotic lesions [17].
We hypothesise that some of the anti-atherogenic effects demonstrated by dietary fish oils may be medi-ated through effects on ICAM-1 and MSR-A expres-sion on macrophages. Therefore, in this study we investigated the effects of diets containing 20% by weight of different oils (fish oil, safflower oil, coconut oil) on surface and mRNA expression of the adhesion molecule ICAM-1 and two scavenger receptors (MSR-A types I and II) by murine peritoneal macrophages, a convenient source of inflammatory macrophages.
2. Methods
2.1. Animals and diets
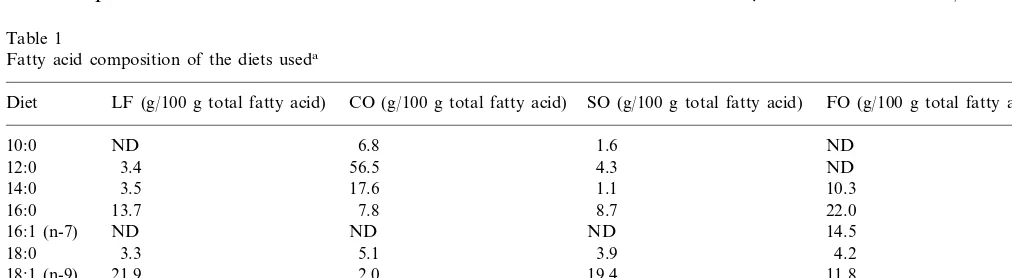
Male C57Bl6 mice (Harlan-Olac, Bicester, UK) were fed on a low fat (LF) diet (containing 25 g/kg corn oil) or on high fat diets containing 200 g/kg coconut oil (CO), safflower oil (SO) or fish oil (FO) (purchased from ICN Biomedicals, High Wycombe, UK). The high fat diets also contained 10 g/kg corn oil to prevent essential fatty acid deficiency. All diets contained identi-cal amounts of protein (200 g/kg), starch (200 g/kg), sucrose (295.8 g/kg) and vitamin E (1.2 g/kg). The fatty acid composition of the diets is shown in Table 1.
Animals were allowed free access to the diets for 12 weeks (n]8/diet), and were then killed by an overdose of CO2. All procedures involving animals were ap-proved by the UK Home Office under the Animals (Scientific Procedures) Act 1986.
2.2. Chemicals
Unless otherwise stated, all chemicals were purchased from Sigma, Poole, UK.
2.3. Macrophage preparation
To elicit macrophage migration to the peritoneal cavity, 4 days prior to sacrifice the mice were injected intraperitoneally with 1 ml of Brewer’s thioglycollate broth. After death the peritoneal exudate was collected by washing out the peritoneal cavity with 4 ml sterile PBS (Oxoid, Unipath, Basingstoke, UK). The cells were washed with sterile PBS, collected by centrifugation, passed through lens tissue (Whatman, Loughborough, UK) and washed again with sterile PBS. Contaminating erythrocytes were lysed by a 5-min incubation with Tris-buffered 0.14 mM ammonium chloride, pH 7.2.
2.4. Fatty acid analysis
Total lipid was extracted from macrophages with chloroform/methanol (2:1 v/v) and washed twice in 0.88% KCl. Fatty acids were prepared by saponification at 70°C in methanolic 0.5 M KOH. Samples were neutralized using concentrated sulfuric acid and fatty acids were extracted into chloroform and washed twice in 0.88% KCl. After evaporation to dryness, fatty acid methyl esters were prepared by reaction with an excess of diazomethane in ether. Fatty acid methyl esters (dissolved in hexane) were separated by gas chromatog-raphy in a Hewlett-Packard 6890 gas chromatograph fitted with a 25-m×0.32-mm BPX70 capillary column, film thickness 0.25mm. Helium at 2.0 ml/min was used
Table 1
Fatty acid composition of the diets useda
Diet LF (g/100 g total fatty acid) CO (g/100 g total fatty acid) SO (g/100 g total fatty acid) FO (g/100 g total fatty acid)
ND
10:0 6.8 1.6 ND
3.4 56.5
12:0 4.3 ND
14:0 3.5 17.6 1.1 10.3
13.7 22.0
16:0 7.8 8.7
ND ND
16:1 (n-7) ND 14.5
18:0 3.3 5.1 3.9 4.2
21.9 2.0
18:1 (n-9) 19.4 11.8
54.3 2.3
18:2 (n-6) 61.0 9.0
18:3 (n-3) ND ND ND 3.5
ND
20:5 (n-3) ND ND 10.6
ND
22:6 (n-3) ND ND 10.1
as the carrier gas and the split/splitless injector was used with a split:splitless ratio of 10:1. Injector and detector temperatures were 250 and 270°C, respectively. The column oven temperature was maintained at 170°C for 12 min after sample injection and was programmed to then increase from 170 to 200°C at 5°C/min before being maintained at 200°C for 15 min. The separation was recorded with HP GC Chem Station software. Fatty acid methyl esters were identified by comparison with standards run previously.
2.5. Extraction of RNA and re6erse transcription
Total RNA was extracted from 2×106
macrophages using TRIzol (Life Technologies, Paisley, UK) in accor-dance with the manufacturer’s instructions. Messenger RNA was then selectively reverse transcribed using an oligo (dT) primer from 4.5 mg of total RNA. Reverse transcription (RT) was achieved with 7.5 U of avian myeloblastosis virus reverse transcriptase (Promega, Southampton, UK) in the presence of 1 mM dNTPs (Pharmacia, Milton Keynes, UK), 5 mM MgCl2 (Promega), RT buffer (10 mM Tris – HCl (pH 8.8), 50 mM KCl and 0.1% Triton X-100 (Promega)) and 0.5mg poly (dT)15 (Promega). RNA was substituted with an equal volume (5 ml) diethyl pyrocarbonate (DePc) treated water as a negative control. Reverse transcrip-tion was carried out for 1 h at 42°C followed by heating at 94°C for 3 min to inactivate the enzyme. The result-ing cDNA was diluted with 15ml of DePc treated water to a final volume of 35 ml and used as a polymerase chain reaction (PCR) template.
2.6. DNA amplification and6isualisation
PCR was performed for a housekeeping gene (cy-clophilin), MSR-A type I, MSR-A type II and ICAM-1. Amplification of 2.5ml of cDNA was achieved using 1 U of Taq polymerase in the presence of 15 pmol of primer, Mg-free buffer (19 mM Tris – HCl (pH 9.0), 50 mM KCl and 0.1% Triton X-100 (Promega)), 1.5 mM MgCl2 (0.5 mM for ICAM-1) (Promega) and 0.2 mM dNTPs (Pharmacia). The reaction cycling was 95°C for 30 s, 56°C (60°C for ICAM-1) for 30 s and 72°C for 1 min in a Hybaid Touchdown Thermocycler. The opti-mised number of cycles used (reflecting the exponential phase of the reaction) was 26 for MRSA type I and type II and 30 cycles for ICAM-1. The primer se-quences used for cyclophilin were 5% -TTGGGTCGC-GTCTCGTTCGA-3% sense and 5% -GCCAGGACC-TGTATGCTTCA-3% antisense. Primers for MSRA type I were 5%-GGGAGACAGAGGGCTTACTGG A-3% sense and 5%-TTGTCCAAAGTGAGCTCTCTTG-3% antisense (389 bp). Primers used for MSRA type II were 5%-GGGAGACAGAGGGCTTACTGGA-3% sense and 5%-ATGTTCAGGGAGTTATACTGATC-3%
anti-sense (223 bp). Primers used for ICAM-1 were 5% -TTTTGCTCTGCCGCTCTGGAG-3%sense and 5% -TA-CACATTCCTGGTGACATTC-3% antisense (287 bp). PCR products were electrophoresed on 2% agarose gels stained with ethidium bromide. The resultant bands were visualised with an UV transilluminator and the image stored with a GDS 5000 gel documentation system (UVP, Cambridge, UK). The images were then analysed by densitometry using Phoretix 2D 4.00 soft-ware (Phoretix International, Newcastle-upon-Tyne, UK). All results are expressed as a ratio of ICAM-1 or scavenger receptor: cyclophilin mRNA where the test cDNA was amplified for cyclophilin concurrently with amplification for the ICAM-1 or scavenger receptors and under the same conditions.
2.7. Receptor expression
The macrophages were washed twice using modified PBS with 0.1% (w/v) bovine serum albumin and 10 mM sodium azide. Aliquots of 2×105 cells were incubated with antibody against murine monocytes and macrophages (F4/80), murine MSR-A type I+II (2F8) or murine ICAM-1 (KAT-1) (all from Serotec, Oxford, UK) for 20 min at 4°C. The cells were washed twice again with the modified PBS and then collected by centrifugation. Cells were incubated with a fluorescently labelled second antibody (STAR-49) (Serotec, Oxford, UK) for 20 min at 4°C. The cells were washed as before and fixed with 200 ml of PBS containing 2% (v/v) formaldehyde. The cells were analysed using a FAC-Scan flow cytometer (BectonDickinson, Oxford, UK). Results are expressed as percent of cells positive for each receptor and as median fluorescence to give an indication of level of receptor expression per cell. The expression index has been calculated as proportion of cells positive for each receptor multiplied by the median fluorescence intensity to reflect overall changes in level of receptor expression within the cell population.
2.8. Sca6enger receptor function
Table 2
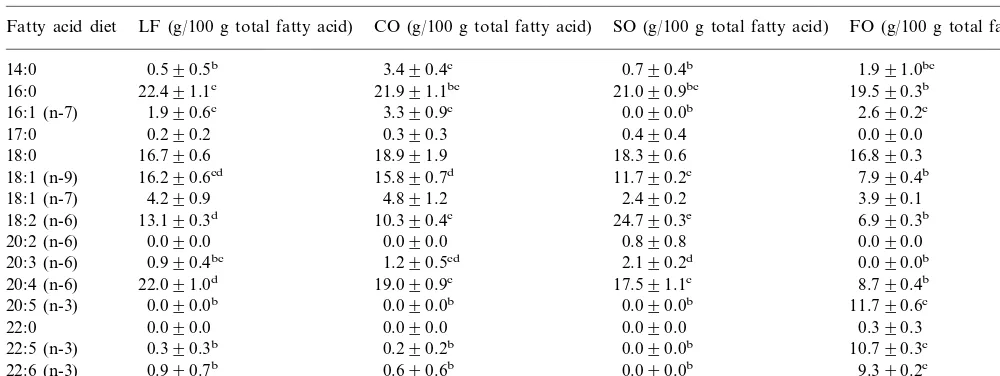
Fatty acid composition of murine peritoneal macrophagesa
CO (g/100 g total fatty acid) SO (g/100 g total fatty acid)
LF (g/100 g total fatty acid) FO (g/100 g total fatty acid)
Fatty acid diet
3.490.4c
14:0 0.590.5b 0.790.4b 1.991.0bc
21.991.1bc 21.090.9bc
22.491.1c 19.590.3b
16:0
3.390.9c 0.090.0b
16:1 (n-7) 1.990.6c 2.690.2c
0.390.3 0.490.4
0.290.2 0.090.0
17:0
18.991.9
18:0 16.790.6 18.390.6 16.890.3
15.890.7d 11.790.2c
16.290.6cd 7.990.4b
18:1 (n-9)
4.891.2 2.490.2
18:1 (n-7) 4.290.9 3.990.1
10.390.4c 24.790.3e
13.190.3d 6.990.3b
18:2 (n-6)
0.090.0 0.890.8
20:2 (n-6) 0.090.0 0.090.0
1.290.5cd 2.190.2d
0.990.4bc 0.090.0b
20:3 (n-6)
19.090.9c 17.591.1c
20:4 (n-6) 22.091.0d 8.790.4b
0.090.0b 0.090.0b
0.090.0b 11.790.6c
20:5 (n-3)
22:0 0.090.0 0.090.0 0.090.0 0.390.3
0.290.2b 0.090.0b
0.390.3b 10.790.3c
22:5 (n-3)
0.990.7b
22:6 (n-3) 0.690.6b 0.090.0b 9.390.2c
24:0 0.890.5 0.390.3 0.490.4 0.090.0
aMice were fed a low fat (LF), coconut oil (CO), safflower oil (SO) or fish oil (FO) diet. All values are means9S.E. forn=3–6 mice. Values across a row not sharing a common alphabetical superscript are significantly different (ANOVA,PB0.05)
Freshly isolated macrophages were washed twice with PBS. Aliquots of 2×105cells were incubated with fluorescently labelled Ac-LDL for 3 h at 37°C. The cells were washed twice again with PBS and fixed in PBS with 2% (v/v) formaldehyde. Uptake of fluorescently labelled Ac-LDL was measured by flow cytometry as described above. Results are expressed as percent of cells positive for Ac-LDL uptake and as median fluorescence to give an indication of level of uptake of Ac-LDL per cell. The uptake index has been calculated as proportion of cells positive for Ac-LDL multiplied by the median fluorescence intensity to reflect overall changes in level of Ac-LDL uptake within the cell population.
2.9. Data analysis
All data are expressed as means9S.E. ofn observa-tions. Data were analysed using a one-way ANOVA with a post-hoc least significant difference test using SPSS version 6.1 for Windows (SPSS, Chicago, USA). A value of PB0.05 was taken to indicate statistical significance.
3. Results
3.1. Yield and purity of macrophages
Cell yields were 19 – 36×106
per animal. The cells were \85% macrophages as assessed by flow cytome-try. There were no statistical differences in cell yield or purity between the diets.
3.2. Fatty acid composition of macrophages
Macrophages from the CO fed mice had a signifi-cantly higher proportion of 14:0 when compared with macrophages from the mice fed on the LF and SO diets (Table 2). Macrophages from mice fed on the FO diet had a significantly lower proportion of 18:1 n-9 com-pared with those from mice fed on each of the other diets. Macrophages from mice fed on the FO diet had significantly lower proportions of the long-chain n-6 fatty acids 18:2 n-6, 20:3 n-6 and 20:4 n-6 when com-pared with those from mice fed on each of the other high fat diets. Linoleic acid (18:2 n-6) and arachidonic acid (20:4 n-6) were also found at significantly lower levels in the macrophages from FO fed mice compared with the LF fed mice. Conversely the long chain n-3 polyunsaturated fatty acids (20:5 n-3, 22:5 n-3 and 22:6 n-3) were found in significantly higher proportions in the macrophages from the FO fed mice compared with those from the mice fed on each of the other diets.
3.3. Receptor expression
The percentage of macrophages expressing ICAM-1 was lowest for mice fed the FO diet (Table 3). The median fluorescence intensity, which indicates level of receptor expression per macrophage, was also lowest for cells from the mice fed the FO diet. The ICAM-1 expression index was lowest for the macrophages from mice fed the FO diet and this was significantly lower than that seen for macrophages from mice fed the CO diet (Table 3).
Table 3
ICAM-1 and MSR-A type I+II expression by murine peritoneal macrophagesa
Diet ICAM-1 MSR-A type I+II
MFI Expression index % Positive MFI Expression index % Positive
74.094.8 63.893.2bc
LF 87.193.0 68.696.8bc 74.1912.2 54.5914.0bc
89.496.5 77.396.6c
CO 86.793.9 79.392.8c 74.198.4 58.396.3c
78.496.0 70.996.6bc 73.692.9c
89.792.6 76.499.9
SO 56.798.1c
FO 79.795.0 73.395.0 59.796.5b 59.094.6b 54.091.9 32.093.0b
aMice were fed on a low fat (LF), coconut oil (CO), safflower oil (SO) or fish oil (FO) diet. Results are means9S.E. for percentage positive (% positive), median fluorescence intensity (MFI) and expression index forn=8–12 mice. Values in a column not sharing a common alphabetical superscript are significantly different (ANOVA,PB0.05).
mice fed on the FO diet having the lowest values (Table 3). A significantly lower proportion of macrophages from mice fed on the FO diet expressed MSR-A type I+II compared to those from mice fed on the CO or SO diets. The median fluorescence intensity, which indicates level of receptor expression per macrophage, was also lowest for cells from the mice fed the FO diet. The MSR-A type I+II expression index was again lowest for mice fed on the FO diet and this was significantly lower than the MSR-A type I+II index for cells from mice fed the CO or SO diets (Table 3).
3.4. Macrophage sca6enger receptor function
Uptake of Ac-LDL was lowest for the macrophages from mice fed the FO diet both by percentage of macrophages positive for Ac-LDL and median fluores-cence intensity (Table 4). The uptake index for Ac-LDL was significantly lower for macrophages from mice fed on the FO diet compared with those from mice fed on the SO diet.
3.5. ICAM-1 and macrophage sca6enger receptor mRNA
Results are shown as the ratio of ICAM-1 or MSR-A mRNA to the constitutively expressed cyclophilin mRNA as assessed by densitometry (Fig. 1A,B,C). Messenger RNA specific for ICAM-1 was significantly lower in macrophages from the FO fed mice compared with those from mice fed on the LF or CO diets (Fig. 1A). ICAM-1 mRNA in macrophages from the FO fed mice was also lower than that from mice fed the SO diet. Freshly isolated macrophages from mice fed on the SO or FO-rich diets had less mRNA specific for MSR-A type I than macrophages from mice fed on CO or LF diet but this difference was not statistically significant. (Fig. 1B). Macrophages from mice fed on the FO diet had significantly less mRNA for MSR-A type II than those from mice fed on the LF diet (Fig. 1C). This was also lower than that found in cells from mice fed on the other high fat diets although this did
not reach significance. Messenger RNA for MSR-A type II was not altered by feeding on the high fat diets containing CO and SO when compared with feeding on the LF diet (Fig. 1C).
4. Discussion
The protective effect of fish oil against the develop-ment of cardiovascular disease in humans has been demonstrated in epidemiological studies showing re-duced incidence in populations with high intakes of oily fish [19,20] and correlations between oily fish intake and a reduced risk [14,15]. The addition of FO to a cholesterol-enriched diet reduces atherosclerotic lesions and cholesterol accumulation in the arteries in animal models of diet-induced atherosclerosis [16,17].
Atherosclerotic plaques are rich in tissue macrophages, which take up oxidised LDL via scav-enger receptors [4 – 6]. These cells become the lipid-laden foam cells, which is perhaps the most characteristic event of early atherosclerosis [21]. Op/op mice (which have low macrophage colony stimulating factor production and so lack macrophages) crossed with atherosclerotic prone apoE− /apoE− knockout mice do not develop fatty streaks, illustrating the im-portance of mature macrophages in the intima for atherogenesis [22]. In this study we have investigated
Table 4
Acetylated-LDL uptake over 3 h by murine peritoneal macrophagesa
% Positive
Diet MFI Uptake index
LF 57.395.4 2585.99258.5 1518.79235.0bc 1911.49369.2bc 2992.59510.3
CO 62.593.9
SO 68.094.8 3381.89655.0 2493.39628.2c 2431.89475.6
59.994.9 1350.29243.6b FO
aMice were fed on a low fat (LF), coconut oil (CO), safflower oil (SO) or fish oil (FO) diet. Results are means9S.E. for percentage positive (% positive), median fluorescence intensity (MFI) and uptake index forn=9–12 mice. Values in a column not sharing a common alphabetical superscript are significantly different (ANOVA, PB
Fig. 1. mRNA levels for (a) intercellular adhesion molecule 1 (ICAM-1), (b) macrophage scavenger receptor A type I (MSR-A type I), (c) macrophage scavenger receptor A type II (MSR-A type II), in macrophages from mice fed on a low fat (LF), coconut oil (CO), safflower oil (SO) or fish oil (FO) diet. Values are ratios of ICAM-1 or MSR-A type I or MSR-A type II: cyclophilin mRNA. Data are mean values and S.E. for n=8 – 12 mice. Values not sharing a common alphabetical superscript are significantly different (ANOVA, PB0.05).
of ICAM-1. Although a dietary FO-induced reduction in ICAM-1 surface expression has been observed previously in both animal and human experiments (see below), this is the first time that reduced expression at the level of mRNA has been described. These observations suggest that a component of FO can affect transcription of the ICAM-1 gene. This conclusion is supported by cell culture experiments where incubation of human saphenous vein endothelial cells with eicosapentaenoic acid or docosa-hexaenoic acid (the two major long-chain n-3 polyunsat-urated fatty acids found in fish oil) resulted in a reduction of interleukin-1binduced ICAM-1 mRNA levels [23]. A modest reduction (23%) of ICAM-1 surface expression on human saphenous vein endothelial cells has been demonstrated after incubation with docosahexaenoic acid [24]. In vitro experiments incubating endothelial cells with docosahexaenoic acid have also shown a reduction in other pro-atherogenic molecules, such as vascular cell adhesion molecule 1, both at the level of surface expres-sion and the level of mRNA [24]. In the current study there was a significant increase in docosahexaenoic acid, eicosapentaenoic acid and other n-3 polyunsaturates in macrophages from the mice fed FO compared with those fed on the other high fat diets and the LF diet.
Hughes et al. have demonstrated that dietary FO supplementation can reduce ICAM-1 expression on human peripheral blood monocytes [25]. We have previ-ously shown that FO feeding reduces ICAM-1 expression on rat dendritic cells [26] and on mitogen-stimulated rat lymphocytes [27] and reduces adhesion of these lymphocytes to macrophage and endothelial cell mono-layers [27]. Thus, a reduction in the expression of ICAM-1 may have important physiological consequences for interactions between monocytes or macrophages with other leukocytes and with endothelial cells during early atherogenic inflammation.
Macrophages from FO-fed animals had the lowest levels of mRNA coding for MSR-A type I and type II and the lowest surface expression of MSR-A type I+type II. This was accompanied by the lowest uptake of Ac-LDL. This suggests that the phenotypic alteration in MSR-A expression has functional consequences. The role of the macrophage scavenger receptors in the develop-ment of atherosclerotic lesions has been well docudevelop-mented [28]. The importance of scavenger receptors in the development of diet-induced atherosclerosis was indi-cated in recent studies where mice with a targeted disruption of the MSR-A gene developed significantly less extensive and severe plaques when compared to mice with the wild type MSR-A gene [12,13].
It was previously shown that macrophage differentia-tion and conversion to foam cells is accompanied by markedly increased cell surface expression of MSR-A type I with only a small increase in surface expression of MSR-A type II [29]. Since the monoclonal antibody we used does not distinguish between MSR-A type I and II, it is not clear what the relative levels of expression of these the effects of dietary FO on the expression of two
functional molecules (ICAM-1 and MSR-A), important in recruitment of macrophages to the aortic intima, and their activation and subsequent conversion to foam cells. Feeding male C57Bl6 mice on high fat diets enriched with different fats resulted in significant changes in the fatty acid profile of peritoneal macrophages, which reflected the fatty acid compositions of the diets. Macrophages from the mice fed on the CO diet contained a greater proportion of saturated fatty acids when compared with macrophages from mice fed on the other diets. Macrophages from the mice maintained on the SO rich diet had a greater proportion of n-6 polyunsaturated fatty acids (particularly linoleic acid) and macrophages from the mice fed on the FO rich diet had a greater proportion of n-3 polyunsaturated fatty acids.
two isoforms are on the cells that we studied. Therefore we cannot make any conclusions about the effects of dietary fats on the surface expression of MSR-A type I or type II. The effects of dietary fat were more pronounced on the expression of mRNA for the type II isoform; fish oil decreased mRNA levels by almost 40% compared with the low fat diet. This diet also decreased MSR-A type I mRNA by 25% although this effect was not statistically significant. However, these data suggest that fish oil might decrease expression of both of these isoforms of MSR-A. Macrophage differentiation and conversion to foam cells is accompanied by increased expression of MSRA type I mRNA with little effect on MSR-A type II mRNA [29]. It will be important to investigate the effects of dietary fats including fish oil on the altered expression of MSR-A isoforms which occurs during macrophage conversion to foam cells.
MSR-A also appears to have a role in macrophage adhesion [30,31]. Blocking with an MSR-A-specific anti-body prevents murine peritoneal macrophages from binding to tissue culture treated plastic [32]. Incubation of murine macrophages with long-chain n-3 polyunsatu-rated fatty acids also decreased macrophage adhesion to tissue culture treated plastic surfaces [33]. It is tempting to suggest that this was due to a reduction in expression of MSR-A involved in this adhesion. Pre-treating sections from lymphoid and non-lymphoid tissues with an anti-MSR-A antibody has been shown to abrogate macrophage binding to these tissues [30]. These authors suggested that MSR-A may have a role in homing or retention of macrophages within MSR-A ligand-rich tissues. Thus, the data in this study showing that feeding mice a FO diet decreases MSR-A type I and type II both at the level of surface expression and mRNA, indicate that this diet may play a role in reducing macrophage extravasation and persistence within the arterial intima, in addition to decreasing the ability of macrophages within the arterial intima to form fatty streaks.
In this study we have used thioglycollate elicited peritoneal macrophages. Clearly these cells demonstrate significant expression of MSR-A at both the mRNA and cell surface protein levels. These cells, although studied immediately after isolation from the animals, have under-gone stimulation in vivo. As such, they are not represen-tative of resident peritoneal macrophages but might be expected to be more like those found in atherosclerotic lesions and other sites of inflammatory activity, since those cells have also been subjected to various stimuli. There are differences in MSR-A expression between fresh resident macrophages and more mature macrophages (resident cells after culture) with the fresh cells expressing low amounts of MSR-A on their surface despite contain-ing significant levels of MSR-A mRNA [34]. This suggests that in fresh resident cells there is a post-transcriptional down-regulation of MSR-A expression. Given that macrophages in atherosclerotic plaques express MSR-A
on their surface [9 – 13] and are able to take up modified LDL, it seems appropriate to study the influence of nutritional factors on cells expressing a similar phenotype, i.e. elicited peritoneal macrophages rather than resident macrophages.
Experiments culturing cells with n-3 polyunsaturated fatty acids have demonstrated many anti-inflammatory effects of these fatty acids including the downregulation of adhesion and co-stimulatory surface molecules and a reduced production of inflammatory mediators [35]. Many of these effects can be mimicked by FO feeding in both animal and human subjects. Thus, the reduction in ICAM-1 and MSR-A type I and type II expression observed in the current study may be a result of the increased incorporation of n-3 polyunsaturated fatty acids into macrophages during FO feeding. It has recently become apparent that n-3 polyunsaturated fatty acids can affect gene transcription for some molecules [36], although the mechanism by which they do so is not clear. In vitro culture of human monocytic cell lines with n-3 polyunsat-urated fatty acids resulted in the reduction of mRNA for the scavenger receptor CD36 [37]. The current study indicates that these fatty acids also alter ICAM-1 and MSR-A type I and type II gene expression. These effects may be mediated through alterations in signal transduc-tion or through a more direct effect on transcriptransduc-tion factors (see review in Ref. [38]). The FO rich diet also lowered the proportion of linoleic acid in the macrophages in this study. Recent experiments have demonstrated that two oxidative metabolites of linoleic acid (9-hydroxyoc-tadecadienoic acid and 13-hydroxyoc(9-hydroxyoc-tadecadienoic acid) mediate the upregulatory signal between oxidised LDL and MSR-A gene transcription [39]. Thus a reduction in linoleic acid, as seen in the macrophages from the FO fed mice in this study, may also reduce levels of scavenger receptor mRNA in macrophages.
This study shows a decrease in ICAM-1 and MSR-A on murine peritoneal macrophages following FO feeding compared with other high fat diets and the LF diet. Such alterations in macrophage phenotype may reduce recruit-ment and activation of macrophages into the aortic intima and thus impede the atherogenic process. Therefore the data supports the hypothesis that the beneficial effect of fish oil on cardiovascular disease may be mediated in part by anti-atherosclerotic effects on macrophages.
Acknowledgements
References
[1] Poston RN, Haskard DO, Cuocher JR, Gall NP, Johnsontidey RR. Expression of intercellular-adhesion molecule-1 in atheroscle-rotic plaques. Am J Pathol 1992;140:665 – 73.
[2] Davies MJ, Gordon JL, Gearing AJH, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-SELECTIN in human atherosclerosis. J Pathol 1993;171:223 – 9.
[3] Nageh MF, Sandberg Et, Marotti KR, Lin AH, Melchior EP, Bullard DC, et al. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1997;17:1517 – 20.
[4] Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol 1981;103:181 – 90.
[5] Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol 1981;103:191 – 200.
[6] Krieger M, Acton S, Ashkenas J, Pearson A, Penman M, Resnick D. Molecular fly paper, host defense, and atherosclerosis. J Biol Chem 1993;268:4569 – 72.
[7] Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem 1994;63:601 – 37.
[8] Gough PJ, Greaves DR, Gordon S. A naturally occurring isoform of the human macrophage scavenger receptor (SR-A) gene gener-ated by alternative splicing blocks modified LDL uptake. J Lipid Res 1998;39:531 – 43.
[9] Hiltunen TP, Luoma JS, Nikkari T, Yla-Herttuala S. Expression of LDL receptor, VLDL receptor, LDL receptor-related protein, and scavenger receptor in rabbit atherosclerotic lesions. Circula-tion 1998;97:1079 – 86.
[10] Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkiola T, Witztum JL, et al. Gene expression in macrophage-rich human atherosclerotic lesions: 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest 1991;87:1146 – 52. [11] Gough PJ, Greaves DR, Suzuki H, Hakkinen T, Hiltunen MO,
Turunen M, et al. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arte-rioscler Thromb Vasc Biol 1999;19:461 – 71.
[12] Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, et al. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest 1998;78:423 – 34. [13] Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, et al. A role for macrophage scavenger receptors in atheroscle-rosis and susceptibility to infection. Nature 1997;396:292 – 6. [14] Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM,
Sweetnam PM, et al. Effects of changes in fat fish and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;ii:757 – 61.
[15] Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 1997;336:1046 – 53.
[16] Renier G, Skamene E, DeSanctis J, Razioch D. Dietary n-3 polyunsaturated fatty acids prevent the development of atheroscle-rotic lesions in mice. Modulation of macrophage secretory activ-ities. Arterioscler Thromb 1993;13:1515 – 24.
[17] Mortensen A, Fischer Hansen B, Fischer Hansen J, Frandsen H, Bartnikowska E, Anderson PS, et al. Comparison of the effects of fish oil and olive oil on blood lipids and aortic atherosclerosis in Watanabe heritable hyperlipidaemic rabbits. Br J Nutr 1998;80:565 – 73.
[18] Lougheed M, Lum CM, Ling W, Suzuki H, Kodama T, Stein-brecher U. High affinity saturable uptake of oxidised low density
lipoprotein by macrophages from mice lacking the scavenger receptor. J Biol Chem 1997;272:12938 – 44.
[19] Kromann N, Green A. Epidemiological studies in the Uperavik district, Greenland. Acta Med Scand 1980;208:401 – 6.
[20] Kromhout D, Bosschieter EB, Coulander CL. The inverse relation-ship between fish consumption and 20-year mortality from coro-nary heart disease. N Engl J Med 1985;312:1205 – 9.
[21] Ross R. Cell biology of atherosclerosis. Annu Rev Physiol 1995;57:791 – 804.
[22] Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA 1995;92:8264 – 8.
[23] Collie-Duguid ESR, Wahle KWJ. Inhibitory effect of fish oil n-3 polyunsaturated fatty acids on the expression of endothelial cell adhesion molecules. Biochem Biophys Res Commun 1996;220:969 – 74.
[24] De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA Jr, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-in-duced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb 1994;14:1826 – 36. [25] Hughes DA, Pinder AC, Piper Z, Johnson IT, Lund EK. Fish oil supplementation inhibits the expression of major histocompatabil-ity complex class II molecules and adhesion molecules on human monocytes. Am J Clin Nutr 1996;63:267 – 72.
[26] Sanderson P, MacPherson GG, Jenkins CH, Calder PC. Dietary fish oil diminishes the antigen presenting activity of rat dendritic cells. J Leukocyte Biol 1997;62:771 – 7.
[27] Sanderson P, Calder PC. Dietary fish oil diminishes adhesion to macrophage and endothelial cell monolayers. Immunology 1998;94:79 – 87.
[28] Hiltunen TP, Yla-Herttuala S. Expression of lipoprotein receptors in atherosclerotic lesions. Atherosclerosis 1998;137(Suppl.):S81 – 8. [29] Geng Y, Kodama T, Hansson GK. Differential expression of scavenger receptor isoforms during monocyte-macrophage differ-entiation and foam cell formation. Arterioscler Thromb 1994;14:798 – 806.
[30] Hughes DA, Fraser IP, Gordon S. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol 1995;25:466 – 73.
[31] Yokata T, Ehlin-Henricksson B, Hansson GK. Scavenger recep-tors mediate adhesion of activated B lymphocytes. Exp Cell Res 1998;239:16 – 22.
[32] Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature 1993;364:343 – 7.
[33] Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocyto-sis. Biochem J 1990;269:807 – 14.
[34] Kim JG, Keshava C, Murphy AA, Pitas RE, Parthasarathy S. Fresh mouse peritoneal macrophages have low scavenger receptor activity. J Lipid Res 1997;38:2207 – 15.
[35] Calder PC. Fat chance of immunomodulation. Immunol Today 1998;19:244 – 7.
[36] Robinson DR, Urakaze M, Huang R, Taki H, Sugiyama E, Knoell CT, et al. Dietary marine lipids suppress continuous expression of interleukin-1bgene transcription. Lipids 1996;31:S23 – 31. [37] Pietsch A, Weber C, Goretzki M, Weber PC, Lorenz RL. N-3 not
n-6 fatty acids reduce the expression of the combined adhesion and scavenger receptor CD36 in human monocytic cells. Cell Biochem Funct 1995;13:211 – 6.
[38] Miles EA, Calder PC. Modulation of immune function by dietary fatty acids. Proc Nutr Soc 1998;57:277 – 92.