www.elsevier.com / locate / bres
Short communication
Cholinergic modulation of the sleep state-dependent P13 midlatency
auditory evoked potential in the rat
b c a a ,
*
Luis Teneud , Hiroshi Miyazato , Robert D. Skinner , Edgar Garcia-Rill
a
Department of Anatomy, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA
b
Department of Physiology, Univ. de Los Andes, Merida, Venezuela
c
Department of Psychiatry, University of the Ryukyus, Okinawa, Japan
Accepted 12 September 2000
Abstract
Injections into the pedunculopontine nucleus (PPN) of the cholinergic receptor agonist, carbachol (CAR), were found to reduce the amplitude of the vertex-recorded, sleep state-dependent P13 midlatency evoked potential in a dose- and time-dependent manner. This effect was blocked or reduced by pretreatment with the muscarinic receptor antagonist, scopolamine, injected into the PPN. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Biological rhythms and sleep
Keywords: Auditory evoked potentials; P50 potential; Pedunculopontine nucleus; Reticular Activating System; Acetylcholine
Recent studies suggest that the P13 midlatency auditory known to receive inhibitory cholinergic (muscarinic re-evoked potential may be the rodent equivalent of the ceptor [31]) input [17], mostly from the contralateral PPN P1 / P50 potential in the human [21–23]. The three main and the laterodorsal tegmental nuclei [11]. The present characteristics of this potential in both species are (1) sleep study was conducted to determine if injections of a state-dependence (it is present during waking and paradox- cholinergic (muscarinic receptor) agonist into the region of ical sleep, but absent during slow wave sleep, i.e. during the PPN would affect the manifestation of the vertex-states of cortical EEG synchronization of high frequencies recorded P13 potential, and whether or not such an effect but absent during synchronization of low frequencies), (2) could be reduced or blocked by pretreatment with a rapid habituation (attenuated at stimulation frequencies cholinergic (muscarinic receptor) antagonist injected into over 1 Hz, i.e. not a primary sensory pathway but rather a the PPN.
‘reticular’ low-conduction security pathway), and (3) The methods employed have been previously published mediated by cholinergic projections (blocked by systemic [19–23]. Briefly, adult male Sprague–Dawley rats (n518, administration of the muscarinic cholinergic agonist 250–350 g, Sasco) were anaesthetized with ketamine HCl scopolamine) [6,8–10,21–23]. This vertex-recorded po- (60 mg / kg, i.m.) and sodium pentobarbital (20 mg / kg, tential can be modulated by localized injections of gabaer- i.p.). Anaesthetic levels were maintained during implanta-gic, noradrenergic and serotonergic agents into the region tion of recording skull screw electrodes bilaterally at the of the pedunculopontine nucleus (PPN) in the rat [19–21], vertex (Vx) referred to a frontal sinus electrode. The in keeping with known inputs to the PPN from the screws were connected to a receptacle anchored to the substantia nigra, locus coeruleus and raphe nuclei [25]. skull. Guide cannulae were implanted stereotaxically 2 mm Cholinergic mesopontine neurons of the PPN are also dorsal to the PPN (A: 1.0, L: 1.9, H: 3.0) [24], and recordings began after a 1 week recovery period. Auditory evoked potentials were recorded from unrestrained, alert
*Corresponding author. Tel.: 11-501-686-5167; fax: 1
1-501-686-rats before and after injection of a compound. In all trials,
6382.
E-mail address: [email protected] (E. Garcia-Rill). evoked potentials from the Vx were amplified (10,000x)
and filtered at 3 Hz–1 KHz. Measurement, by an in-dividual blind to treatment, of the amplitude of auditory evoked potentials was made from the beginning of the wave to its peak as previously described [19–23]. All subjects were tested between 9 a.m. and 1 p.m. to control for possible time-of-day effects.
Auditory stimuli (rarefaction clicks 0.1 ms in duration at 103 dB) were delivered via speakers on opposite walls of the sound-attenuating recording chamber once every 5 s until 32 evoked potentials were acquired for averaging. After control recordings were completed, animals received bilateral injections (0.3 ml ea) using a 32-gauge injection
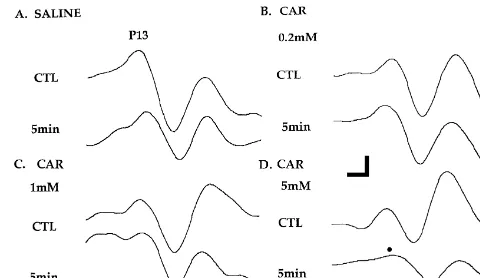
cannula which extended beyond the tip of the implanted Fig. 1. Effects of injections of various concentrations of CAR into the 25-gauge guide cannula. The vehicle for all drug injections PPN on the amplitude of the vertex-recorded P13 potential. Recordings
was physiological saline. The pre-injection P13 potential before (CTL) and 5 min after saline injections into the PPN showed no significant changes in P13 potential amplitude (A. upper left). However,
amplitude was designated as 100% and subsequent
post-while 0.2 mM (B. upper right) or 1 mM (C. lower left) CAR injections
injection amplitudes were calculated as a percent
am-did not significantly change the amplitude of the P13 potential compared
plitude of this control recording, i.e. following saline to saline, P13 potential amplitude was significantly reduced (filled circle) injection. In dose-response studies (n56), 0.2, 1 or 5 mM by injections of 5 mM (D. lower right) CAR. Calibration bars — vertical
carbachol (CAR) was injected bilaterally into the PPN. In 20mV and horizontal 5 ms.
studies of cholinergic interactions (n56), animals were pretreated (injections into PPN) with 20 mM scopolamine
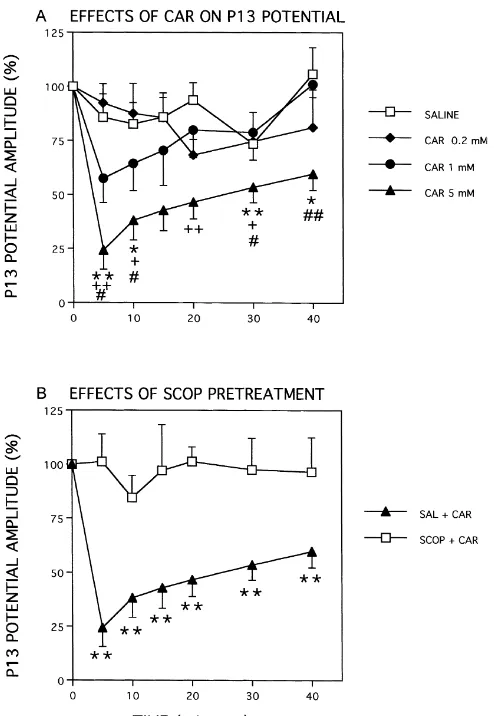
(SCOP) or saline (SAL). Fifteen minutes after pretreat- 7.77, P,0.001), and the presence of an interaction be-ment, 5 mM CAR was injected into the PPN and record- tween dose and time (F18,9051.86, P,0.02). According to ings were performed thereafter. In studies of anatomical repeated measures, one-way ANOVA at each time point, specificity (n56), 5 mM CAR was injected bilaterally, significant dose effects occurred at 5, 10, 30 and 40 min using different-length injection cannulae, into three sites: after injection. Post-hoc (Scheffe’) tests indicated that: (1) 1.3 mm above the PPN (H: 4.3), within the PPN (H: 3.0) the 5 mM CAR dose effect was significantly greater than or 1.3 mm below the PPN (H: 1.7). The administration of the SAL effect at 5 (P,0.01), 10 (P,0.05), 30 (P,0.01) different concentrations of neuroactive compounds or and 40 (P,0.05) min; (2) had a greater effect than the 0.2 saline was made in random order and no animal was mM CAR dose at 5 (P,0.01), 10 (P,0.05), 20 (P,0.01) injected more than once per week or more than five times and 30 (P,0.05) min; and (3) had a greater effect than the overall. It should be noted that injections required that the 1 mM CAR dose at 5 (P,0.05), 10 (P,0.05), 30 (P, animal be removed from the chamber, making saline 0.05) and 40 (P,0.01) min.
Fig. 3. Effects on P13 potential amplitude of bilateral injections of 5 mM CAR into the PPN following pretreatment with SCOP or SAL (n56). Averaged recordings before (CTL) and 15 min after bilateral injections of 5 mM CAR. A. Pretreatment with 20 mM SCOP reduced or blocked the suppressive effect of CAR on P13 potential amplitude. B. Pretreatment with SAL failed to reduce or block the suppressive effect of CAR on P13 amplitude (filled circle). Calibration bars — vertical 20mV and horizontal 5 ms.
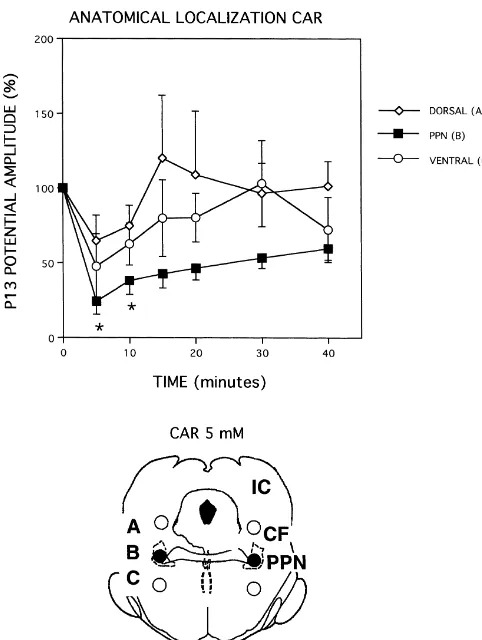
effective in decreasing P13 potential amplitude than in-jection into the dorsal site (A) (P,0.02) at 5 and 10 min after treatment. The ineffective dorsal injection site (A) included the ventral part of the inferior colliculus, cuneiform nucleus and mesencephalic V nucleus. However, injection into the PPN (B) did not differ statistically (only
Fig. 2. (A) Dose- and time-dependent effects of injections of CAR into
numerically) from 5 mM CAR injection into the ventral
the PPN on P13 potential amplitude. The P13 potential was averaged over
32 trials before and after bilateral injections of saline (SAL, open squares) site (C), which included the dorsal and oral part of the
or CAR (0.2, 1 or 5 mM, filled diamonds, filled circles and filled pontine reticular nucleus (immediately ventral to the PPN). triangles, respectively) into the PPN (n56). Each point is the mean6S.E.
One possibility for this lack of statistical difference is that
of the P13 potential amplitude as a percent of pre-injection P13 potential
diffusion of CAR dorsally along the cannula in typical
amplitude at various times after injection. Post hoc Scheffe’ test
signifi-11 1 tear-drop fashion, may have activated muscarinic receptors cance: **P,0.01 or *P,0.05 compared to SAL, P,0.01 or P,0.05
[[ [
compared to 0.2 mM CAR, and P,0.01 or P,0.05 compared to 1 in ventral PPN, leading to a partial diminution of P13
mM CAR. (B) Effects of SAL or SCOP pretreatment on injections of 5 potential amplitude. It should also be noted that there are mM CAR into the PPN. Each point is the mean6S.E. of the P13 potential
diffuse descending projections from cholinergic
mesopon-amplitude as a percent of pre-injection mesopon-amplitude. The effects of 5 mM
tine nuclei throughout the oral pontine reticular formation
CAR after SAL injection are shown as filled triangles, while the effects of
[1,16,27], so that injections into site C may have activated
5 mM CAR after 20 mM SCOP injection are shown as open squares. Post
hoc significance: **P,0.01 for SAL1CAR compared to SCOP1CAR. cholinergic receptors in the region which somehow in-fluenced the generation of the P13 potential. There may be other explanations for the lack of statistical difference following injection of CAR after SAL or SCOP pretreat- between CAR injections into site B compared to site C. ment are shown in Fig. 3. Nevertheless, the specificity of localization is evident in Fig. 4 shows the relationship between injection sites and the statistical difference between injection at A and those changes in P13 potential amplitude (n56). While localized at B.
the N40, also has been proposed to be the rodent equiva-lent of the P1 potential [18]. This wave can be recorded at the vertex but has been localized to the hippocampus [2,18]. The N40 does show sensory gating properties which can be modulated by cholinergic and noradrenergic agents [29,30]. In contrast to the P13 potential, however, the N40 is not affected by scopolamine, but it is affected by nicotinic antagonists [13,18]. In addition, the facts that the N40 can be recorded in anaesthetized animals [13,18], and is not sleep state-dependent [21], suggest that this] wave may be independent of the RAS, although it is obviously a useful measure of hippocampal sensory gating. Our results suggest that CAR may activate muscarinic cholinergic receptors at the level of the PPN in order to reduce the amplitude of the P13 potential by inhibiting neurons which generate the waveform. Injections of SCOP into the region of the PPN presumably block inhibitory muscarinic cholinergic receptors located on neurons modu-lating the P13 potential, thus preventing the reduction in amplitude induced by injections of CAR into the same region. This effect, however, should not be confused with that of systemic administration of SCOP, which is known to reduce or block the manifestation of the P13 potential [22]. The P13 potential is thought to be generated by output projections of the PPN [26]. The rationale for this suggestion stems from studies in the cat, in which the equivalent waveform to the P50 potential in the human and
Fig. 4. Anatomical localization of the effects on P13 potential amplitude the P13 potential in the rat is the feline ‘wave A’, which of bilateral injections of CAR (5 mM) into the PPN (B) and outside of
has a latency intermediate to these values, of 25 ms [10].
the PPN (A and C). Top: The P13 potential was averaged over 32 trials
However, PPN neurons in the cat respond to auditory
before (control, CTL) and after bilateral injections of 5 mM CAR dorsal
stimuli at latencies from 10 to 20 ms, suggesting that that
to (open diamonds), within (filled squares), or ventral to (open circles) the
PPN. Post hoc Scheffe’ test significance: *P,0.05 for B compared to A, ‘wave A’ is generated, at least in part, by PPN outputs and
n.s. for B compared to C. Bottom: Locations of 5 mM CAR injection sites not by activation of PPN itself [26]. The PPN has diffuse A, B and C in relation to NADPH diaphorase-positive neurons (PPN).
ascending projections to virtually every thalamic nucleus
Abbreviations: CF — cuneiform nucleus, IC — inferior colliculus, PPN
as well as to basal ganglia and basal forebrain structures
— pedunculopontine nucleus. Section is approximately 1.0 mm anterior
[25]. It is these multiple cholinergic PPN projections
to the interaural line [24].
which systemic administration of SCOP may block in order to lead to a reduction in the P50 potential in the physiological studies demonstrating the presence of an human, ‘wave A’ in the cat and the P13 potential in the rat inhibitory cholinergic input to mesopontine PPN neurons [6,22].
Pawelak, T.W. Freeman, C.N. Karson, F.A. Boop, E. Garcia-Rill,
modulation in the up- or down-regulation of the RAS in
Combat veterans with posttraumatic stress disorder exhibit
de-the disorders mentioned [14].
creased habituation of the P1 midlatency auditory evoked potential, Life Sci. 61 (1997) 1421–1434.
[16] B.E. Jones, Immunohistochemical study of choline acetyltransfer-Acknowledgements ase-immunoreactive processes and cells innervating the pontomedul-lary reticular formation in the rat, J. Comp. Neurol. 295 (1990) 485–514.
Supported by USPHS grant NS20246.
[17] C.S. Leonard, R.R. Llinas, Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study, Neuroscience 59 (1994) 309–330. References [18] V. Luntz-Leybman, P.C. Bickford, R. Freedman, Cholinergic gating of responses to auditory stimuli in rat hippocampus, Brain Res. 587 (1992) 130–136.
[1] H.A. Baghdoyan, Location and quantification of muscarinic receptor
[19] H. Miyazato, R.D. Skinner, T. Crews, K. Williams, E. Garcia-Rill, subtypes in rat pons: implications for REM sleep generation, Am. J.
Serotonergic modulation of the P13 midlatency auditory evoked Physiol. 273 (1997) R896–R904.
potential in the rat, Brain Res. Bull. 51 (2000) 387–391. [2] P.C. Bickford-Wymer, H. Nagamoto, R. Johnson, L.E. Adler, M.
[20] H. Miyazato, R.D. Skinner, E. Garcia-Rill, Sensory gating of the Egan, G.M. Rose, R. Freedman, Auditory sensory gating in
hip-P13 midlatency auditory evoked potential and the startle response in pocampal neurons: a model system in the rat, Biol. Psychiat. 27
the rat, Brain Res. 822 (1999) 60–71. (1990) 183–192.
[21] H. Miyazato, R.D. Skinner, E. Garcia-Rill, Neurochemical modula-[3] F.A. Boop, E. Garcia-Rill, R. Dykman, R.D. Skinner, The P1:
tion of the P13 midlatency auditory evoked potential in the rat, insights into attention and arousal, Pediat. Neurosurg. 20 (1994)
Neuroscience 92 (1999) 911–920. 57–62.
[22] H. Miyazato, R.D. Skinner, N.B. Reese, F.A. Boop, E. Garcia-Rill, [4] J.S. Buchwald, R.J. Erwin, J. Schwafel, P. Tanguay, Abnormal P1
A middle-latency auditory-evoked potential in the rat, Brain Res. potentials in autistic subjects, Neurosci. Abst. 14 (1988) 771.
Bull. 37 (1995) 247–255. [5] J.S. Buchwald, R.J. Erwin, D. Van Lancker, J.L. Cummings,
[23] H. Miyazato, R.D. Skinner, N.B. Reese, J. Mukawa, E. Garcia-Rill, Midlatency auditory evoked responses: differential abnormality of
Midlatency auditory evoked potentials and the startle response in the P1 in Alzheimer’s disease, Electroenceph. clin. Neurophysiol. 74
rat, Neuroscience 75 (1996) 289–300. (1989) 378–384.
[24] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, [6] J.S. Buchwald, E.H. Rubinstein, J. Schwafel, R.J. Strandburg,
4th Edition, Academic Press, New York, 1998. Midlatency auditory evoked responses: differential effects of a
[25] N.B. Reese, E. Garcia-Rill, R.D. Skinner, The pedunculopontine cholinergic agonist and antagonist, Electroenceph. clin.
nucleus auditory input, arousal and pathophysiology, Prog. Neuro-physiol. 80 (1991) 303–309.
biol. 42 (1995) 102–133. [7] J. Clothier, J.T. Scruggs, L.J. Gamble, R.D. Skinner, E. Garcia-Rill,
[26] N.B. Reese, E. Garcia-Rill, R.D. Skinner, Auditory input to the The P1 / P50 midlatency auditory evoked potential in depression,
pedunculopontine nucleus I. Evoked potentials, Brain Res. Bull. 37 Sleep 21 (1998) 224.
(1995) 257–264. [8] R.J. Erwin, J.S. Buchwald, Midlatency auditory evoked responses:
[27] K. Semba, Aminergic and cholinergic afferents to REM sleep Differential effects of sleep in the human, Electroenceph. clin.
induction regions of the pontine reticular formation in the rat, J. Neurophysiol. 65 (1986) 383–392.
Comp. Neurol. 330 (1993) 543–556. [9] R.J. Erwin, J.S. Buchwald, Midlatency auditory evoked responses:
[28] R.D. Skinner, L. Rasco, J. Fitzgerald, C.N. Karson, M. Matthew, Differential recovery cycle characteristics, Electroenceph. clin.
D.K. Williams, E. Garcia-Rill, Reduced sensory gating of the P1 Neurophysiol. 64 (1986) 417–423.
potential in rape victims and combat veterans with posttraumatic [10] R.J. Erwin, J.S. Buchwald, Midlatency auditory evoked responses in
stress disorder, Depress. Anxiety 9 (1999) 122–130. the human and in the cat model, in: R. Johnson, J.W. Rohrbaugh, R.
[29] K.E. Stevens, L.L. Fuller, G.M. Rose, Dopaminergic and norad-Parasweraman (Eds.), Current Trends in Event-Related Potential
renergic modulation of amphetamine-induced changes in auditory Research, Elsevier, Amsterdam, 1987, pp. 461–467.
gating, Brain Res. 555 (1991) 91–98. [11] H.C. Fibiger, K. Semba, Afferent connections of the
pedunculopon-[30] K.E. Stevens, J. Meltzer, G.M. Rose, Disruption of sensory gating tine and laterodorsal tegmental nuclei in the rat, Neurosci. Abst. 14
by the alpha-2 selective noradrenergic antagonist yohimbine, Biol. (1988) 633.
Psychiat. 33 (1993) 130–132. [12] R. Freedman, L.E. Adler, M.C. Waldo, E. Pachtman, R.D. Franks,
[31] M.T. Vilaro, J.M. Palacios, G. Mengod, Multiplicity of muscarinic Neurophysiological evidence for a defect in inhibitory pathways in
receptor subtypes? Comparison of the distribution of cholinergic schizophrenia: Comparison of medicated and drug-free patients,
cells and cells containing mRNA for five receptor subtypes of Biol. Psychiat. 18 (1983) 537–551.
muscarinic receptors in the rat brain, Molec. Brain Res. 21 (1994) [13] R. Freedman, L.E. Adler, P. Bickford, W. Byerley, H. Coon, C.M.
30–46. Cullum, J.M. Griffith, J.G. Harris, S. Leonard, C. Miller, M.
Myles-[32] S.R. Vincent, K. Satoh, D.M. Armstrong, H.C. Fibiger, NADPH-Worsley, H.T. Nagamoto, G. Rose, M. Waldo, Schizophrenia and
diaphorase: A selective histochemical marker for the cholinergic nicotinic receptors, Harvard Rev. Psychiat. 2 (1994) 179–192.
neurons in the pontine reticular formation, Neurosci. Lett. 43 (1983) [14] E. Garcia-Rill, Disorders of the reticular activating system, Med.
31–36. Hypoth. 49 (1997) 379–387.