Glycosylation of the cationic peanut peroxidase gene expressed in
transgenic tobacco
Bao Lige
a, Shengwu Ma
b, Robert B. van Huystee
a,*
aDepartment of Plant Sciences,Uni6ersity of Western Ontario,London,Ont., Canada N6A 5B7 bSiebens-Drake Research Institute,1400 Western Road,London,Ont., Canada N6G 2V4 Received 19 August 1999; received in revised form 22 February 2000; accepted 22 February 2000
Abstract
The major cationic peanut (Arachis hypogaea) peroxidase, secreted into the extracellular space, is a glycoprotein with three N-linked glycans (polysaccharides) which are connected to the peptide backbone at Asn-60, Asn-144 and Asn-185. In this report, a C-terminal histidine-tagged cationic peanut peroxidase gene was expressed in transgenic tobacco (Nicotiana tabacum). Tissue of the transgenic tobacco was cultured in suspension culture and the his-tagged peroxidase was purified in large quantities from 14-day-old suspension culture. The number of glycans, glycosylation sites and the chemical nature of glycan moieties attached to cationic peanut peroxidase expressed in transgenic tobacco were examined. Cationic peanut peroxidase isolated from the above transgenic tobacco had the identical number of complex glycans, attached at the same glycosylation sites as on cationic peanut peroxidase isolated from peanut suspension culture. Monosaccharide components of these glycans are N-acetylglucosamine (GlcNAc), mannose (Man), fucose (Fuc), xylose (Xyl) and galactose (Gal), the same sugars as found in native cationic peanut peroxidase. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Peroxidase; Peanut; N-linked glycans; Monosaccharide; Glycosylation; Transgenic tobacco
www.elsevier.com/locate/plantsci
1. Introduction
Glycosylation is one of the most important co-and post-translational modifications to proteins. Proteins, including plant proteins can be glycosy-lated at either O- or N-glycan binding sites, as reviewed [1 – 4]. Many functions have been sug-gested for protein glycosylation, including sig-nalling for intracellular targeting, protection from proteolytic breakdown, maintenance of protein stability, configuration and correct folding [5 – 9].
Proteins are direct gene products. Therefore, their sequences are determined by a template. In glycan biosynthesis, no such template is involved in the processing of the oligosaccharides. More-over, the biosynthesis of complex N-linked glycans can be affected by various environmental factors [6]. Therefore, glycoproteins may not always be similarly glycosylated when comparisons are made between different cell types from the same organ-ism [6,10,11]. Thus, if a glycoprotein is expressed in foreign host cells, its glycosylation could be very different from the original glycoprotein [12,13], and these modifications may alter the biological activity of a protein. In addition, the same oligosaccharide on different proteins may have quite different properties, depending on the orien-tation with respect to its polypeptide [7]. The sequence and linkage of each of the monosaccha-ride components and the mature oligosacchamonosaccha-ride structure at a specific glycosylation site are compli-Abbre6iations: ABEE, p-aminobenzoic ethyl ester; CM,
car-boxymethylcellulose; cPrx, cationic peanut peroxidase; cPrx-his6, six histidine-tagged cationic peanut peroxidase; Fuc, fucose; Gal, galac-tose; GlcN, glucosamine; GlcNAc,N-acetylglucosamine; Man, man-nose; NTA, nitrilotriacetic acid; PVDF membrane, polyvinylidene difluoride membrane; TFA, trifluoroacetic acid; TPCK, L-1-tosy-lamido-2-phenylethylchloromethyl ketone; Xyl, xylose.
* Corresponding author. Tel.: +1-519-6792111, ext. 6490; fax: +1-519-6613935.
E-mail address:[email protected] (R.B. van Huystee)
cated [14] and many factors may be involved. We are interested in understanding the role of glycosy-lation in the action and formation of cationic peanut peroxidase (cPrx). cPrx, a 40-kDa protein, is the major secreted isozyme of peanut peroxidases [15]. Like many plant extracellular proteins, cPrx is also an intensively studied glycoprotein with three N-linked glycans which are connected to the polypeptide backbone at 60, 144 and Asn-185 [16]. While the function of heme and calcium in the enzyme are now known [17], little if anything is known about the role of the glycans [18]. The glycans belong to the complex type and sugar composition analysis shows that N -acetylglu-cosamine (GlcNAc), mannose (Man), fucose (Fuc), galactose (Gal) and xylose (Xyl) are present in all three glycans [19], in agreement with its structure [14]. The cPrx is synthesised as a pre-protein con-taining a 22-amino acid N-terminal signal peptide [20]. When its cDNA was expressed in transgenic tobacco, the signal peptide of cPrx was able to direct the secretion of cPrx into the extracellular space [21]. As a first step towards the analysis of the biological role of glycans in the function of cPrx, a C-terminal histidine-tagged cPrx gene was ex-pressed in transgenic tobacco and purified in large quantities from the tobacco cell suspension culture. Then, the number of glycans, glycosylation sites and the chemical nature of carbohydrate moieties at-tached to cPrx expressed in transgenic tobacco were examined.
2. Materials and methods
2.1. Genetic manipulation and production of
transgenic plants
For Agrobacterium-mediated transformation, a binary plasmid was assembled as shown in Fig. 1. A portion was designed to include the full length of cPrx coding gene sequence with its N-terminal signal peptide facilitating secretion into extracellu-lar medium, plus the codons for C-terminal six consecutive histidine residues. The latter was added so as to form a recombinant protein permitting simple identification and purification. The strategy and procedure for DNA manipulation, analysis of transgenic expression and localization were com-pleted as described [21].
2.2. Production of transgenic tobacco callus
suspension culture
For the establishment of callus suspension cul-ture, leaf discs from young transformed plants known to be expressing cPrx were cultured on agar plates of callus inducing medium supplemented with 250 mg/ml of carbenicilin and 100 mg/ml of kanamycin as described [21]. After 5 – 6 weeks, suspension cultures were initiated in 250 ml liquid callus inducing medium containing 50 mg/ml of kanamycin from the developed calli at 25°C with continuous shaking (120 rpm) and maintained for 4 – 6 weeks until uniform dividing cell cultures had been established. Large scale cell cultures were started by expanding the culture from the continu-ously established cell suspension in 250 ml medium. The cells were grown in same but antibiotic-free liquid medium at room temperature with continu-ous shaking (140 rpm), and were routinely subcul-tured every 7 days by adding 250 ml of fresh medium to 250 ml of 14-day-old cell culture (grown in 500 ml medium) in an 1000-ml flask.
2.3. Purification of the expressed protein from the
spent medium
The 14-day-old spent culture medium was sepa-rated from the tobacco cells by vacuum filtration and subsequently the macromolecular components in the medium were precipitated in 70% acetone at 4°C overnight. The precipitate was centrifuged at 9000×gfor 15 min, and the pellet was resuspended
in 20 mM Na-acetate buffer (pH 5.0).
After removing the insolubles by centrifugation at 10 000×g, the supernatant was brought to 80% saturated ammonium sulphate and cen-trifuged at 13 000×g for 10 min. The pellet was resolubilized in 20mM Na-acetate buffer (pH 5.0), then dialysed against double distilled water overnight at 4°C, and the flocculated material which appeared during dialysis was removed by centrifugation at 13 000×g [15].
The dialysate was placed on a 2×15-cm car-boxymethylcellulose (CM) (BioRad) column pre-equilibrated with 20 mM Na-acetate buffer (pH 5.0), and intensively washed with 20 mM Na-ac-etate buffer (pH 5.0) to remove the unbound proteins. Then, a continuous gradient from 20 to 300 mM Na-acetate buffer (pH 5.0) was used to elute the bound protein. The CM column was regenerated with 1 M Na-acetate buffer (pH 5.0) and re-equilibrated with 20 mM Na-acetate buffer (pH 5.0) [19].
The eluate from the gradient of 20 – 300 mM was precipitated in 70% acetone at −20°C. The pellet from centrifugation at 13 000×g was air dried and resuspended in 10 mM imidazole (pH 7.0). Then, the protein solution was loaded on a 1.5×15-cm column of Ni2+-nitrilotriacetic acid (NTA) resin pre-equilibrated with 10 mM imida-zole (pH 7.0). Prior to elution, the bound
mate-rial was washed extensively with 30 mM
imidazole (pH 6.3) to remove the non-specifically bound proteins. The bound protein was eluted with 300 mM imidazole (pH 5.0) [21]. The eluate was precipitated in 70% acetone at −20°C overnight and the pellet from centrifugation at 13 000×g was air dried, and resuspended in 1 mM NH4-acetate (pH 5.0) for analysis. The column was regenerated with 600 mM imidazole (pH 5.0) and re-equilibrated with 10 mM imida-zole (pH 7.0). The purity of six histidine-tagged cationic peanut peroxidase (cPrx-his6) was exam-ined by SDS-PAGE [22] and measuring the RZ value.
2.4. Trypsin digestion and gel filtration
chromatography
The purified cPrx-his6 (30 mg) was treated with acidic acetone (0.5% HCl in pure acetone, v/v) to remove its heme component as described [23]. Then the mixture was centrifuged at 13 000×g for 15 min and the pelleted protein
was resuspended in 3 ml of 0.1 M NH4HCO3 buffer (pH 8.0). If any reddish color remained, the acidic acetone treatment was repeated until all heme moiety was completely removed. The apoprotein was collected by centrifugation, then air dried and resuspended in 3 – 4 ml of 0.1 M NH4HCO3 buffer (pH 8.0), and digested with L -1-tosylamido-2-phenylethylchloromethyl ketone-(TPCK) treated trypsin (2 mg/ml, trypsin dis-solved in 0.1 M NH4HCO3 buffer, pH 8.0) (sub-strate:enzyme=30:1, w/w) [16] at 37°C for 2 h. Then the tryptic peptides were loaded on a Bio-Gel P-6 column (1.5×90 cm, molecular weight range 1000 – 6000 Da, 50 – 150 mesh) pre-equili-brated with 0.1 M NH4HCO3 buffer (pH 7.0). The same buffer was also used as a mobile phase, and the elution was carried out at room temperature, at a flow rate of 8 ml/h. Glycopep-tide-containing fractions were identified using a modified phenol sulphuric acid method and col-lected. In this method, 50 ml of sample was mixed with 50 ml of 5% phenol in a clear 1.5-ml microtube, and 400 ml of pure sulphuric acid was added. The color of the reaction solution was observed 15 min later [24]. The fractions con-taining glycopeptides were then dialysed against HPLC grade distilled water and lyophilized for further analysis.
2.5. Separation of glycopeptides by re6erse phase
HPLC
2.6. Identification of glycopeptides by N-terminal sequence analysis
The three glycopeptides separated by HPLC were loaded on SDS-gel electrophoresis, respec-tively, and then transferred onto polyvinylidene difluoride (PVDF) membranes (BioRad). Follow-ing stainFollow-ing with Coomassie blue, the peptide bands were cut out, destained in 40% methanol, and sequenced on an Applied Biosystem Model 475 sequencing apparatus equipped with an on-line model 120A HPLC for phenylthiohydatoin amino acid identification.
2.7. Labelling of sugars with p-aminobenzoic ethyl
ester (ABEE)
The preparation of ABEE reagent and the derivatization of sugars were completed as de-scribed [19]. To prepare the stock for ABEE reagent, 330 mg ABEE and 70 mg sodium cyanoborohydride were dissolved in 700 ml methanol, and then 82 ml glacial acetic acid was added. The stock was stored at 4°C. Before use, the ABEE reagent was warmed to 25°C to dissolve any crystals formed during storage.
To derivatize standard sugars, 80 ml of ABEE reagent was added to aliquots of 20ml of different concentrations of standard sugars containing 800 nmol lactose. Lactose was used as an internal standard and derivatized along with other sugars. Following a brief vortexing, the mixture was incu-bated in a heating block at 80°C for 55 min. Then 380 ml HPLC grade distilled water was added to the reaction vials and mixed by vortexing. Chloro-form (1 ml) was added to the mixture and vor-texed vigorously to extract the free ABEE reagent. The chloroform and aqueous phase were separated by placing the vials on the bench or by brief centrifugation and the chloroform (lower phase) was carefully removed by pipette. The chloroform extraction was repeated one more time and, finally, the clear aqueous phase containing the sugar derivatives was carefully collected and filtered for HPLC use.
2.8. Acid hydrolysis of glycoprotein and
glycopeptides
In order to analyse the sugar composition of the histidine-tagged cPrx as a whole or the sugar
composition of its individual glycans, cPrx or its glycans were hydrolysed to their individual monosaccharides. Glycoprotein (4 mg) or gly-copeptides (2 mg) from the HPLC separation were dissolved in 100 ml of HPLC grade distilled water in a Pierce Reacti-vial (1×3 cm), and 100 ml of 4 M TFA was added to give a final concentration of 2 M TFA. The vial was capped tightly, vortexed briefly and placed in a heating block (Fisher Scien-tific) at 100°C for 6 h [25]. The hydrolysate was cooled to room temperature and dried completely using a vacuum dessicator.
For the derivatization of glycoprotein or gly-copeptide hydrolysate, the hydrolysates obtained from acid hydrolysis was dissolved in 20 ml of 40 mM lactose, and 80 ml of ABEE reagent was added. Other procedures were the same as the derivatization of standard sugars as described above, and the samples containing the sugar derivatives were also analyzed by HPLC.
2.9. Re6erse phase HPLC analysis of the sugar
deri6ati6es and construction of calibration cur6es
for quantitati6e analysis
The samples of ABEE sugar derivatives in the aqueous phase were subjected to HPLC C-18 column for sugar analysis using a 20-ml sample loop. The chromatography was performed at room temperature in an isocratic mode with 86% solvent A (50 mM sodium acetate buffer, pH 4.5) and 14% solvent B (50 mM sodium acetate buffer, pH 4.5/acetonitrile/methanol=40/40/20, v/v/v) at a flow rate of 2.4 ml/min for 60 min. The sugar derivatives were detected at 254 nm [19].
To set up standard curves, a range of standard sugars, Man, Gal, GlcNAc, Xyl, Fuc, from 200 to 800 nmol in 20-ml solutions containing 800 nmol of lactose as an internal standard, were derivatized with ABEE reagent, respectively, and 5% (20 ml) of the derivatives was analyzed using HPLC as described above.
3. Results
3.1. Purification of the histidine-tagged cPrx from
the tobacco medium
intro-Fig. 2. Verification of the cPrx purification from suspension culture medium of transgenic tobacco cells. After sequential acetone and ammonium sulphate precipitation, and CM and Ni2+-NTA chromatography, various amounts of purified
protein were visualised on SDS-PAGE stained with Coomassie blue: lane 1, 5mg; lane 2, 10mg; lane 3, 15mg; lane 4, 20mg; lane 5, 25mg.
described in [21]. Cell-culture makes the large scale isolation of the expressed protein with high purity easy and effective. Large amounts of glycoprotein were needed to identify the number, the glycan binding sites, and the sugar composition. Follow-ing the steps described in Section 2, the result of purification was visualized on SDS-PAGE stained with Coomassie blue as shown in Fig. 2. When protein samples ranging from 5 to 25 mg were loaded on SDS-PAGE, only a single band was detected and such purity was believed to be satis-factory to conduct the further analysis of its glyco-sylation pattern.
3.2. HPLC separation of glycopeptide
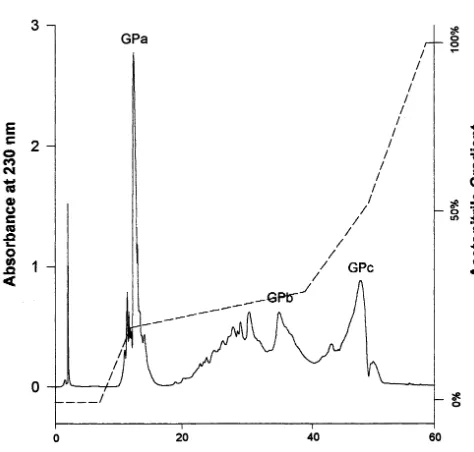
According to the deduced amino acid sequence from the cDNA, cPrx has four potential N-glyco-sylation sites with the consensus sequence of Asn-X-Thr/Ser [16]. Studies showed, however, that only three of them (Asn-60, Asn-144 and Asn-185) are indeed glycosylated, and the glycans can be separated into three glycopeptides with trypsin treatment [16]. Therefore, purified cPrx-his6 was digested with TPCK-treated trypsin and Bio-Gel P-6 filtration chromatography was used to sepa-rate the glycopeptides from the majority of other tryptic peptides. This eliminated a number of tryp-tic peptide fragments which could have caused excessive noise on the profiles of the subsequent HPLC separation. As a result, the designation of glycopeptide peaks on the HPLC profile was sim-plified to a large extent. The elution pattern for the HPLC separation of glycopeptides from re-combinant cPrx-his6 (Fig. 3) is very similar to that of wild type cPrx on HPLC observed by Sun et al. [19]. A total of three glycopeptides were detected at similar retention times in the same gradient ranges, and they were also designated as GPa, GPb and GPc, representing N-185, -60, and -144 [16]. This result indicates that the histidine-tagged cPrx expressed in transgenic tobacco has the same three glycans as found in wild type cPrx.
3.3. Identification of glycopeptides after HPLC
separation
The three tryptic glycopeptides separated on HPLC were further identified by N-terminal se-quence analysis. A comparison of the amino acid sequencing results, summarized in Table 1, with
Fig. 3. Separation of the three glycopeptides of cPrx-his6 by reverse phase HPLC. The glycopeptides generated by trypsin digestion and preliminarily purified through Bio-gel filtration were separated by HPLC with a C-18 column as described in Section 2. The acetonitrile gradient is indicated as dashed lines. Elution was monitored at wavelength 230 nm for pep-tides. The detected glycopeptides peaks are designated as GPa, GPb and GPc, respectively.
Table 1
Partial N-terminal sequencing analysis of tryptic glycopeptides obtained by HPLC from cationic peanut peroxidasea
aGlycan linkages for GPb and GPc occur at an ASN well beyond the analyzed peptide sequence.
the amino acid sequence deduced from the cPrx suggests strongly that cDNA sequence verified the occurrence of glycopeptides GPa, GPb and GPc at the glycosylation sites 184, 60 and
Asn-144, respectively. These data provided further evi-dence for the conclusion from earlier experiments with monoclonal antibodies recognizing the carbo-hydrate-containing motifs [21].
3.4. ABEE deri6atization of sugars
The oligosaccharide chains in glycoprotein or glycopeptides were first hydrolysed by TFA into their monosaccharides. Then the monosaccharides were coupled to the ultraviolet-absorbing com-pound ABEE by reductive amination with sodium cyanoborohydride [25]. As a result, each monosac-charide ABEE derivative was detectable at 254 nm using the UV detector on the HPLC, which gave a good resolution in single chromatographic step. The derivatization procedure of reducing sugars with ABEE in the presence of sodium cyanoboro-hydride has some advantages in that it is very simple and results in no side products [25].
3.5. Analysis of monosaccharide composition and
construction of standard cur6es for quantitati6e
analysis
In N-linked plant glycoproteins some specific abundant monosaccharides such as Gal, Man, Xyl, Fuc, GlcNAc and GalNAc are present [14,26 – 28]. But not all these monosaccharides, especially Gal, were always found in plant glyco-proteins [29,30]. Prior to quantitative analysis, a qualitative analysis of monosaccharides was, therefore, needed to determine the exact compo-nents. Intact protein of cPrx-his6 was hydrolysed and derivatized, and the sugar derivatives were applied to HPLC using 86% solvent A and 14%
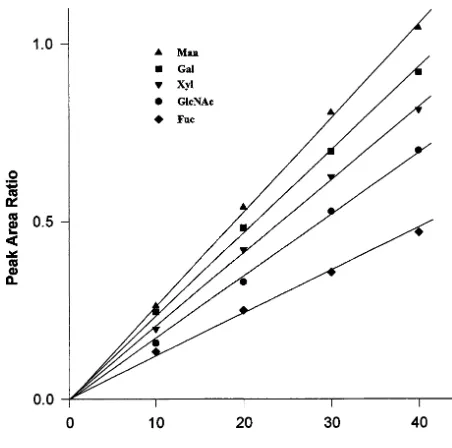
Fig. 5. Calibration curves for quantitative analysis of monosaccharides. Varying amounts of standard sugar deriva-tives (200 – 800 nmol) in 20-ml aqueous solutions containing 800 nmol lactose were derivatized with ABEE reagent, and 5% of the derivatives was subjected to HPLC. The peak area ratios were expressed relative to the peak area of 40 nmol lactose.
represent GlcNAc because N-acetylated amino sug-ars like GalNAc and GlcNAc are deacetylated during the process of hydrolysis, but could be analyzed without reacetylation in single chromato-graphic step [19,25].
With these results on the monosaccharide of cPrx-his6 as a basis, the quantitative analysis was started with the construction of standard curves. Aliquots of 20 ml of solutions containing various amounts (200 – 800 nmol) of standard monosaccha-rides and 800 nmol lactose used as an internal standard were converted to their ABEE derivatives and 5% (10 – 40 nmol) was subjected to reverse phase HPLC. Then the average of three determina-tions was used to construct the standard curves. The high sensitivity and reproducibility of sugar separa-tion provided a linear profile of the standard curves, when the peak area ratio was expressed, relative to 40 nmol lactose upon reverse phase HPLC separa-tion of 5% of the sample (10 – 40 nmol) (Fig. 5).
3.6. Monosaccharide composition of cPrx-his6
glycans
To determine the monosaccharide composition for individual glycans, the three tryptic glycopep-tides obtained from the trypsin digestion of cPrx expressed in transgenic tobacco were separated on HPLC, hydrolysed with TFA and ABEE deriva-tized. Then, the sugar derivatives were subjected to reverse phase HPLC for analysis. Lactose was used as an internal standard to calculate the peak area ratios of the detected monosaccharides. The molar percentages calculated according to the standard curves are presented in Table 2. Monosaccharide components of native cPrx [19], and NMR analysis of the glycan structure [14] show that the native cPrx glycans are complex glycans. The sugar composi-tion of GPc from cPrx-his6 is essentially the same as that of native GPc. GPa and GPb in transgenic tobacco have the same amounts of Man, Gal, and GlcNAc, but they have increased amounts of Fuc and smaller amounts of Xyl. These data strongly indicate that the complex type of oligosaccharides is present on all the glycosylation sites. Further-more, a comparison of these data with some of the complex glycan structures established for some plant glycoproteins [4,30,31] also suggested the existence of complex glycans in cPrx-his6. That is to say, cPrx in tobacco cells was targeted to ER and proceeded through the processing in the Golgi compartments.
Table 2
Monosaccharide compositions (mol %) of glycans from cPrx-his6a
GlcNAc
Glycans Gal Man Xyl Fuc
38.4
GPa 8.3 33.3 7.7 12.3 39.8 8.5
GPb 26.9 9.1 15.7
27.9 5.1 12.2 GPc 48.5 6.4
aTryptic glycopeptides were separated by HPLC,
hy-drolysed and derivatized with ABEE, then the molar percent-ages were calculated according to the standard curves.
4. Discussion
In order to examine the glycosylation and glycan modifications of cPrx later [18] a histidine-tagged cPrx was expressed in transgenic tobacco from which a suspension culture was derived. The recom-binant protein was purified by nickel-affinity chro-matography from 14-day-old medium of these cultured tobacco cells. Tobacco cell suspension culture provided a rich source for the isolation of the expressed cPrx-his6, because a small number of proteins exist in the medium as compared to many in the plant extract [32]. Therefore, the simplicity of isolating the expressed protein from the spent suspension culture medium rather than from the cell extract was obvious. After standard purification and Ni2+-NTA chromatography, a large amount of cPrx with a high purity was obtained. Considering that cPrx contains 20% carbohydrates spread over three glycans, much protein is needed for indepen-dent monosaccharide analysis. Thus without an effective purification method, it would be impossi-ble to carry out the subsequent purification of glycopeptides and sugar composition analysis.
To assure that subsequent studies on cPrx with one specific glycan missing is reliable there is a need to verify that the remaining glycans from the native enzyme and that from the transgenic product are the same. The question was: (1) whether the same glycosylation sites are used for the attachment of N-linked glycans in the transgenic tobacco; and (2) whether the monosaccharide composition of the glycans from the transgenic product was closely similar to the original cPrx. Purification of tryptic glycopeptides from cPrx-his6 and subsequent anal-ysis by N-terminal sequencing, qualitative and quantitative analysis of the monosaccharide com-position have confirmed that the same glycosylation sites were used in transgenic tobacco. Moreover, cPrx-his6 has similar complex type of glycans with GlcNAc, Man, Fuc, Xyl and Gal found in native cPrx. Oligosaccharides of plant glycoproteins are formed by modifying a common core structure Mana3(Mana6)(Xylb2)Manb4GlcNAcb4(Fucb3)
GlcNAc, and one or more of other sugar residues
are attached to this core structure [4,18,29,33,34]. Based on the data from Table 2, in addition to an obvious Gal, it can be concluded that Fuc, GlcNAc and Man are also involved in the further modifica-tions of the core structure [18]. Protein glycosylation
is influenced by three main factors: the overall protein conformation, the effect of local conforma-tion, and the available repertoire of glycosylation-processing enzymes [7]. N-glycosylation mostly occurs at an Asn residue which is in ab-turn of the polypeptide in an Asn-X-Ser/Thr sequence [35]. According to the 3D structure of cPrx from X-ray crystallography [36], the three glycans are indeed attached to the loop areas. Therefore, cPrx ex-pressed in transgenic tobacco, at least, has to have the same b-turns of cPRX in tobacco cells to be glycosylated at the same sites as they occur in peanut. While1H-NMR of 12 sugar glycans of the cPRX is a most complex and time consuming job [14], in the meanwhile the monosaccharide compo-nents and type of glycans at those glycosylation sites in transgenic tobacco suggest that they are the same as that of native cPrx from peanut. In addition the initial signal peptide for cPrx was able to direct its targeting and secretion in tobacco cells. Considering these results it is appropriate to state that the mechanisms of protein targeting, and folding, the accessibility of the glycans to the modifications and the glycosidases and glycosyltransferases are very much conserved between peanut and tobacco plants. The next study on ablation of a single glycan and its effect on the enzyme may now proceed.
Acknowledgements
We thank Dr R. Esnault (Institute des Sciences Vegetales, CNRS, Gif-sur-Yvette, France) for providing us with the plasmid containing the cPrx cDNA. This work was supported by grant from the Natural Sciences and Engineering Council of Canada.
References
[1] J. Montreuil, J.F.G. Vliegenthart, H. Schachter, Glyco-proteins, in: A. Neuberger, L.L.M. van Deenen (Eds.), New Comprehensive Biochemistry, vol. 29a, Elsevier, Amsterdam, 1995.
[2] F.M. Klis, O-glycosylation in plants, in: A. Neuberger, L.L.M. van Deenen (Eds.), New Comprehensive Bio-chemistry, vol. 29a, Elsevier, Amsterdam, 1995, pp. 511 – 520.
[4] P. Lerouge, L. Faye, Recent development in structural analysis of N-glycans from plant glycoproteins, Plant Physiol. Biochem. 34 (1996) 263 – 271.
[5] R.A. Dwek, Glycobiology: toward understanding the function of sugars, Chem. Rev. 96 (1996) 683 – 720. [6] P.M. Rudd, R.A. Dwek, Glycosylation: heterogeneity and
the 3D structure of proteins, Crit. Rev. Biochem. Mol. Biol. 32 (1997) 1 – 100.
[7] R.A. Dwek, Glycobiology: more functions for oligosac-charides, Science 269 (1995) 1234 – 1235.
[8] H. Lis, N. Sharon, Protein glycosylation: structural and functional aspects, Eur. J. Biochem. 218 (1993) 1 – 27. [9] R.T. Schwarz, R. Datema, The lipid pathway of protein
glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates, Adv. Carbohydr. Chem. Biochem. 40 (1982) 287 – 379.
[10] C. Goulut-Chassaing, R. Bourrillon, Structural differences between complex-type Asn-linked glycan chains of glyco-proteins in rat hepatocytes and Zajdela hepatoma cells, Biochem. Biophys. Acta 1244 (1995) 30 – 40.
[11] P. Lerouge, M. Cabaes-Macheteau, C. Rayon, A.-C. Fischette-Lain, V. Gomord, L. Faye, N-glycoprotein biosynthesis in plants: recent developments and future trends, Plant Mol. Biol. 38 (1998) 31 – 48.
[12] C.G. Haidaris, D.J. Fisher, F. Gigliotti, P.J. Simpson-Haidaris, Antigenic properties of recombinant glycosy-lated and non-glycosylated Pneumocystis carinii
glycoprotein A polypeptides expressed in baculovirus-in-fected insect cells, Mol. Biotechnol. 9 (1998) 91 – 97. [13] L. Zeitlin, S.S. Olmsted, T.R. Moench, M.S. Co, B.J.
Martinelli, V.M. Paradkar, D.R. Russell, C. Queen, R.A. Cone, K.J. Whaley, A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against herpes, Nat. Biotechn. 16 (1998) 1361 – 1364.
[14] G.S. Shaw, Y. Sun, K.R. Barber, R.B. van Huystee, Sequence specific analysis of the heterogeneous glycan chain from peanut peroxidase by1H-NMR,
Phytochem-istry 53 (2000) 135 – 144.
[15] P.A. Sesto, R.B. van Huystee, Purification and yield of a cationic peroxidase from a peanut suspension cell culture, Plant Sci. 61 (1989) 163 – 168.
[16] L. Wan, R.B. van Huystee, A study on glycosylation of cationic peanut peroxidase, Biochem. Biophys. Res. Com-mun. 194 (1993) 1398 – 1405.
[17] K.R. Barber, M.J. Rodriquez Maranon, G.S. Shaw, R.B. van Huystee, Structural influence of calcium on the heme cavity of cationic peanut peroxidase as determined by H-NMR spectroscopy, Eur. J. Biochem. 232 (1995) 825 – 833.
[18] R.B. van Huystee, M.T. McManus, Glycans of higher plant peroxidases: recent observations and future specula-tions, Glycoconjugate J. 15 (1998) 101 – 106.
[19] Y. Sun, B. Lige, R.B. van Huystee, HPLC determination of the sugar compositions of the glycans on the cationic peanut peroxidase, J. Agric. Food Chem. 45 (1997) 4196 – 4200.
[20] D. Buffard, C. Breda, R.B. van Huystee, O. Asemota, M. Pierre, H.D.B. Dang, R. Esnault, Molecular cloning of complementary DNAs encoding two cationic peroxidases from cultivated peanut cells, Proc. Natl. Acad. Sci. USA
87 (1990) 8874 – 8878.
[21] B. Lige, S. Ma, D. Zhao, R.B. van Huystee, Cationic peanut peroxidase: expression and characterization in transgenic tobacco and purification of the histidine-tagged protein, Plant Sci. 136 (1998) 159 – 168.
[22] U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[23] R.N. Chibbar, R. Cella, R.B. van Huystee, The heme moiety in peanut peroxidase, Can. J. Biochem. Cell Biol. 62 (1984) 1046 – 1050.
[24] M.K. Dubois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem. 28 (1956) 350 – 356.
[25] H. Kwon, J. Kim, Determination of monosaccharides in glycoproteins by reverse-phase high-performance liquid chromatography, Anal. Biochem. 215 (1993) 243 – 252. [26] W.T. Wang, N.C. Ledonne, B. Ackerman, C.C. Sweeley,
Structural characterization of oligosaccharides by high-performance liquid chromatography, fast atom bombard-ment-mass spectrometry, and exoglycosidase digestion, Anal. Biochem. 141 (1984) 366 – 381.
[27] J.F.G. Vliegenthart, J. Montreuil, Primary structure of glycoprotein glycans, in: J. Montreuil, J.F.G. Vliegen-thart, H. Schachter (Eds.), Glycoprotein, Elsevier, New York, 1995, pp. 13 – 25.
[28] U. Sonnewald, A. Sturm, M.J. Chrispeels, L. Willmitzer, Targeting and glycosylation of patatin, the major potato tuber protein in leaves of transgenic tobacco, Planta 179 (1989) 171 – 180.
[29] L. Faye, K.D. Johnson, A. Sturm, M.J. Chrispeels, Structure, biosynthesis, and function of asparagine-linked glycans on plant glycoproteins, Physiol. Plant. 75 (1989) 309 – 314.
[30] D. Ashford, R.A. Dwek, J.K. Welply, S.W. Homans, H. Lis, G.N. Taylor, T.W. Rademacher, 1,2-D-xylose and a-1,3-L-fucose substituted N-linked oligosaccharides from
Erythrina cristagalli lectin, Eur. J. Biochem. 166 (1987) 311 – 320.
[31] B. Fournet, Y. Leroy, J.-M. Wieruszeski, J. Montreuil, R.D. Portez, R. Goldberg, Primary structure of an N-glycosidic carbohydrate unit derived from Sophora japonicalectin, Eur. J. Biochem. 166 (1987) 321 – 324. [32] R.B. van Huystee, A.S.K. Tam, Peptides released by
cultured peanut cells during culture, J. Plant Physiol. 133 (1988) 645 – 647.
[33] A.R. Kermode, Mechanisms of intracellular protein trans-port and targeting in plant cells, in: B.V. Conger (Ed.), Critical Reviews in Plant Science, vol. 15, CRC Press, Boca Raton, FL, 1996, pp. 285 – 423.
[34] C. Rayon, V. Gomord, L. Faye, P. Lerouge, N-glycosyla-tion of phytohemagglutinin expressed in bean cotyledons or in transgenic tobacco plants, Plant Physiol. Biochem. 34 (1996) 273 – 281.
[35] R. Kornfeld, S. Kornfeld, Assembly of asparagine-linked oligosaccharides, Annu. Rev. Biochem. 54 (1985) 631 – 664.
[36] D.J. Schuller, N. Ban, R.B. van Huystee, A. McPherson, T.L. Poulos, The crystal structure of peanut peroxidases, Structure 4 (1996) 311 – 321.