BIOGEOGRAPHY AND CONSERVATION OF
OXUDERCINE GOBIES (GOBIIDAE: OXUDERCINAE) IN
LESSER SUNDA, MOLUCCAS, AND SULAWESI
YULIADI ZAMRONI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
STATEMENT LETTER
I hereby declare that dissertation entitled “Biogeography and Conservation of Oxudercine gobies (Gobiidae: Oxudercinae) in Lesser Sunda, Moluccas and Sulawesi” is original result of my own research supervised by supervisory committee and has never been submitted in any form at any institution before. All information from other authors cited here are mentioned in the text and listed in the reference at the end part of the dissertation.
SUMMARY
YULIADI ZAMRONI. Biogeography and Conservation of Oxudercine Gobies (Gobiidae: Oxudercinae) in Lesser Sunda, Moluccas and Sulawesi. Supervised by BAMBANG SURYOBROTO, ZEEHAN JAAFAR, and KADARWAN SOEWARDI.
Oxudercine gobies (Gobiiformes: Gobiidae) comprises of 42 species in 10 genera. They are distributed throughout the tropical Indo-Pacific, with one species in the Eastern Atlantic. The highest diversity of oxudercines, i.e. 31 species, occurs in the Indo-Australian Archipelago (IAA). The tectonic change of these areas during Pliocene and the sea level cycles due to glacial phenomenon during Pleistocene limit the gene flow of oxudercines. This constraint triggers the speciation and results in high diversity and endemicity of oxudercines and the other faunas in IAA. The species richness of these fishes dropped in the center of IAA which is covered area of Lesser Sunda, Moluccas, and Sulawesi island groups. In this study, we collected 10 species of oxudercine gobies (Apocryptodon madurensis, Boleophthalmus boddarti, Periophthalmodon freycineti, Periophthalmus argentilineatus, Periophthalmus gracilis, Periophthalmus kalolo, Periophthalmus malaccensis, Periophthalmus minutus, Periophthalmus pusing, and Scartelaos histophorus) where one species, Periophthalmus pusing, is reported as a new species from Lesser Sunda. Hence, there are total of 13 species of oxudercine gobies are found in these three island groups currently.
Based on oxudercines distribution, the center of IAA does not recover a single area monophyly. It other words, these oceanic islands are not separated biogeographical region as mentioned in the Wallacea concept. These areas are contact zone between the eastern Sahulian and the western Sundan species. It results in uniqueness of oxudercines species composition here. The Northern Moluccas shows a closer relationship to Sahulian region, whereas the Sulawesi, Lesser Sunda and Southern Moluccas have a closer relationship to Sundan.
iv
Key words: Mudskippers, Zoogeography, Wallacea, Mangrove Degradation,
RINGKASAN
YULIADI ZAMRONI. Biogeografi dan Konservasi Ikan Glodok (Gobiidae: Oxudercinae) di Sunda Kecil, Maluku dan Sulawesi. Dibimbing oleh BAMBANG SURYOBROTO, ZEEHAN JAAFAR, dan KADARWAN SOEWARDI.
Ikan glodok terdiri dari 42 species dalam 10 genus. Ikan ini terdistrubusi luas di daerah tropis Indo-Pasifik, dengan satu species mendiami Atlantik bagian barat. Keanekaragaman jenis teringgi (31 spesies) dari kelompok ikan ini terdapat di wilayah kepulauan Indo-Australian (IAA). Sejarah tektonik selama era Pliosen dan variasi turun-naiknya muka air laut selama periode Pliestosen menghambat terjadinya aliran gen (gen flow) antar populasi antar pulau di wilayah IAA, hal ini memicu terjadinya spesiasi antar populasi dan menghasilkan keanekaragaman jenis dan spesies endemic yang tinggi di wilayah IAA. Keanekaragaman jenis ikan gelodok menurun di kepulauan samudra (seperti Sunda Kecil, Maluku dan Sulawesi) yang terletak diantara dangkalan Sunda dan Sahul diwilayah IAA. Pada penelitian ini kami mengoleksi 10 species ikan glodok (Apocryptodon madurensis, Boleophthalmus boddarti, Periophthalmodon freycineti, Periophthalmus argentilineatus, Periophthalmus gracilis, Periophthalmus kalolo, Periophthalmus malaccensis, Periophthalmus minutus, Periophthalmus pusing, dan Scartelaos histophorus) dimana Periophthalmus pusing merupakan species baru yang dilaporkan distribusinya di wilayah Sunda Kecil. Jika digabungkan dengan laporan dari publikasi sebelumnya, total 13 spesies ikan glodok terdapat di ketiga kelompok kepulauan samudra ini.
Berdasarkan pola distribusi dari ikan glodok, kepulauan samudra di wilayah IAA tidak membentuk satu area monofiletik. Dengan kata lain, wilayah ini tidak menjadi zona beogeografi sendiri sebagaimana yang disebutkan dalam konsep Wallacea. Wilayah ini adalah daerah pertemuan antara dangkalan Sunda dan dangkalan Sahul. Hal ini menyebabkan komposisi spesies di wilayah ini sangat unik. Maluku Utara memiliki komposisi spesies yang mirip dengan wilayah Sahul sedangkan wilayah Sunda Kecil, Maluku Selatan dan Sulawesi memiliki komposisi spesies yang mirip dengan wilayah Sunda.
vi
untuk menambah tiga wilayah lagi (Teluk Lembar, Selindungan dan Kawangu) untuk mengkonservasi ikan gobi khususnya yang terancam punah.
Copyright © 2016. Bogor Agricultural University.
All Rights Reserved
Prohibited to cite all or a part of this dissertation without referring to and mentioning the source. Citation permits to the purpose of education, research, scientific paper, report, or critism writing only; and it does not defame the name and honor of Bogor Agricultural University.
viii
BIOGEOGRAPHY AND CONSERVATION OF
OXUDERCINE GOBIES (GOBIIDAE: OXUDERCINAE) IN
LESSER SUNDA, MOLUCCAS AND SULAWESI
YULIADI ZAMRONI
Dissertation
submitted in partial fulfillment of the requirements for Doctoral Degree
in
Major of Animal Bioscience
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
x
Examiners in the closed examination: Dr Ir Ibnul Qayim
Dr Ir Mohammad Mukhlis Kamal, MSc
Examiners in the dissertation defense: Prof Ir H Surya Hadi, MSc Ph.D
Dr Rudhy Gustiano
Title : Biogeography and Conservation of Oxudercine Gobies (Gobiidae: Oxudercinae) in Lesser Sunda, Moluccas and Sulawesi
Name : Yuliadi Zamroni Student ID : G362110071 Major : Animal Biosciences
Certified by Supervisory Committee
Dr Drs Bambang Suryobroto Chair
Dr Zeehan Jaafar Prof Dr Ir Kadarwan Soewardi Member Member
Acknowledged by
Chair of Animal Biosciences Dean of Graduate School
Dr Ir RR Dyah Perwitasari, MSc Dr Ir Dahrul Syah, MScAgr
FOREWORD
On the blessing of ALLOH S.W.T., the Lord of the universe, I am able to finish my dissertation. This paper is made to fulfill the requirement for doctoral
degree at Bogor Agricultural University. The title of my paper is “Biogeography
and Conservation of Oxudercine Gobies (Gobiidae: Oxudercinae) in Lesser Sunda, Moluccas and Sulawesi”.
I would like to thank my advisor committee Dr Bambang Suryobroto, Dr Zeehan Jaafar and Prof Dr Ir Kadarwan Soewardi. I am indebted to Directorate General of Higher Education (DIKTI), Indonesia for the Graduate Scholarship (BPPS); Balai Taman Nasional Komodo (BTNK) and Balai Besar Konservasi Sumber Daya Alam Nusa Tenggara Timur (BBKSDA-NTT) for permitting me to conduct research in East Nusa Tenggara islands; UPT Balai Konservasi Biota Laut LIPI Ambon for their facility during sampling in the Moluccas islands; Museum Zoologicum Bogoriense (MZB), Indonesian Institute of Science (LIPI) Cibinong for permitting me to examined the museum specimens; and Institut de Rechherche pour le Développement (IRD) for sponsored my training in Phylogenetics and Population Genetics with R at Institut Català de Paleontologia Miquel Crusafont, Barcelona, Spain.
I express my gratitude to my parents, my wife (Novita Tri Artiningrum) and my daughter (Annisa Hishnul Izza) for their greatly support during my study; special thanks to Mr. Fahri (Tadulako University), Mr. Vendy Eko Susilo and Mr. Fendi for their help during field work in Sulawesi and Java; Mrs Tini and Mrs Retno for their help in laboratory; and all of my friends in the Major of Animal Biosciens for the wonderful of friendships.
May this paper is usefull for all who study on gobies and biogeography. Bogor, August 2016
TABLE OF CONTENT
2 AMBLYOPINE AND OXUDERCINE GOBIES (TELEOSTEI: GOBIIDAE) FROM THE LESSER SUNDA, MOLUCCAS, AND SULAWESI GROUP OF
ISLANDS, INDONESIA 3
3 BIOGEOGRAPHY OF OXUDERCINE GOBIES (TELEOSTEI: GOBIIDAE) IN THE LESSER SUNDA, MOLUCCAS, AND SULAWESI GROUP OF
ISLANDS 27
4 A CASE FOR THE CONSERVATION MANGROVE FORESTS IN THE
xvi
LIST OF TABLES
Table 2.1 Records of amblyopines and oxudercines in the oceanic
islands of IAA base on published literature. 6
Table 2.2 Records of amblyopines and oxudercines based on present
sampling and museum materials. 8
Table 3.1 Nine areagrams of six taxa used in analysis of biogeographical
pattern. 33 indicates the oceanic islands between Sunda and Sahul shelves. Dash-lines are the limit of three oceanic island groups. 5 Figure 2.2 Sampling locations (close circles) and localities of museum
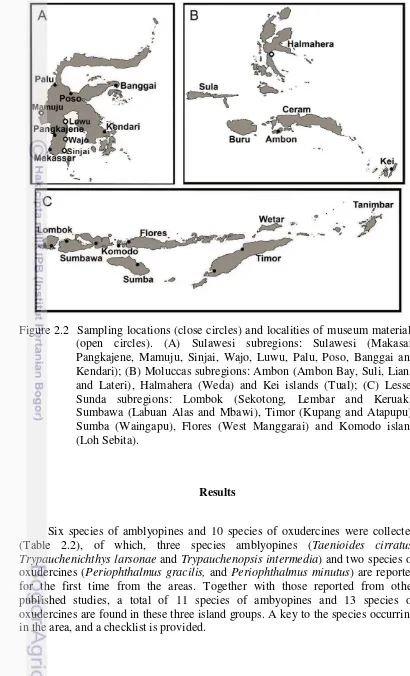
materials (open circles). (A) Sulawesi subregions: Sulawesi (Makasar, Pangkajene, Mamuju, Sinjai, Wajo, Luwu, Palu, Poso,
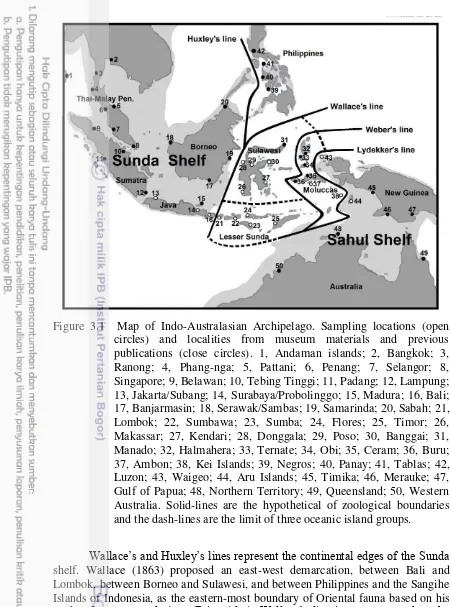
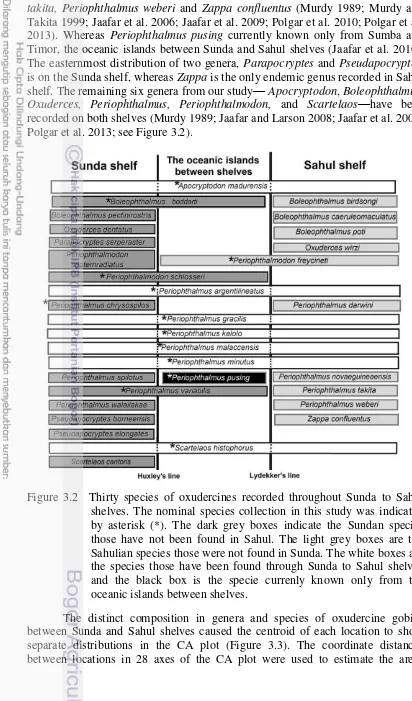
Figure 3.1 Map of Indo-Australasian Archipelago. Sampling locations (open circles) and localities from museum materials and previous publications (close circles). 1, Andaman islands; 2, Bangkok; 3, Ranong; 4, Phang-nga; 5, Pattani; 6, Penang; 7, Selangor; 8, Singapore; 9, Belawan; 10, Tebing Tinggi; 11, Padang; 12, Lampung; 13, Jakarta/Subang; 14, Surabaya/Probolinggo; 15, Madura; 16, Bali; 17, Banjarmasin; 18, Serawak/Sambas; 19, Samarinda; 20, Sabah; 21, Lombok; 22, Sumbawa; 23, Sumba; 24, Flores; 25, Timor; 26, Makassar; 27, Kendari; 28, Donggala; 29, Poso; 30, Banggai; 31, Manado; 32, Halmahera; 33, Ternate; 34, Obi; 35, Ceram; 36, Buru; 37, Ambon; 38, Kei Islands; 39, Negros; 40, Panay; 41, Tablas; 42, Luzon; 43, Waigeo; 44, Aru Islands; 45, Timika; 46, Merauke; 47, Gulf of Papua; 48, Northern Territory; 49, Queensland; 50, Western Australia. Solid-lines are the hypothetical of zoological boundaries and the dash-lines are the limit of three oceanic island groups. 28 Figure 3.2 Thirty species of oxudercines recorded throughout Sunda to
indicated by asterisk (*). The dark grey boxes indicate the Sundan species those have not been found in Sahul. The light grey boxes are the Sahulian species those were not found in Sunda. The white boxes are the species those have been found through Sunda to Sahul
shelves. 32
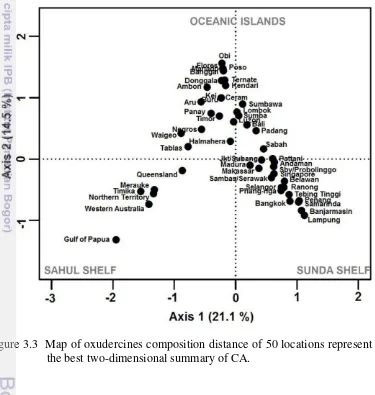
Figure 3.3 Map of oxudercines composition distance of 50 locations represent in the best two-dimensional summary of CA. 33 Figure 3.4 Area relationships of 50 locations throughout Sunda to Sahul
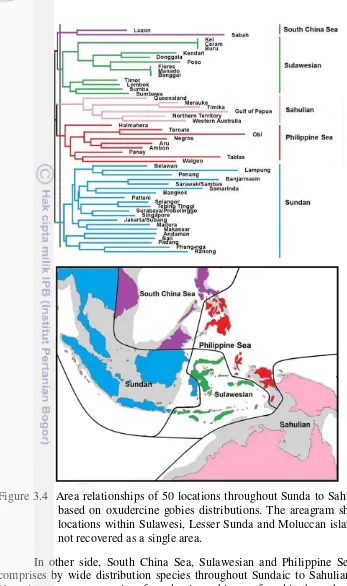
shelves based on oxudercine gobies distributions. The areagram showed the locations within Sulawesi, Lesser Sunda and Moluccan
island groups not recovered as a single area. 34
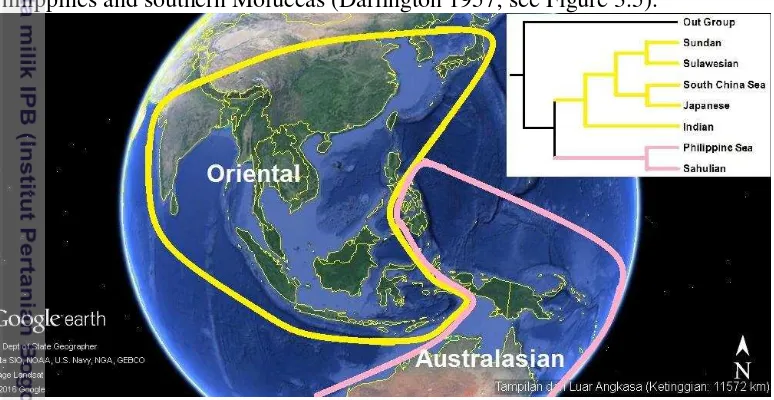
Figure 3.5 General areagram, or biogeographical pattern of the area throughout Sunda to Sahul shelves, representing the combined area relationships of eleven clades (Map modified from Google Earth
2016). 35
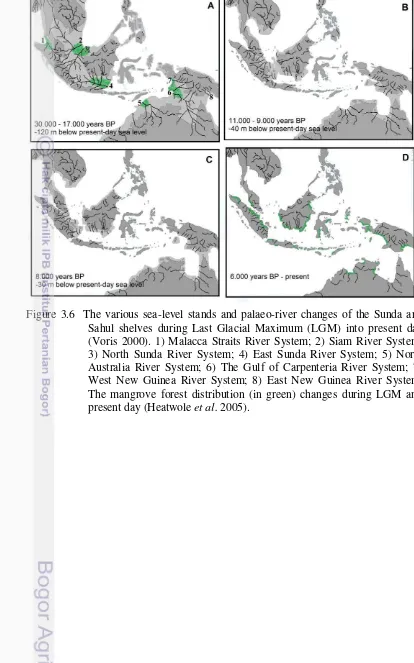
Figure 3.6 The various sea-level stands and palaeo-river changes of the Sunda and Sahul shelves during Last Glacial Maximum (LGM) into present day (Voris 2000). 1) Malacca Straits River System; 2) Siam River System; 3) North Sunda River System; 4) East Sunda River System; 5) North Australia River System; 6) The Gulf of Carpenteria River System; 7) West New Guinea River System; 8) East New Guinea River System. The mangrove forest distribution (in green) changes during LGM and present day (Heatwole et al. 2005). 37 Figure 4.1 Map of sampling areas were conducted throughout LSI (open
circles). 43
Figure 4.2 Divergence of mangrove habitats within the Lesser Sunda
Islands based on gobioid fishes 45
LIST OF APPENDICES
Appendix 3.1 Geographical distribution of Oxudercines based on present
1
GENERAL INTRODUCTION
Some common problems in biogeography study are delimiting a zoogeographical realm and providing certain evidence based on distribution of closely related species. These difficulties also arise in aquatic organism researches. The present distribution of aquatic organisms are influenced by some factors such as their dispersal capabilities, paleogeography of the region, rise and fall cycle of sea level during glacial and interglacial periods, and long-distance transport of pelagic larvae by ocean currents (Hall 2009). These factors contribute significantly to the determination of zoogeographical boundaries. While general and large-scale patterns of organismal distribution are typically accepted, the boundaries demarcating those concepts are often disputed. This problem is ensued in oceanic islands between Sunda and Sahul shelves. The uniqueness of faunal distribution reflects the complexities of geological history of these regions. The faunal composition is also marked differently in the Sunda and Sahul shelves, with Oriental type on the former, and Australasian on the later. The faunas from both realms are met and intermingled in the islands between Sunda and Sahul shelves (Parenti and Ebach 2009). Seven hypothetical lines have been proposed as zoological boundaries in this area, four of the most often discussed are Wallace’s,
Huxley’s, Weber’s, and Lydekker’s lines (Simpson 1977).
Oxudercine gobies are intertidal fishes whose migration is unconstrained by marine ecosystem. However, the availability of mudflat and mangrove ecosystem, which function as their habitat delimits their distributions (Murdy 1989). This limitation results in uniqueness of their distribution pattern in oceanic islands, which reflect the evolution of this area and provides unique evidence for the hypotheses on the zoogeographical boundaries. Unfortunately, the distribution of oxudercine gobies in the islands between the Sunda and Sahul shelves are poorly known, partially because of insufficient ichtyofauna studies conducted in coastal areas. In areas where scientific collections has been established, the foci were on either strictly freshwater or reef ichthyofauna. Consequently, coastal intertidal areas of these islands (Lesser Sunda, Moluccas, and Sulawesi group of islands) suffer from large gaps in their baseline biodiversity data (Peristiwady 2012).
2
AMBLYOPINE AND OXUDERCINE GOBIES
occuring in the center of the Indo-Australian Archipelago, an area where coastal ichthyological collections are poorly represented. Targeted collections of amblyopines and oxudercines gobies were carried out in the Lesser Sunda Islands, Moluccas islands and the island of Sulawesi. Seventeen species comprises by six species of amblyopines (Caragobius urolepis, Odontamblyopus rubicundus, Paratrypauchen microcephalus, Taenioides cirratus, Trypauchenichthys larsonae and Trypauchenopsis intermedia) and 10 species of oxudercines (Apocryptodon madurensis, Boleophthalmus boddarti, Periophthalmodon freycineti, Periophthalmus argentilineatus, Periophthalmus gracilis, Periophthalmus kalolo, Periophthalmus malaccensis, Periophthalmus minutus, Periophthalmus pusing and Scartelaos histophorus) were collected. Whereas, five species of amblyopine (Brachyamblyopus brachysoma, Karsten totoyensis, Taenioides anguillaris, Taenioides eruptionis and Trypauchen vagina) and three species of oxudercines (Periophthalmodon schlosseri, Periophthalmus variabilis and Pseudapocryptes borneensis) were reported in literature but not collected in this present sampling.Key words: Sunda shelf, Sahul shelf, Indo-Australian Archipelago, Oriental
fauna, Australasian fauna.
Introduction
The family Gobiidae, one of the largest fish families, comprises some 2000 species of typically small, freshwater, estuarine, and marine fishes (Nelson 2006). Five subfamilies—Amblyopinae, Gobiinae, Gobionellinae, Oxudercinae, and Sicydiinae—are traditionally recognized (Pezold 1993) although present classification is still volalite due to incongruences between morphology and molecular datasets. Amblyopine gobies, also known as eel gobies (Teleostei: Gobiidae: Amblyopinae), are considered the putative sister taxa to the monophyletic oxudercine gobies, also known as mudskippers (Teleostei: Gobiidae: Oxudercinae), based on osteology of the jaw suspensorium (Harrison 1989; Murdy 2011a,b). Molecular analyses of gobioid relationships, however, recovered these two groups as paraphyletic, yet form a monophyletic clade
4
between these taxa and thus simultaneously report their distribution in the Lesser Sunda, Sulawesi, and Moluccas group of islands.
Amblyopine and oxudercine gobies both occur in tropical and subtropical mangrove forests and mudflat habitats. Except for one species found along the western coast of Africa, Periophthalmus barbarus, these fishes are absent in the Atlantic Ocean (Murdy 1989). Amblyopine gobies are presumed to spend most of their time within the mud substrate, and are difficult to collect. Consequently, museum collections of these fishes are relatively rare. For example, three recent descriptions of new amblyopine taxa, Biendongella iljini, Gymnoamblyopus novaeguinea and Taenioides kentalleni, were all described from a single specimen (Murdy and Randall 2002; Murdy and Ferraris 2003; Prokofiev 2015). Little is known of the ecology and biology of amblyopine fishes. Oxudercines gobies are relatively well studied when compared to amblyopine gobies. Their general distribution and some aspects of their ecology and biology are known (Murdy 1989).
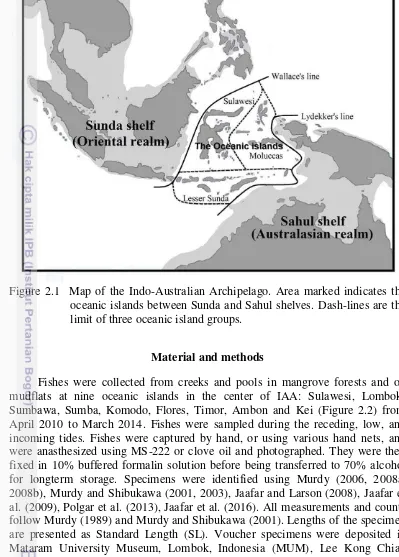
The highest diversity of these fishes is in the Indo-Australian Archipelago (IAA), an area within the larger Indo-Pacific region (Briggs 2005). The high diversity of many marine taxa within the IAA has been attributed to the complex geological history of the area during the Paleocene to Pliocene (60-5 mya), and the sea level fluctuations during the Pleistocene (Voris 2000; Hall 2002). In contrast to the high diversity throughout the IAA, the oceanic islands in the center of IAA, especially three major island groups, Lesser Sunda, Moluccas, and the Sulawesi subregions are poorly known (Monk et al. 2000; Figure 2.1). For example, prior to this study, nineteen species of amblyopines and 31 species of oxudercines have been reported from IAA but only eight species of amblyopines and nine species of oxudercines gobies have been reported from these three island groups (Table 2.1). While this may be a true indication of the biodiversity in the area, a more likely explanation for the pattern is the absence of a rigorous and systematic sampling effort for ichthyofauna for the islands in central IAA (Peristiwady 2012). Our study reviews the diversity and distribution of amblyopine and oxudercine gobies based on published literature, museum material, and new material from our systematic collection trips to the three island groups – Lesser Sunda, Moluccas, and the Sulawesi. In addition, we examined specimens of amblyopine and oxudercine taxa in the Museum Zoologicum Bogoriense, Indonesian Institute of Science (LIPI), in Bogor, Indonesia (MZB) as comparative material. We include that data in the checklist (see subheading
Figure 2.1 Map of the Indo-Australian Archipelago. Area marked indicates the oceanic islands between Sunda and Sahul shelves. Dash-lines are the limit of three oceanic island groups.
Material and methods
6
Table 2.1 Records of amblyopines and oxudercines in the oceanic islands of IAA base published literature.
Species Locality Publication
Apocryptodon madurensis Halmahera Murdy (1989)
Boleophthalmus boddarti Sulawesi (Makassar) Bleeker (1853a); Weber (1913); Koumans (1953)
Brachyamblyopus brachysoma Sulawesi (Manado) Koumans (1953) Sulawesi Kottelat et al. (1993)
Caragobius urolepis Timor (near Kupang), Ceram, Sulawesi Koumans (1953) Lesser Sunda Kottelat et al. (1993) Sulawesi (Bitung) Kimura and Matsuura (2003)
Karsten totoyensis Sumbawa (Bima Bay) Weber (1913)
Sumbawa (Bima Bay), Sulawesi Murdy (2002)
Odontamblyopus rubicundus Ambon Koumans (1953)
Paratrypauchen microcephalus Sulawesi (Raha) Popta (1922); Kottelat et al. (1993); Murdy (2008b)
Sulawesi (Raha, Kwandang, Selayar) Koumans (1953)
Periophthalmodon freycineti Timor Cuvier and Valenciennes (1837); Bleeker (1858a)
Periophthalmus argentilineatus Ceram Bleeker (1853c)
Buru Bleeker (1857)
Flores Günther (1861)
Ambon, Flores, Sulawesi Eggert (1935) Lombok (Labuan Tereng, Ekas, Labuhan
Haji), Sumbawa (Bima), Flores (Larantuka, Mbawa), Adonara, Timor (Atapupu), Sulawesi (Donggala, Makassar, Gorontalo, Palima), Halmahera (Ternate, Tidore), Obi, Ambon, Ceram (Saparua), Banda, Buru
Koumans (1953)
Lesser Sunda, Ambon Murdy (1989) Sulawesi Kottelat et al. (1993) Sulawesi (Bitung) Kimura and Matsuura (2003)
Periophthalmus kalolo Ambon Bleeker (1853b); Eggert (1935)
Lombok (Labuan Tereng), Sumbawa (Bima), Flores (Labuan Bajo), Sulawesi (Donggala/Palu)
Weber (1913)
Flores (Labuan Bajo), Timor, Sulawesi (Pare-pare), Tongian islands, Talaud islands, Ambon, Halmahera (Ternate), Obi island
Koumans (1953)
Sulawesi Kottelat et al. (1993)
Periophthalmus malaccensis Ambon Murdy (1989)
Periophthalmus variabilis Halmahera (Pulau Babu) Eggert (1935); Koumans (1953)
Pseudapocryptes borneensis Timor Koumans (1953)
Scartelaos histophorus Sulawesi (Makassar) Weber (1913)
Halmahera Murdy (1989)
Taenioides anguillaris Sulawesi (Strait Makassar) Koumans (1953) Lombok (Kuta beach) Matsuura et al. (2000) Sulawesi (Bitung) Kimura and Matsuura (2003)
Taenioides eruptionis Ambon Koumans (1953)
Trypauchen vagina Sulawesi (Makassar) Bleeker (1853a)
Ceram Günther (1861)
Lombok (Labuan Tereng), Sulawesi (Makassar, Selayar, Kwandang)
Weber (1913)
Lombok (Labuan Tereng), Flores, Sulawesi (Makassar, Malawa, Bajo), Ceram, Ambon
Figure 2.2 Sampling locations (close circles) and localities of museum materials (open circles). (A) Sulawesi subregions: Sulawesi (Makasar, Pangkajene, Mamuju, Sinjai, Wajo, Luwu, Palu, Poso, Banggai and Kendari); (B) Moluccas subregions: Ambon (Ambon Bay, Suli, Liang and Lateri), Halmahera (Weda) and Kei islands (Tual); (C) Lesser Sunda subregions: Lombok (Sekotong, Lembar and Keruak), Sumbawa (Labuan Alas and Mbawi), Timor (Kupang and Atapupu), Sumba (Waingapu), Flores (West Manggarai) and Komodo island (Loh Sebita).
Results
Six species of amblyopines and 10 species of oxudercines were collected (Table 2.2), of which, three species amblyopines (Taenioides cirratus, Trypauchenichthys larsonae and Trypauchenopsis intermedia) and two species of oxudercines (Periophthalmus gracilis, and Periophthalmus minutus) are reported for the first time from the areas. Together with those reported from other published studies, a total of 11 species of ambyopines and 13 species of oxudercines are found in these three island groups. A key to the species occurring in the area, and a checklist is provided.
8
Table 2.2 Records of amblyopines and oxudercine based on present sampling and museum materials. Periophthalmodon; Ps= Periophthalmus; S= Scartelaos; T= Taenioides; Tr= Trypauchenichthys; Ts= Trypauchenopsis
Key to species of amblyopines and oxudercines occurring in the area
The key is modified from Murdy (2002), Larson and Murdy (2001), Murdy and Ferraris (2003), Jaafar and Larson (2008), and Koumans (1953).
6a. Canine teeth in outer row of lower jaw 4–5 on each side; length of head longer than distance from base of pelvic fin to anal fin ... ... Taenioides anguillaris 6b. Canine teeth in outer row of lower jaw 3–4 on each side; length of head shorter than distance from base of pelvic fin to anal fin ... ... Taenioides eruptionis 7a. Pectoral-fin rays simple, free from fin membrane and silklike; head and body subcylindrical, and long; pectoral-fin rays 20-65, usually more than 23 ... Odontamblyopus rubicundus 7b. Pectoral-fin rays branched, not forming free rays; head and body compressed; pectoral-fin rays 23 or fewer ... Brachyamblyopus brachysoma 8a. A pouch-like cavity at dorsal margin of operculum present ... 9 8b. No pouch-like cavity at dorsal margin of operculum ... 11 9a. Frontal crest prominently exposed; pelvic fin separated to base... ... Trypauchenichthys larsonae 9b. Frontal crest subdermal or only slightly exposed; pelvic fins united ... 10 10a. Pelvic fins fully united forming a disc; abdomen fully scaled ... ... Trypauchen vagina 10b. Pelvic fins emarginated posteriorly; abdomen without scales ... ... Paratrypauchen microcephalus 11a. Pectoral fin rounded, symmetrical dorsoventrally; eyes present but rudimentary; segmented caudal-fin rays usually 7+6, including 6+5 branched rays ... Caragobius urolepis 11b. Pectoral fin pointed posteriorly, not symmetrical; eyes lacking; segmented caudal-fin rays 8+7, including 7+6 branched rays ... Karsten totoyensis 12a. Dermal cup absent ... 13 12b. Dermal cup present ... 14 13a. The cephalic sensory system showing the presence of posterior interorbital pore; First dorsal fin with 5 spines; second dorsal with 29-33 total elements; anal fin with 27-29 total elements .... Pseudapocryptes borneensis 13b. The cephalic sensory system showing the presence of supraorbital pore; First dorsal fin with 6 spines; second dorsal with 24 or fewer total elements; anal fin with 23 or fewer total elements ... ... Apocryptodon madurensis 14a. Two canine teeth internal to lower jaw symphysis present; anal and second dorsal-fin bases 34% of standard length or greater ... 15 14b. No canine teeth internal to lower jaw symphysis; anal and second
dorsal-fin bases 27% of standard length or less ... 16 15a. Barbels present on underside of head ... Scartelaos histophorus 15b. No barbels on underside of head ... Boleophthalmus boddarti 16a. Two rows of teeth in upper jaw; outermost teeth large and curved ... 17 16b. A single row of teeth in upper jaw; teeth blunt, not curved ... 18 17a. Spinous dorsal fin IV, rarely V; length of first dorsal-fin base less than 10% of standard length; pectoral-fin rays 15-17 ... ... Periophthalmodon freycineti 17b. Spinous dorsal fin modally VIII-IX, rarely VI or VII; length of first
10
18a. Frenum present visible without magnification; pelvic fins are united and emarginated posteriorly ... 19 18b. Frenum absent, or visible only with magnification; pelvic fins totally separate ... 21 19a. First dorsal fin with numerous white spots ... 20 19b. First dorsal fin with orange spots that turn brown in preservative ... ... Periophthalmus variabilis 20a. First dorsal fin with XI – XV spines, black stripe inframarginally with numerous white spots basally; pelvic frenum vestigial; branchiostegal membrane not pigmented; lateral scales typically more than 70 ... ... Periophthalmus kalolo 20b. First dorsal fin with X spines, brown stripe inframarginally with numerous white spots broad distributed; pelvic frenum prominent; branchiostegal membrane pigmented; lateral scales typically fewer than 70 ... ... Periophthalmus malaccensis 21a. First dorsal fin rounded, prominent black spot posteriorly ... 22 21b. First dorsal fin pointed, black spot absent ... 23 22a First dorsal fin with X spines or fewer, D1 shorter than depth of body at anus; interdorsal distance more than half the length of the first dorsal-fin spine ... Periophthalmus gracilis 22b First dorsal fin with XI-XV spines, D1 taller than depth of body at anus; interdorsal distance less than half the length of the first dorsal-fin spine... ... Periophthalmus pusing 23a. First dorsal fin with black stripe inframarginally; peritoneum densely black ventrally; lateral scales 75 or more ... Periophthalmus argentilineatus 23b. First dorsal fin with light brown stripe inframarginally; peritoneum lightly pigmented ventrally; lateral scales 75 or fewer ... Periophthalmus minutus
Taxonomy Family Gobiidae Subfamily Amblyopinae
Brachyamblyopus brachysoma Bleeker, 1854
Amblyopus brachysoma Bleeker, 1854a: 510 (Indonesia: Sumatra: Priaman)
Material Examined. None.
Comparative Material Examined: SUMATRA: MZB 2270; 1 (89.8 mm), Way Belukang, Kuala Sekampung, Lampung, coll. Soetikno, 1 November 1975.
Distribution. Sumatra, Indonesia (Bleeker 1854a); Singapore (Larson et al. 2008); Sulawesi (Manado), New Guinea (Lorentz River), Thailand (Bangkok), Hong Kong, Persian Gulf and India (Koumans 1953).
Caragobius urolepis (Bleeker, 1852)
Amblyopus urolepis Bleeker, 1852: 581 (Indonesia: Sumatra: Palembang).
Caragobius typhlops Smith & Seale, 1906: 81 (Philippines: Mindanao: Rio Grande).
Taenioides chilkensis Hora, 1923: 757, fig. 34 (India: Orissa: Chilka Lake).
Brachyamblyopus olivaceus Herre, 1927: 329, pl. 25, fig. 3 (Philippines: Oriental Negros: La
Libertad).
Caragobius geomys Fowler, 1935: 161, figs. 129-130 (Thailand: Bangkok).
Nudagobioides monserrati Roxas & Ablan, 1940: 309, pl. 8 (Philippines: Luzon: Pangasinan
Province).
Material Examined: LOMBOK: MUM 121-00025, 10 (40.5–52.7 mm), mangrove forest at Kelapa beach, Labuan Tereng, Lembar, West Lombok, coll. Y. Zamroni, 21 July 2012; TIMOR: MUM 121-00013, 5 (26.6–46.8 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012; USNM 427003, 2 (44.8–45.6 mm), Bipolo village, East Kupang, coll. Z. Jaafar & Y. Zamroni, 13 November 2012; ZRC 53909, 2 (not measured), data as USNM 427003.
Distribution. India (Hora 1923); Mindanao and Negros, Philippines (Herre 1927; Murdy and Shibukawa 2003); Thailand and Fiji (Murdy and Shibukawa 2003); Japan (Koumans 1953); New Guinea (Allen 1991); Northern Australia (Larson et al. 2013); Nha Trang Bay, Vietnam (Prokofiev 2015); Sumatra, North Sulawesi, Lombok and Timor, Indonesia (Murdy and Shibukawa 2003; Kimura and Matsuura 2003; this study).
Karsten totoyensis (Garman, 1903)
Gobioides totoyensis Garman, 1903: 235, Pl. 3, figs. 1–2 (Fiji: Totoya Island)
Taenioides coecus Weber, 1913: 486 (Indonesia: Sumbawa: Bima Bay)
Material Examined. None.
Distribution. Totoya Island, Fiji (Garman 1903); Philippines (Murdy 2002); Sulawesi, and Sumbawa, Indonesia (Weber 1913; Murdy 2002).
Odontamblyopus rubicundus (Hamilton, 1822)
Gobioides rubicundus Hamilton, 1822: 365, pl. 5, fig. 9 (India: estuaries of the Ganges River)
Amblyopus mayenna Valenciennes in Cuvier & Valenciennes, 1837: 163 (Myanmar: Rangoon:
Ganges River)
Amblyopus taenia Günther, 1861: 135 (East Indies)
Material Examined: LOMBOK: MUM 121-00026, 1 (71.6 mm), mangrove forest at Kelapa beach, Labuan Tereng, Lembar, West Lombok, coll. Y. Zamroni, 21 July 2012; TIMOR: MUM 121-00016, 2 (40.0–48.4 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012.
12
Paratrypauchen microcephalus (Bleeker, 1860)
Trypauchen microcephalus Bleeker, 1860: 62 (Indonesia: Kalimantan: Sungi-Duri)
Trypauchen wakae Jordan & Snyder, 1901: 127, fig. 32 (Japan: Wakayama Prefecture: Wakanoura)
Trypauchen raha Popta, 1922: 37 (Indonesia: Sulawesi: Muna Island: Raha)
Ctenotrypauchen barnardi Hora, 1926: 221, figs. 1-2 (South Africa: KwaZulu-Natal: Tugela River)
Trypauchen wakae chantungensis Fang, 1942: 85 (China: Shantung Province: Chefoo)
Material Examined. TIMOR: MUM 121-00014, 5 (30.0–49.4 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012.
Distribution. Japan, Taiwan, Philippines, Australia, Singapore, Malaysia, India, Mozambique and South Africa (Murdy 2008b); Nha Trang Bay, Vietnam (Prokofiev 2015); Sumatra, Java, Borneo, Sulawesi, and Timor, Indonesia (Popta 1922; Koumans 1953; this study).
Taenioides anguillaris (Linnaeus, 1758)
Gobius anguillaris Linnaeus, 1758: 264 (China)
Gobioïdes anguilliformis La Cepède, 1800: 577 (China)
Taenioides hermannii La Cepède, 1800: 532 Pl. 14 (no locality data)
Cepola coecula Bloch & Schneider, 1801: 241, pl. 54 (India: Tharangambadi)
Amblyopus Hermannianus Valenciennes, in Cuvier & Valenciennes, 1837: 159, pl. 350 (India:
Calcutta; Burma: Rangoon)
Amblyopus rugosus Richardson, 1846: 207 (China: Macao)
Material Examined. None.
Comparative Material Examined: BORNEO: MZB 951, 1 (220.0 mm), Sembiangan, North Borneo, coll. Mohari, 1912.
Distribution. China (Günther 1861); India (Günther 1861; Day 1876); Philippines (Herre 1927); Penang, Peninsular Malaysia (Koumans 1953); Borneo, East Malaysia (this study); New Guinea (Allen 1991); Sumatra, Java, Sulawesi, and Lombok, Indonesia (Koumans 1953; Matsuura et al. 2000; Kimura and Matsuura 2003).
Taenioides cirratus (Blyth, 1860)
Amblyopus cirratus Blyth, 1860: 147 (India: Calcutta)
Amblyopus brachygaster Günther, 1861: 134 (East Indies)
Gobioides cirratus Day, 1876: 318, pl. 69, fig. 4 (India: Calcuta)
? Amblyopus sumatranus Volz, 1903: 554 (Indonesia: Sumatra: Palembang: Banju Asin)
Taenioides snyderi Jordan & Hubbs, 1925: 310 (Japan: Wakayama Prefecture: Wakanoura)
Material Examined. SULAWESI: MUM 121-00113, 1 (95.8 mm), Wanggu River, Andonuhu, Kendari Bay- South East Sulawesi, coll. Y. Zamroni, 18 January 2014.
MZB 2089 (in part), 1 of 3 specimens (220.0 mm), Way Belukang, Kuala Sekampung, Palas, Lampung, coll. Soetikno, 14 June 1975; MZB 2133 (in part), 1 of 2 specimens (225.0 mm), Way Belukang, Kuala Sekampung, Lampung, coll. Soetikno, A. Budiman and F. Sabar, 15 June 1975.
Distribution. East Indies (Günther 1861); India (Day 1876); Philippines (Herre 1927); Australia (Larson et al. 2013); New Guinea (Allen 1991); Borneo, Sumatra, Java, and Sulawesi, Indonesia (Koumans 1953; this study).
Taenioides eruptionis (Bleeker, 1849)
Amblyopus eruptionis Bleeker, 1849: 38 (Indonesia: Java: Kalimas River or Kali Kediri [Brantas],
near Surabaya)
Material Examined. None.
Comparative Material Examined: BORNEO: MZB 2056, 1 (197.0 mm), Pangembangan river (Taluk river), South Kalimantan, coll Umar, 14 March 1975;
SUMATRA: MZB 2089 (in part), 2 of 3 specimens (257.0–312.0 mm), Way
Belukang, Kuala Sekampung, Palas, Lampung, coll. Soetikno, 14 June 1975; MZB 2133 (in part), 1 of 2 specimens (227.0 mm), Way Belukang, Kuala Sekampung, Lampung, coll. Soetikno, A. Budiman and F. Sabar, 15 June 1975. Distribution. Borneo, Java, Sumatra, and Ambon, Indonesia (Koumans 1953; this study).
Trypauchen vagina (Bloch & Schneider, 1801)
Gobius vagina Bloch & Schneider, 1801: 73 (India: Tranquebar)
Gobioides ruber Hamilton, 1822: 38, 365 (India: Ganges River)
Trypauchen vagina Valenciennes in Cuvier & Valenciennes, 1837: 152 (new combination)
Material Examined. None.
Distribution. China (Günther 1861); India (Day 1876); Philippines (Herre 1927); Persian Gulf, Formosa, Nicobar, Penang (Koumnas 1953); Singapore (Larson et al. 2008); Kuwait, Myanmar, Thailand (Murdy 2006); Mediterranean Sea (Akamca et al. 2011); Vietnam (Prokofiev 2015); Borneo, Java, Sulawesi, Bali, Ceram, Madura, Lombok, Flores, Ambon, Indonesia (Bleeker 1853a, Bleeker 1858b; Günther 1861; Weber 1913; Koumans 1953).
Trypauchenichthys larsonae Murdy, 2008
Trypauchenichthys larsonae Murdy, 2008a: 62, fig.1 (Northern Territory, Australia)
Material Examined. TIMOR: MUM 121-00076, 1 (40.0 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012.
14
Remarks. This record represents a range extension for this species, and a new record for the Lesser Sunda Islands and Indonesia. Specimens were obtained from approximately 60 cm deep mud substrate.
Trypauchenopsis intermedia Volz, 1903
Trypauchenopsis intermedius Volz, 1903: 555 (Indonesia: Sumatra: Palembang: Banju Asin).
Brachyamblyopus burmanicus Hora, 1926: 455, fig. 2 (Myanmar: Rangoon river).
Tanioides jacksoni Smith, 1943: 70, fig. 2 (South Africa: KwaZulu-Natal: St. Lucia Estuary).
Taenioides limicola Smith, 1964: 145, fig. 1 (Guam: Acfayan Bay).
Material Examined. TIMOR: MUM 121-00017, 3 (33.4–50.0 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012; USNM 410689, 1 (26.2 mm), locality as MUM 121-00017, coll. Z. Jaafar & Y. Zamroni, 14 November 2012; USNM 410704, 3 (45.1–60.2 mm), Bipolo village, East Kupang, coll. Z. Jaafar & Y. Zamroni, 13 November 2012; SULAWESI: MUM 121-00080, 3 (63.0–85.9 mm), Wanggu river, Andonuhu, Kendari Bay- South East Sulawesi, coll. Y. Zamroni, 18 January 2014; MUM 121-00257, 1 (62.3 mm), Banggai, Central Sulawesi, coll. W.S. Enot, 17 January 2014.
Comparative Material Examined:JAVA: MZB 4938, 1 (52.3 mm), Cisiin river, Sumur, Pandeglang-West Java, 21 August 1982.
Distribution. –South Africa, Philippines, Caroline Islands, Marianas Islands, and Ryukyu Islands, Japan (Shibukawa and Murdy 2012); Sumatra, Java, Sulawesi, Bali, and Timor, Indonesia (Shibukawa and Murdy 2012; this study).
Subfamily Oxudercinae
Apocryptodon madurensis (Bleeker, 1849)
Apocryptes madurensis Bleeker, 1849: 35 (Indonesia: Java: Madura Strait).
Apocrytpes glyphisodon Bleeker, 1849: 36 (Indonesia: Java: Jakarta).
Apocryptes bleekeri Day, 1876: 300, pl. 64, fig. 3 (India: Madras).
Apocryptodon montalbani Herre, 1927: 277, pl. 22, fig. 2 (Philippines: Panay).
Apocryptodon sealei Herre, 1927: 278 (Philippines: Luzon).
Apocryptodon taylori Herre, 1927: 279, pl. 22, fig. 3 (Philippines: Tablas).
Apocryptodon malcolmi Smith, 1932: 47, fig. 22 (Thailand: Chantaburi River).
Apocryptodon lomboyi Ablan, 1940: 373, pl.1 (Philippines: Luzon).
Material Examined. LOMBOK: MUM 121-00023, 11 (28.7–57.0 mm), mangrove forest at Kelapa beach, Labuan Tereng, Lembar-West Lombok, coll. Y. Zamroni, 23 May 2013; TIMOR: MUM 121-00048, 10 (21.1–43.7 mm), Oebelo mangrove forest, Neilbaki, Kupang Bay, coll. Y. Zamroni, 13 September 2012; MUM 121-00021, 2 (44.5–48.1 mm), Maubesi mangrove forest, Atapupu, coll. Y. Zamroni, 16 September 2012; USNM 427011, 1 (40.0 mm), Bipolo village, East Kupang, coll. Z. Jaafar & Y. Zamroni, 13 November 2012.
Distribution. India (Day 1876); Thailand (Murdy 1989); Singapore (Larson et al. 2008); Panay, Luzon, and Tablas, Philippines (Herre 1927); Northern Territory, Australia (Murdy 1989; Larson et al. 2013); Madura, Java, Lombok and Timor, Indonesia (Günther 1861; Koumans 1953; this study).
Boleophthalmus boddarti (Pallas 1770)
Gobius boddarti Pallas, 1770: 11, pl. 2, figs 4-5 (Indian Ocean).
Gobius striatus Bloch & Schneider, 1801: 71, pl. 16 (India: Tharangambadi).
Gobius plinianus Hamilton, 1822: 45, pl. 35, fig. 13 (India: Ganges Delta).
Boleophthalmus inornatus Blyth, 1860: 148 (Myanmar: Tenasserim).
Boleophthalmus sculptus Günther, 1861: 104 (India).
Apocryptes punctatus Day, 1867: 941 (India: Madras).
Material Examined. –LOMBOK: MUM 121-00022, 27 (38.8–101.2 mm), mangrove forest at Kelapa beach, Labuan Tereng, Lembar-West Lombok, coll. Y. Zamroni, 14 April 2010; MUM 121-00024, 4 (30.9–81.4 mm), location and collector as MUM 121-00022, 21 May 2013; SUMBAWA: MUM 121-00035, 7 (40.0–110.4 mm), Mbawi mangrove forest, Dompu, coll. Y. Zamroni, 15 November 2013; SULAWESI: MUM 121-00100, 1 (76.0 mm), Barombang river, Makassar, coll. Y. Zamroni, 21 January 2014; MUM 121-00091, 8 (54.4–82.6 mm), Maccini Baji harbor, Labakkang, Pangkajene, South Sulawesi, coll. Y. Zamroni, 22 January 2014.
Comparative Material Examined: JAVA: MUM 121-00003, 3 (50.3–94.8 mm), Gending beach, Probolinggo-East Java, coll. Y. Zamroni, 15 October 2013; MUM 121-00004, 3 (76.1–85.9 mm), Klaseman beach, Probolinggo-East Java, coll. Y. Zamroni, 16 October 2013; MUM 121-00006, 2 (46.1–50.0 mm), Tambak Sari beach, Pajarakan, Probolinggo-East Java, coll. Y. Zamroni, 17 October 2013; MZB (uncatalogued), 10 (117.4–129.9 mm), Segara Anak river, Alas Purwo National Park, eastern Java, coll. Maulana, S.Y., October 2015.
Distribution. Singapore (Larson et al. 2008); India, Thailand, Malaysia, Vietnam and Borneo (Murdy 1989); Sumatra, Java, Sulawesi, Lombok, Sumbawa, and Halmahera, Indonesia (Takita et al. 1999; Polgar et al. 2013; Wahyudewantoro et al. 2014; this study).
Remarks. This is the first record of Boleophthalmus boddarti from Lombok and Sumbawa. In Labuan Tereng in Lombok, this species was found in abundance on the mangrove-forest floor, on open mudflats, and on the banks of streams, canals and fishponds. In Mbawi in Sumbawa however, they were found only on the banks of rivers and canals.
Periophthalmodon freycineti (Quoy & Gaimard, 1824)
Periophthalmus freycineti Quoy & Gaimard, 1824: 257 (Indonesia: Timor: Babao River)
Periophthalmus australis Castelnau, 1875: 22 (Australia: Queensland).
16
Material Examined. TIMOR: MUM 121-00018, 2 (48.8–49.4 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012; MUM 121-00056, 2 (33.1–66.3 mm), Maubesi mangrove forest, Atapupu, coll. Y. Zamroni, 15 September 2012; AMBON: MUM 121-00112, 1 (31.0 mm), Passo, Ambon Bay, Moluccas province, coll. Y. Zamroni, 27 February 2014.
Distribution. Philippines, Australia and Indonesia (Murdy 1989); Papua, Halmahera, Timor and Ambon, Indonesia (Eggert 1935; Murdy 1989; this study).
Periophthalmodon schlosseri (Pallas, 1770)
Gobius schlosseri Pallas, 1770: 5, pl. 1, figs 1–4 (Indonesia: Ambon).
Periophthalmus ruber Bloch & Schneider, 1801: 64 (India: Tharangambadi).
Periophthalmus phya Johnstone, 1903: 296, pl. 14 (Malay Peninsula: southern Thailand: estuaries
of Jambu and Pattani Rivers).
Periophthalmodon schlosseri argentiventralis Eggert, 1935: 49 (Indonesia: Java: Edam Island).
Material Examined. None.
Comparative Material Examined: JAVA: MUM 121-00077, 1 (93.4 mm), Mayangan beach, Legon Kulon, Subang-West Java, coll. Y. Zamroni, 28 December 2013; MZB (uncatalogued), 1 (141.76 mm), Muara Angke, Jakarta, 16 May 2010; SUMATRA: MZB 2078, 2 (160.0–165.0 mm), Muara Way Belak, Palas, South Lampung, coll. Soetikno, 13 June 1975; MZB 1736, 2 (170.0–177.0 mm), interchange between Way river & Belukak river, Lampung, coll. Soetikno, 13 June 1975; MZB 5397, 1 (163.0 mm), Rantau Panjang Bayeun, East Aceh, coll. D. Wowor, 11 February 1984.
Distribution. Thailand, Singapore, Borneo and Malay Peninsula (Murdy 1989); Sumatra, Java, Sulawesi, and Ambon (Pallas 1770; Koumans 1953; Murdy 1989; Wahyudewantoro 2014; this study).
Periophthalmus argentilineatus Valenciennes, 1837
Periophthalmus argentilineatus Valenciennes in Cuvier & Valenciennes, 1837: 191 (Indonesia:
Waigeo)
Periophthalmus dipus Bleeker, 1854b: 320 (Indonesia: Java: Banten; Sumatra: Padang; Flores:
Larantuka)
Euchoristopus kalolo regius Whitley, 1931: 326 (Australia: King George Sound)
Periophthalmus vulgaris vulgaris Eggert, 1935: 81, pls. 6 & 7, figs. 23–28 (Indonesia: Java:
Jakarta)
Periophthalmus vulgaris notatus Eggert, 1935: 83, pl. 7, fig. 29 (Malaysia: Klappa Island)
Periophthalmus vulgaris ceylonensis Eggert, 1935: 85 (Sri Lanka: Galle)
Periophthalmus dipus parvus Eggert, 1935: 88, pl. 8, fig. 32 (Indonesia: Sumatra: Belawan)
Periophthalmus dipus angustiformis Eggert, 1935: 89, fig. 14 (Indonesia: Flores: Mbawa)
Periophthalmus argentilineatus striopunctatus Eggert, 1935: 94, pl. 9, fig. 36 (Indonesia:
Kalimantan: Balikpapan)
Periophthalmus sobrinus Eggert, 1935: 95, pl. 9, figs. 37–38 (Red Sea: Loe Arafali)
5 (31.7–52.8 mm), Selindungan mangrove forest, Sekotong – West Lombok, coll. Y. Zamroni, 26 May 2013; MUM 121-00064, 12 (35.0–61.6 mm), Mangrove forest at Kelapa beach, Labuan Tereng, Lembar – West Lombok, coll. Y. Zamroni, 21 July 2012; MUM 121-00065, 6 (32.4–60.0 mm), Mangrove forest at Cemara beach, Lembar – West Lombok, coll. Y. Zamroni, 22 May 2013; MUM 121-00073, 10 (33.4–55.8 mm), Telong-elong mangrove forest, Keruak – East Lombok, coll. Y. Zamroni, 10 November 2013; SUMBAWA: MUM 121-00062, 6 (43.0–55.0 mm), Labuan Alas mangrove forest, coll. Y. Zamroni, 13 November 2013; MUM 121-00047, 6 (55.4–61.0 mm), Mbawi mangrove forest, Dompu, coll. Y. Zamroni, 15 November 2013; TIMOR: MUM 121-00067, 12 (49.6–63.0 mm), mangrove forest at Paradiso beach, Kupang, coll. Y. Zamroni, 17 September 2012; MUM 121-00069, 7 (36.7–50.2 mm), Oebelo mangrove forest, Neilbaki,
West Manggarai, coll. Y. Zamroni, 25 September 2012; KOMODO: MUM 121-00055, 7 (39.6–56.3 mm), Loh Sebita, Komodo Island, coll. Y. Zamroni, 22 September 2012; AMBON: MUM 121-00104, 15 (29.8–59.8 mm), Hunut mangrove forest, Ambon Bay, Ambon, Moluccas province, coll. Y. Zamroni, 11 March 2014; MUM 121-00097, 1 (55.4 mm), Suli-Ambon, Moluccas province, coll. Y. Zamroni, 15 March 2014; MUM 121-00105, 13 (31.3–57.3 mm), Lateri, Ambon, Moluccas province, coll. Y. Zamroni, 28 February 2014; MUM 121-00102, 11 (30.0–55.9 mm), Passo, Ambon Bay, Moluccas province, coll. Y. Zamroni, 27 February 2014; KEI: MUM 121-00110, 4 (49.0–67.9 mm), Fair, Tual, Kei islands, Moluccas province, coll. Y Zamroni, 8 March 2014;
HALMAHERA: MZB 18768 (in part), 2 of 4 specimens (23.5–31.0 mm), Muara
18
Comparative Material Examined: SUMATRA: MZB 22862 (in part), 9 of 14 specimens (30.1–39.2 mm), Kahyapu beach, Kahyapu village, Enggano subdistric, northern Bengkulu, 28 April 2015; JAVA: MUM 121-00058, 4 (34.3– 52.1 mm), Gending beach, Probolinggo-East Java, coll. Y. Zamroni, 15 November 2013; MUM 121-00071, 4 (40.3–52.5 mm), Tambak Sari beach, Pajarakan, Probolinggo-East Java, coll. Y. Zamroni, 17 October 2013; MUM 121-00072, 7 (36.6–52.0 mm), Klaseman, Probolinggo-East Java, coll. Y. Zamroni, 16 October 2013; MZB 4339, 1 (48.7 mm), Cisumur, Sumur-Pandeglang, coll. Soetikno & D. I. Hartoto, 17 January 1982; MZB 4333, 1 (49.3 mm), Cisumur river, Sumur-Pandeglang, coll. D. I. Hartoto, 14 January 1982; MZB 4335, 5 (52.00–59.64 mm), Muara Baru river, Sumur-Pandeglang, coll. D. I. Hartoto & Soetikno, 18 January 1982; MZB 21580, 1 (50.4 mm), Sokel river, Watukarung village, Pringkuku subdistrict, Pacitan-eastern Java, coll. Renny K.H. & Sopian, 27 July 2013; MZB 21622, 1 (56.0 mm), Teleng river, Pacitan, eastern Java, coll. Renny K.H. & Sopian, 29 July 2013; BALI: MUM 121-00059, 6 (36.1–39.4 mm), Benoa Bay-Denpasar, coll. Y. Zamroni, 5 June 2013; MUM 121-00060, 28 (40.1–52.0 mm), Terima Bay, Labuan Lalang-Buleleng, coll. Y. Zamroni, 19 October 2013; MUM 121-00061, 6 (38.9–65.0 mm), Gilimanuk Bay-Jembrana, coll. Y. Zamroni, 19 October 2013; PAPUA: MUM 121-00046, 2 (50.2–54.0 mm), Gom island near Waigeo island, coll. K. Krey, 7 October 2011; ARU
ISLANDS: MUM 121-00111, 12 (75.0–78.0 mm), Babi island, Aru Islands, coll.
Y. Zamroni, 7 March 2014.
Distribution. South Africa, Tanzania, Kenya, Somalia, Eritrea, Seychelles, Madagascar, Pakistan, Myanmar, Sri Lanka, Thailand, Malaysia, Japan, Philippines, Indonesia, Australia, Papua New Guinea and Oceania (Murdy 1989). In Indonesia, this species could be found in Sumatra and their satelit islands such as Weh island, Bangka, Nias, Mentawai and Simalur; Java, Madura, Bali, Borneo; in Lesser Sunda islands such as Lombok, Sumbawa, Sumba, Timor, Flores, Komodo and Adonara; in Sulawesi and their satelit islands such as Tongian, Salibabu and Talaud; Moluccas islands such as Ambon, Buru, Ceram, Saparua, Banda, Obi, Halmahera, Ternate, Kei and Aru islands; New Guinea and Waigeo (Cuvier and Valenciennes 1837; Bleeker 1854b; Eggert 1935; Koumans 1953; Allen 1991; Kimura and Matsuura 2003; Wahyudewantoro 2009; this study).
Periophthalmus gracilis Eggert, 1935
Periophthalmus gracilis Eggert, 1935: 79, Pl.6 fig. 22 (Indonesia: Java: Cilacap and Jakarta;
Sumatra: Siboga).
Material Examined.- LOMBOK: MUM 121-00051, 5 (22.0–34.0 mm), Sepi Bay, Sekotong, West Lombok, coll. Y. Zamroni, 6 April 2010; SUMBAWA: MUM 121-00075, 16 (28.2–46.3 mm), Mbawi mangrove forest, Dompu, coll. Y. Zamroni, 15 November 2013.
coll. Y. Zamroni, 15 October 2013; MUM 121-00070, 8 (34.0–38.9 mm), Tambak Sari beach, Pajarakan, Probolinggo-East Java, coll. Y. Zamroni, 17 October 2013. Distribution. Malay Peninsula, Luzon (Philippines) and Queensland (Australia) (Murdy 1989); Singapore (Larson et al. 2008); Sumatra, Nias, Lombok, Sumbawa and Java, Indonesia (Eggert 1935; Koumans 1953; Takita et al. 1999, this study).
Periophthalmus kalolo Lesson, 1830
Periophthalmus kalolo Lesson, 1830: 146 (Indonesia: Papua: Waigeo).
Periophthalmus kallopterus Bleeker, 1853b: 342 (Indonesia: Ambon).
Periophthalmus fuscatus Blyth, 1858: 271 (Andaman Islands).
Periophthalmus harmsi Eggert, 1929: 402 (Indonesia: Java: Poeloe Popole).
Periophthalmus koelreuteri albostriatus Eggert, 1935: 73, fig. 10 (Indonesia: Java: Poeloe Popole).
Periophthalmus koelreuteri velox Eggert, 1935: 75 (Indonesia: Java: sand beach opposite
Amsterdam Island near Jakarta).
Periophthalmus koelreuteri africanus Eggert, 1935: 78, pl. 5, fig. 21 (Tanzania: Dar es Salaam).
Periophthalmus musgravei Whitley, 1961: 69 (Papua New Guinea: Misima island).
Material Examined. TIMOR: MUM 121-00020, 5 (40.3–76.7 mm), mangrove
Wasea, Central Weda, Central Halmahera district, North Moluccas province, coll. Renny K. H., D. Wowor & Sopian, 4 February 2010; SULAWESI: MUM 121-island, Ujung Kulon-West Java, coll. Soetikno, 27 February 1971; MZB 5566, 1 (102.0 mm), Buluran beach-East Java, coll. Moelyadi, July 1984; SUMATRA:
MZB 22868 (in part), 3 of 5 specimens (26.7–74.3 mm), Malakoni beach, Malakoni village, Enggano subdistric, northern Bengkulu, Bengkulu province, coll. Renny K.H. & Sopian, 26 April 2015; MZB 22862 (in part), 5 of 14 specimens (21.5–55.2 mm), Kahyapu beach, Kahyapu village, Enggano subdistric, northern Bengkulu, coll. Renny K.H. & Sopian, 28 April 2015.
20
Periophthalmus malaccensis Eggert, 1935
Periophthalmus malaccensis Eggert, 1935: 62, figs. 3–4 (Singapore)
Material Examined.- LOMBOK: MUM 121-00049, 6 (58.7–68.9 mm), Sepi Bay, Sekotong, West Lombok, coll. Y. Zamroni, 6 April 2010; MUM 121-00050, 10 (50.7–75.0 mm), Sepi Bay, Sekotong, West Lombok, coll. Y. Zamroni, 3 June 2013; SUMBAWA: MUM 121-00036, 12 (32.8–64.7 mm), Mbawi, Dompu, coll. Y. Zamroni, 15 November 2013; KOMODO: MUM 121-00052, 13 (58.6–67.5 mangrove fores, Ambon Bay, Moluccas province, coll. Y. Zamroni, 27 February 2014; MUM 121-00103, 5 (55.0–80.0 mm), Hunut mangrove forest, Ambon Bay, Moluccas province, coll. Y. Zamroni, 11 March 2014; HALMAHERA: MZB 18768 (in part), 1 of 4 specimens (61.3 mm), Muara Wasea, Central Weda, Central Halmahera district, North Moluccas province, coll. Renny K. H., D. Wowor & Sopian, 4 February 2010; SULAWESI: MUM 121-00089, 2 (54.9– 88.5 mm), Donggala, Palu, Central Sulawesi, coll. Y. Zamroni, 12 January 2014; MUM 121-00090, 3 (58.5–74.4 mm), Wanggu river, Andonuhu, Kendari Bay, SE Sulawesi, coll. Y. Zamroni, 18 January 2014; MUM 121-00092, 1 (79.5 mm), Poso beach, Central Sulawesi, coll. Y. Zamroni, 14 January 2014; MZB 10683, 2 (38.0–71.3 mm), Kujuk Kujunge river, Paojepe village, Wajo-South Sulawesi, coll. Haryono, 26 December 2000; MZB 10480 (in part), 1 of 8 specimens (74.6 mm), Mangrove forest Tongke-Tongke, Sinjai, South Sulawesi, coll. Haryono & Harun, 13 June 1975; MZB 10675 (in part), 12 of 14 specimens (74.0–98.0 mm), Masic river, Paojepe village, Keera distric, Wajo-South Sulawesi, coll. Haryono, 23 September 2000.
Comparative Material Examined: JAVA: MUM 121-00002, 4 (39.8–90.7 mm), Gending beach, Probolinggo-East Java, coll. Y. Zamroni, 15 October 2013; MUM 121-00007, 6 (50.5–90.1 mm), Klaseman beach, Probolinggo-East Java, coll. Y. Zamroni, 16 October 2013; MUM 121-00012, 6 (36.2–90.5 mm), Tambak Sari beach, Pajarakan, Probolinggo-East Java, coll. Y. Zamroni, 17 October 2013;
PAPUA: MUM 121-00045, 1 (64.5 mm), Gom island near Waigeo island, coll.
K. Krey, 7 October 2011;
Distribution. Singapore (Eggert 1935); Philippines (Murdy 1989); Java, Sulawesi, Lombok, Sumbawa, Sumba, Komodo, Flores, Ambon, Halmahera, and Papua (Waigeo), Indonesia (Murdy 1989; this study).
(Murdy 1989). Polgar (2012) presumed this species to be extinct in the Sundaland ecoregion, which we disagree with as individuals were observed in Probolinggo, eastern Java (YZ pers. obs.).
Periophthalmus minutus Eggert, 1935
Periophthalmus minutus Eggert, 1935: 90, figs. 15-16, Pl. 8, fig. 33 (Indonesia: Sumatra: Belawan:
Deli River). Zamroni, 17 September 2012; MUM 121-00085, 1 (40.9 mm), Oebelo mangrove forest, Neilbaki, Kupang, coll. Y. Zamroni, 13 September 2012; SULAWESI:
MUM 121-00094, 7 (30.5–51.1 mm), Donggala, Palu, Central Sulawesi, coll. Y. Zamroni, 12 January 2014; MZB 10675 (in part), 2 of 14 specimens (46.26–53.38 mm), Masic River, Paojepe village, Keera subdistrict, Wajo, South Sulawesi, coll. Haryono 23 September 2000.
Comparative Material Examined: BALI: MUM 121-00082, 2 (47.0–50.1 mm), Gilimanuk Bay, Jembrana, coll. Y. Zamroni, 19 October 2013; MUM 121-00086, 11 (33.1–48.5 mm), Terima Bay, Labuan Lalang-Buleleng, coll. Y. Zamroni, 19 October 2013.
Distribution. Andaman Islands, Thailand, and Palawan (Murdy 1989); Australia (Takita et al. 2011); Java, Sumatra, Bali, Lombok, Timor and Sulawesi, Indonesia (Eggert 1935; Murdy 1989; this study).
Periophthalmus pusing Jaafar, Polgar, Zamroni 2016
Periophthalmus pusing Jaafar, Polgar & Zamroni 2016 (Indonesia: Sumba).
22
mangrove forest, Kupang, coll. Z. Jaafar & Y. Zamroni, 12 November 2012; USNM 427009, 10 (31.5–38.6 mm), data as USNM 410706; MUM 121-00043, 3 (32.2–36.0 mm), data as USNM 410706.
Distribution. –Currently known from Sumba and Timor.
Periophthalmus variabilis (Eggert, 1935)
Periophthalmus variabilis variabilis Eggert, 1935: 64, Fig. 5, Pl. 3, fig. 13 (Indonesia: Java:
Cilacap).
Periophthalmus variabilis sumatranus Eggert, 1935: 65, Fig. 6, Pl. 4, fig. 14–15 (Indonesia:
Sumatra: Belawan, Padang and Medan; Java: Jakarta).
Periophthalmus variabilis asiaticus Eggert, 1935: 66, Fig. 7 (Thailand: Paknam).
Periophthalmus variabilis tidemani Eggert, 1935: 67, Fig. 8 (Indonesia: Halmahera: Baboe Island).
Periophthalmus novemradiatus –Murdy, 1989: 43, Fig. 42 (in part).
Material Examined. None.
Comparative Material Examined: JAVA: MUM 121-00019, 1 (57.4 mm), Tambak Sari beach, Pajarakan, Probolinggo-East Java, coll. Y. Zamroni, 17 October 2013; MUM 121-00078, 6 (49.1–62.5 mm), Mayangan beach, Legon Kulon, Subang-West Java, coll. Y. Zamroni, 29 December 2013.
Distribution. Peninsular Malaysia and Singapore (Jaafar et al. 2009); Thailand (Jaafar et al. 2009); Java, Sumatra, and Halmahera (Eggert 1935; Jaafar et al. 2009; this study).
Pseudapocryptes borneensis (Bleeker, 1855)
Apocryptes borneensis Bleeker, 1855: 421 (Indonesia: Borneo: Banjarmasin).
Material Examined. None
Distribution. Penang, Peninsular Malaysia (Koumans 1953); Singapore (Murdy 1989); Bandjarmasin and Timor, Indonesia (Günther 1861; Koumans 1953).
Scartelaos histophorus (Valenciennes, 1837)
Gobius viridis Hamilton, 1822: 42, 366, pl. 32, fig. 12 (India: Ganges River) [invalid; preoccupied
by Gobius viridis Otto 1821]
Boleophthalmus histophorus Valenciennes, in Cuvier & Valenciennes, 1837: 210 (India: Bombay)
Boleophthalmus sinicus Valenciennes, in Cuvier & Valenciennes, 1837: 215 (China: Canton)
Boleophthalmus chinensis Valenciennes, in Cuvier & Valenciennes, 1837: 215 (China: Canton)
Boleophthalmus aucupatorius Richardson, 1845: 148, pl. 62, figs. 1–4 (China: Woosung and
Canton)
Boleophthalmus campylostomus Richardson, 1846: 209 (China: Canton)
Apocryptes macrophthalmus Castelnau, 1873: 87 (Australia: Northern Territory: Port Darwin)
Gobiosoma guttulatum Macleay, 1878: 357, pl. 9, fig. 6 (Australia: Northern Territory: Darwin)
Gobiosoma punctularum De Vis, 1884: 449 (South Sea Islands)
Material Examined.- TIMOR: MUM 121-00015, 20 (58.6–89.1 mm), Oebelo mangrove forest, Kupang, coll. Y. Zamroni, 13 September 2012; SUMBA: MUM 121-00034, 1 (101.3 mm), Kampung Bugis mangrove forest, Waingapu, coll. Z. Jaafar & Y. Zamroni, 9 November 2012; USNM 410673, 2 (99.8–107.0 mm), data as MUM 121-00034; ZRC 53912, 1 (98.8 mm), data as MUM 121-00034;
SULAWESI: MZB 5816, 1 (58.8 mm), Lariang river [probably in Mamuju],
South Sulawesi, 13 August 1989.
Comparative Material Examined: JAVA: MZB 1179, 2 (82.5–84.0 mm), Banten Bay, West Java, coll. Soetikno, 4 July 1970.
Distribution. China and Malaysia (Günther 1861); India (Day 1876); Philippines (Herre 1927); New Guinea and Thailand (Koumans 1953); Pakistan and Australia (Murdy 1989); Singapore (Larson et al. 2008); Java, Sulawesi, Borneo, Madura, Timor, Sumba, and Papua, Indonesia (Weber 1913; Koumans 1953, this study).
DISCUSSION
Central IAA, and the islands therein, is a region with complex geological history. The northern Lesser Sunda islands such as Lombok, Sumbawa, and Flores, are estimated to have formed during the Pliocene 15–5 mya when the Australiana and Asian plates collided (Audley-Charles 1981). The southern Lesser Sunda islands—Timor, Tanimbar, and Sumba—are continental fragments of those plates (Hall 2002). Morotai, Bacan, and Halmahera islands in the Moluccas island group originated from volcanic activities in the Pacific ocean, moved to the north of New Guinea and arrived at their current position about 5 mya (Audley-Charles 1981). The southern Moluccas islands, such as Ambon, Ceram, and Buru, from the fragmentation of the Australian continent (Hall 1998). Sulawesi is a composite island with three major tectonic provinces, (1) the West Sulawesi Plutono-Volcanic Arc, (2) the East Sulawesi Ophiolite Belt, and (3) the Central Sulawesi Metamorphic Belt (Stelbrink et al. 2012).
24
groups. Periophthalmodon schlosseri, an Oriental species, was recorded in Sulawesi. The type locality of this species listed as Ambon (Murdy 1989), but Kottelat (2013) argues that ‘Ambon’ is used to loosely refer to the East Indies in historical texts. The Australasian congener, Periophthalmodon freycineti, is recorded in Timor, Ambon and Halmahera. The report of this species from Myanmar (part of Oriental region) is probably erroneous (Kottelat 2013).
3
BIOGEOGRAPHY OF OXUDERCINE GOBIES
(TELEOSTEI: GOBIIDAE) IN THE LESSER SUNDA,
MOLUCCAS, AND SULAWESI GROUP OF ISLANDS
Abstract
One of the problems in biogeographical study is providing the evidence of boundaries delimitation of closely related species. The oceanic island between Sunda and Sahul shelves reflects the biogeographical problem because distinctly Oriental and Australasia faunas intermingle in this area. The coastal fauna such as Oxudercines are thus excellent model organisms to aid in learning the ecological processes and biogeographical distribution in the oceanic islands. Their distribution may reflect the gradual change of faunal distribution, linking the island between them and providing the evidences for hypotheses on the zoogeographic boundaries. This study recorded the highest diversity of Oxudercine in both Sunda and Sahul shelves and their diversity reduced in the oceanic islands. These islands consisted of widely distributed and no endemic Oxudercine. These area not recover a single area monophyly and included to a part of three area of endemisms: Sundan, Sulawesian and Philippinesian. It means these oceanic islands cannot withstand as a unique biogeographical region such as
“Wallacea concept”. The distributional pattern of oxudercines gobies was tested use the geographical congruence between areagrams of seven taxa and resulted the Oriental realm covered area from western coast of India northward to Japan, southward to Lesser Sunda and eastward to northwestern Philippines and southern Moluccas, whereas the Australasian realm covered area of Sahulland northward to northern Moluccas and southeastern Philippines.
Key words: Boundaries, Gobies, Sundaland, Sahulland
Introduction
Adjacent Sunda and Sahul continental shelves are widely-accepted to be populated with Oriental and Australasian fauna respectively (Darlington 1957). Yet, the boundaries that demarcate these two regions are often disputed (Simpson 1977). The islands between these land masses are interfaces in which Oriental and Australasian organisms colonize to varying degrees (Parenti and Ebach 2009),
28
Figure 3.1 Map of Indo-Australasian Archipelago. Sampling locations (open circles) and localities from museum materials and previous publications (close circles). 1, Andaman islands; 2, Bangkok; 3, Ranong; 4, Phang-nga; 5, Pattani; 6, Penang; 7, Selangor; 8, Singapore; 9, Belawan; 10, Tebing Tinggi; 11, Padang; 12, Lampung; 13, Jakarta/Subang; 14, Surabaya/Probolinggo; 15, Madura; 16, Bali; 17, Banjarmasin; 18, Serawak/Sambas; 19, Samarinda; 20, Sabah; 21, Lombok; 22, Sumbawa; 23, Sumba; 24, Flores; 25, Timor; 26, Makassar; 27, Kendari; 28, Donggala; 29, Poso; 30, Banggai; 31, Manado; 32, Halmahera; 33, Ternate; 34, Obi; 35, Ceram; 36, Buru; 37, Ambon; 38, Kei Islands; 39, Negros; 40, Panay; 41, Tablas; 42, Luzon; 43, Waigeo; 44, Aru Islands; 45, Timika; 46, Merauke; 47, Gulf of Papua; 48, Northern Territory; 49, Queensland; 50, Western Australia. Solid-lines are the hypothetical of zoological boundaries and the dash-lines are the limit of three oceanic island groups.
Wallace’s and Huxley’s lines represent the continental edges of the Sunda
shelf. Wallace (1863) proposed an east-west demarcation, between Bali and Lombok, between Borneo and Sulawesi, and between Philippines and the Sangihe Islands of Indonesia, as the eastern-most boundary of Oriental fauna based on his
study of parrot populations (Psittacidae); Wallace’s line is now accepted as the
line between Borneo and the Sulu archipelago through the Mindoro strait between Mindoro Island and Calamines Islands, Philippines.
Lydekker’s and Weber’s lines borders the Sahul shelf. Lydekker’s line
represents the western-most boundary of strictly Australasian fauna based on the distribution of marsupial mammals (Whitten et al. 1987). Weber’s line was proposed as a boundary that demarcates an equal ratio of Oriental and Australasian fauna based on mammals and mollusks distribution patterns (Moss and Wilson 1998). While the majority of non-flowering and flowering plants do not conform to the distribution patterns described by these lines (George 1981), distribution patterns of some faunal taxa have corroborated the conditions of these hypotheses. The population of the giant freshwater prawn Macrobrachium rosenbergii east of Huxley’s line for example, was found to be genetically distinct from the western population (de Bruyn et al. 2004). Homalopsine snake populations in Sulawesi and the Philippine Islands are phylogenetically closer to Oriental, rather than to Australasian populations (Karns et al. 2000; Alfaro et al. 2004; Alfaro et al. 2008). Overlapping distribution was showed between Sundaic fishes subfamily Phallostethinae with their Sahulian sister taxa subfamily Denatherininae in area of eastern Borneo, southern Philippines and western Sulawesi (Parenti and Ebach 2013). Weber’s line limits the distribution of the Asian water monitor lizards (Varanus salvator complex) to the west, with the Sahulian sister taxa Varanus indicus complex to the east (Cota 2008; Welton et al. 2014).